Effects of Escherichia coli Alkaline Phosphatase PhoA on the Mineralization of Dissolved Organic Phosphorus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Alkaline Phosphatase PhoA
2.1.2. Organic Phosphorus
2.1.3. Amino Acids
2.2. Experimental Methods
2.2.1. Specificity of PhoA to Hydrolyze Phosphate Monoesters
2.2.2. Reaction Time of PhoA to Completely Mineralize Phosphate Monoesters
2.2.3. Influence of Organic Phosphorus Concentration on PhoA Mineralization
2.2.4. Effects of Soluble Amino Acids on PhoA Mineralization
2.3. Data Processing and Analysis
3. Results and Discussion
3.1. The Specificity of PhoA to Hydrolyze Phosphate Monoesters
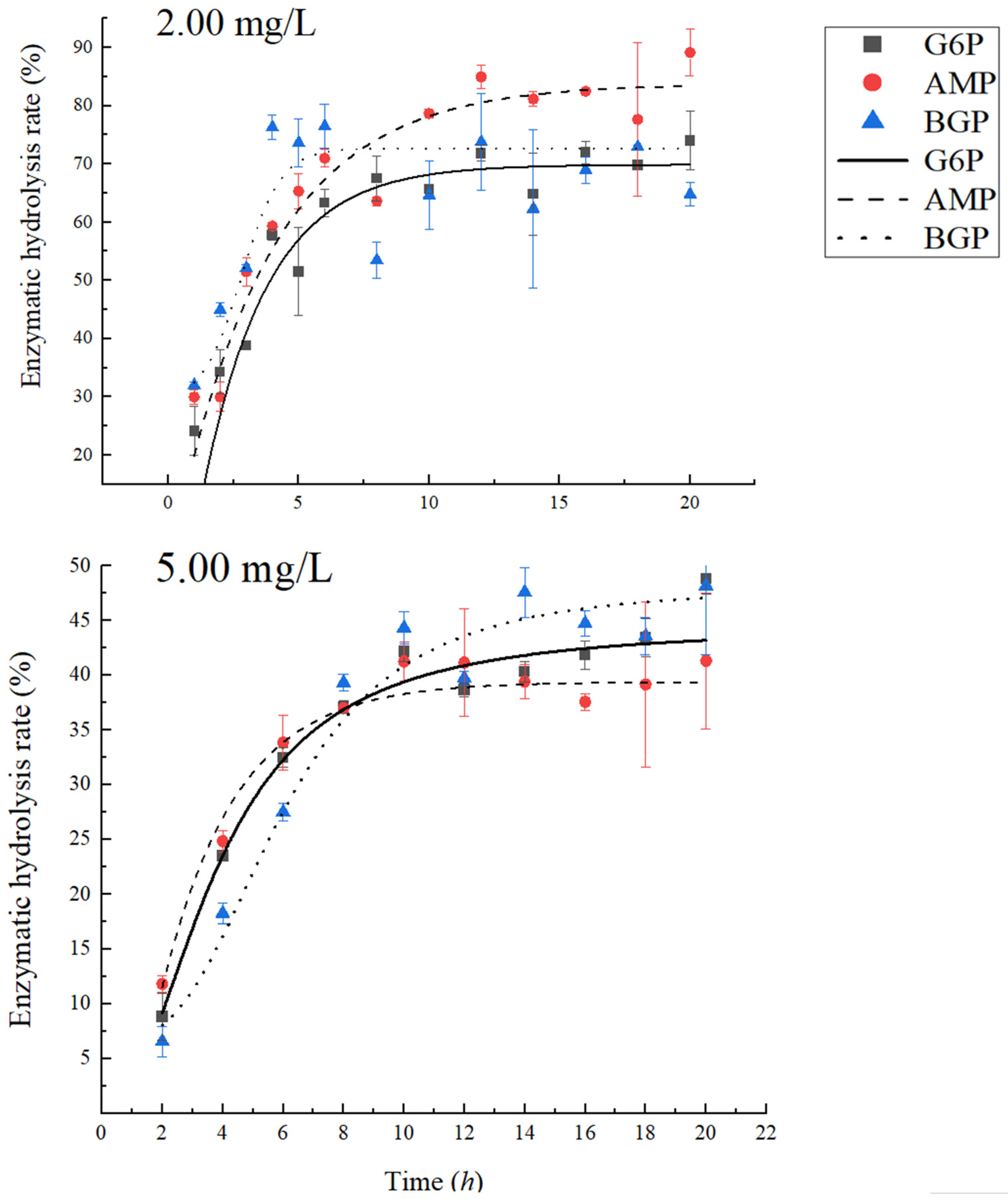
3.2. The Reaction Time of PhoA to Completely Mineralize Phosphate Monoesters
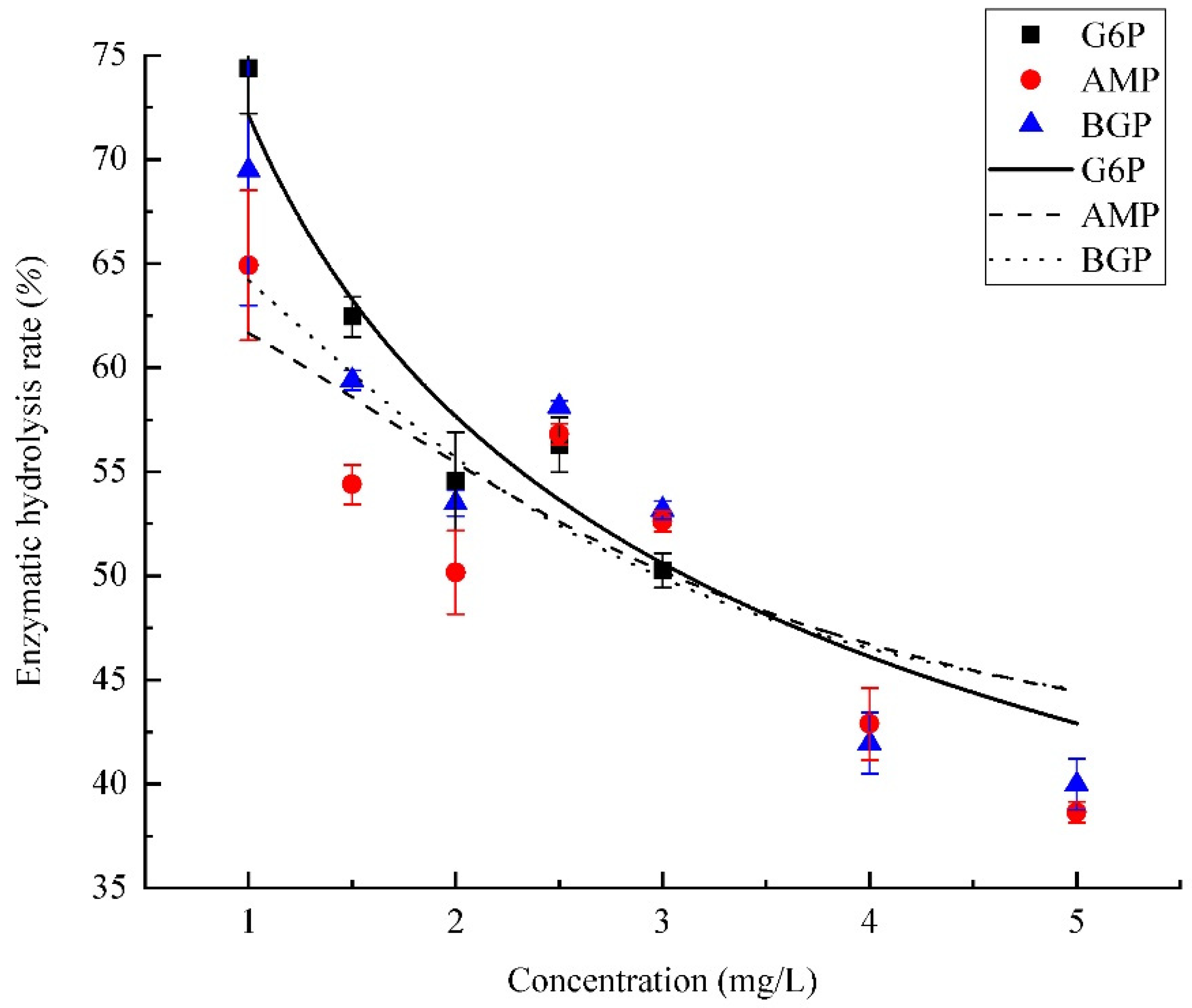
3.3. Effects of Organic Phosphorus Concentration on PhoA Mineralization
3.4. Effects of Different Soluble Amino Acids on PhoA Mineralization of Organic Phosphorus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Smith, D.C.; Steward, G.F.; Azam, F. Variability in ectohydrolytic enzyme activities of pelagic marine bacteria and its significance for substrate processing in the sea. Aquat. Microb. Ecol. 1996, 10, 223–230. [Google Scholar] [CrossRef]
- Luo, H.; Benner, R.; Long, R.; Hu, J. Subcellular localization of marine bacterial alkaline phosphatases. Proc. Natl. Acad. Sci. USA 2009, 106, 212–219. [Google Scholar] [CrossRef]
- Dai, J.Y.; Gao, G.; Wu, S.Q.; Wu, X.F.; Zhou, J.; Xue, W.Y.; Yang, Q.Q.; Chen, D. Bacterial alkaline phosphatases and affiliated encoding genes in natural waters: A review. J. Lake Sci. 2016, 28, 1153–1166. [Google Scholar]
- Ragot, S.A.; Kertesz, M.A.; Bünemann, E.K. PhoD alkaline phosphatase gene diversity in soil. Appl. Environ. Microb. 2015, 81, 7281–7289. [Google Scholar] [CrossRef]
- Sebastian, M.; Ammerman, J.W. The alkaline phosphatase PhoX is more widely distributed in marine bacteria than the classical PhoA. ISME J. 2009, 3, 563–572. [Google Scholar] [CrossRef]
- Skouri-Panet, F.; Benzerara., K.; Cosmidis, J.; Férard, C.; Caumes, G.; DeLuca, G.; Heulin, T.; Duprat, E. In vitro and in silico evidence of phosphatase diversity in the biomineralizing bacterium ramlibactertataouinensis. Front. Microbiol. 2018, 8, 2592. [Google Scholar] [CrossRef] [PubMed]
- Lidbury, I.D.E.A.; Borsetto, C.; Murphy, A.R.J.; Bottrill, A.; Jones, A.M.E.; Bending, G.D.; Hammond, J.P.; Chen, Y.; Wellington, E.M.H.; Scanlan, D.J. Niche-adaptation in plant-associated Bacteroidetes favours specialisation in organic phosphorus mineralisation. ISME J. 2021, 15, 1040–1055. [Google Scholar] [CrossRef]
- Cosmidis, J.; Benzerara, K.; Guyot, F.; Skouri-Panet, F.; Duprat, E.; Férard, C.; Guigner, J.-M.; Babonneau, F.; Coelho, C. Calcium-phosphate biomineralization induced by alkaline phosphatase activity in escherichia coli: Localization, kinetics, and potential signatures in the fossil record. Front. Earth Sci. 2015, 3, 84. [Google Scholar] [CrossRef]
- Lin, W.T.; Zhao, D.D.; Luo, J.F. Distribution of alkaline phosphatase genes in cyanobacteria and the role of alkaline phosphatase on the acquisition of phosphorus from dissolved organic phosphorus for cyanobacterial growth. J. Appl. Phycol. 2018, 30, 839–850. [Google Scholar] [CrossRef]
- Hong, S.J.; Pan, Q.H.; Chen, S.Y.; Zu, Y.; Xu, C.X.; Li, J.H. Identification and characterization of alkaline phosphatase gene phoX in Microcystis aeruginosa PCC7806. 3. Biotech 2021, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, B.W.; Vonk, J.A.; Beusekom, S.A.M.V.; Ibanez, M.; de Lucas Pardo, M.A.; Noordhuis, R.; Manders, E.M.M.; Verspagen, J.M.H.; Geest, H.G.V.D. Benthic hotspots in the pelagic zone: Light and phosphate availability alter aggregates of microalgae and suspended particles in a shallow turbid lake. Limnol. Oceanogr. 2018, 9999, 1–12. [Google Scholar] [CrossRef]
- McMahon, K.D.; Read, E.K. Microbial contributions to phosphorus cycling in eutrophic lakes and wastewater. Annu. Rev. Microbiol. 2013, 67, 199–219. [Google Scholar] [CrossRef]
- Kim, E.E.; Wyckoff, H.W. Reaction mechanism of alkaline phosphatase based on crystal structures. Two-metal ion catalysis. J. Mol. Biol. 1991, 218, 449–464. [Google Scholar] [CrossRef]
- Karunakaran, T.; Gunasekaran, P. Cloning and expression in Escherichia coli of an alkaline phosphatase (phoA) gene from Zymomonas mobili. Curr. Microbiol. 1992, 25, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M.; Ammerman, J.W. Role of the phosphatase PhoX in the phosphorus metabolism of the marine bacterium Ruegeria pomeroyi DSS-3[R]. Environ. Microbiol. Rep. 2011, 3, 535–542. [Google Scholar] [CrossRef]
- Apel, K.A.; Sola-Landa, A.; Rodriguez-Garcia, A.; Martin, J.F. Phosphate control of phoA, phoC and phoD gene expression in Streptomyces coelicolor reveals significant differences in binding of PhoP to their promoter regions. Microbiology 2007, 153, 3527–3537. [Google Scholar] [CrossRef]
- Luo, M.; Guo, Y.C.; Deng, J.Y.; Wei, H.P.; Zhang, Z.P.; Leng, Y.; Men, D.; Song, L.R.; Zhang, X.E.; Zhou, Y.F. Characterization of a monomeric heat-labile classical alkaline phosphatase from Anabaena. Biochemistry 2010, 75, 655–664. [Google Scholar] [CrossRef]
- Kageyama, H.; Tripathi, K.; Rai, A.K.; Cha-Um, S.; Waditee-Sirisattha, R.; Takabe, T. An alkaline phosphatase/phosphodiesterase, PhoD, induced by salt stress and secreted out of the cells of Aphanothece halophytica, a halotolerant cyanobacterium. Appl. Environ. Microb. 2011, 77, 5178–5183. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhu, G.W.; Gao, G.; Qin, B.Q. Spatial distribution of dissloved amino acids in Lake Taihu, China. Acta Ecol. Sin. 2013, 33, 5802–5807. [Google Scholar]
- Yao, X.; Zhu, G.W.; Cai, L.L.; Zhu, M.Y.; Zhao, L.L.; Qin, B.Q. Geochemical characteristics of amino acids in sediments of Lake Taihu, a large, shallow, eutrophic freshwater lake of China. Aquat. Geochem. 2012, 18, 263–280. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wang, S.R.; Shen, H.Y.; Yang, Y.L.; Zhao, K. Distribution of amino acids in sediments from Poyang Lake and its response to the River-Lake relationship. Acta Sci. Circumst. 2015, 35, 1302–1309. [Google Scholar]
- Cowie, G.; Hedges, J. Biochemical indicators of diagenetic alteration in natural organic matter mixtures. Nature 1994, 369, 304–307. [Google Scholar] [CrossRef]
- Dauwe, B.; Middelburg, J.J. Amino acids and hexosamines as indicators of organic matter degradation state in North Sea sediments. Limnol. Oceanogr. 1998, 43, 782–798. [Google Scholar] [CrossRef]
- Pantoja, S.; Lee, C. Amino acid remineralization and matter lability in Chilean coastal sediments. Geochemistry 2003, 34, 1047–1056. [Google Scholar] [CrossRef]
- Fernandes, J.; Amorim, R.; Azevedo, I.; Martins, M.J. In vitro modulation of alkaline phosphatase activity of Saccharomyces cerevisiae grown in low or high phosphate medium. Braz. J. Med. Biol. 2008, 41, 41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, X.C.; Tu, Q.Y. The Standard Methods for Observation and Analysis of Lake Eutrophication, 2nd ed.; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Sone, M.; Kishigami, S.; Yoshihisa, T.; Ito, K. Roles of disulfide bonds in bacterial alkaline phosphatase. Biol. Chem. 1997, 272, 6174–6178. [Google Scholar] [CrossRef]
- Eder, S.; Shi, L.; Jensen, K.; Yamane, K.; Hulett, F.M. A Bacillus subtilis secreted phosphodiesterase alkaline phosphatase is the product of a Pho regulon gene, phoD. Microbiology 1996, 142, 2041–2047. [Google Scholar] [CrossRef]
- Wu, J.R.; Shien, J.H.; Shieh, H.K.; Hu, C.C.; Chang, P.C. Cloning of the gene and characterization of the enzymatic properties of the monomeric alkaline phosphatase (PhoX) from Pasteurella multocida strain X-73. FEMS Microbiol. Lett. 2007, 267, 113–120. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, Y.G.; Liu, Z.Q. The research development of production and application of phytase. Chin. Agric. Sci. Bull. 2011, 27, 257–261. [Google Scholar]
- Menezes-Blackburn, D.; Jorquera, M.A.; Greiner, R.; Gianfreda, L.; de la Luz Mora, M. Phytases and phytase-labile organic phosphorus in manures and soils. Crit. Rev. Environ. Sci. Technol. 2013, 43, 916–954. [Google Scholar] [CrossRef]
- Azeem, M.; Riaz, A.; Chaudhary, A.Z.; Hayat, R.; Hussain, Q.; Ibrahim Tahir, M.; Imran, M. Department of plant patholog microbial phytase activity and their role in organic p mineralization. Arch. Acker Pflanzenbau Bodenkd. 2015, 61, 751–766. [Google Scholar]
- Jørgensen, C.; Jensen, H.S.; Andersen, F.; Egemose, S.; Reitzel, K. Occurrence of orthophosphate monoesters in lake sediments: Significance of myo- and scyllo-inositol hexakisphosphate. J. Environ. Monit. 2011, 13, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition, a challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y. Regulation of Alkaline Phosphatase Gene in Response to Inorganic Phosphorus Starvation in Anabaena FACHB 709; Jiangsu University: Zhenjiang, China, 2012. [Google Scholar]
- Li, Y.F. The Effect of Alkaline Phosphatase on Phosphorus Uptake by Spiraea and Its Influencing Factors; Chongqing Universitiy: Chongqing, China, 2016. [Google Scholar]
- Ericab, Y.; Rebeccac, T.; Loria, P. Alkaline phosphatase in freshwater cladophore epiphyte assemblages: Regulationin response to phosphorus supply and localization. J. Phycol. 2010, 46, 93–101. [Google Scholar]
- Lu, N.; Hu, W.P.; Deng, J.C.; Zhai, S.H.; Chen, X.M.; Zhou, X.P. Spatial distribution characteristics and ccological significance of alkaline phosphatase in water column of Taihu Lake. Chin. J. Environ. Sci. 2009, 30, 2898–2903. [Google Scholar]
- Lin, L.R.; Liao, J.H.; Chen, Q.X. Inhibition of alkaline phosphatase by amino acids. J. Nat. Sci. 2004, 43, 8–11. [Google Scholar]


| Name | Ester Bond | Solubility | Molecular Weight | pH |
|---|---|---|---|---|
| G6P | Monoester | Soluble | 304.10 | 7.8 |
| RNA | Diester | Soluble | 20,000–30,000 | 7.2 |
| IP6-Na | Monoester | Soluble | 923.82 | 9.5–11.5 |
| BGP | Monoester | Soluble | 306.11 | 7.6 |
| AMP | Monoester | Soluble | 365.24 | 3.5 |
| G6P | AMP | BGP | |
|---|---|---|---|
| L-Tyr | 0.642 | 0.325 | 0.707 |
| L-His | 0.384 | 0.014 | −0.554 |
| L-Lys | −0.653 | −0.508 | −0.685 |
| L-Arg | −0.715 | −0.323 | −0.743 |
| L-Ser | 0.282 | 0.157 | 0.426 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, T.; Jin, S.; Chen, S.; Zhang, Y. Effects of Escherichia coli Alkaline Phosphatase PhoA on the Mineralization of Dissolved Organic Phosphorus. Water 2021, 13, 3315. https://doi.org/10.3390/w13233315
Zhou Y, Zhang T, Jin S, Chen S, Zhang Y. Effects of Escherichia coli Alkaline Phosphatase PhoA on the Mineralization of Dissolved Organic Phosphorus. Water. 2021; 13(23):3315. https://doi.org/10.3390/w13233315
Chicago/Turabian StyleZhou, Yanwen, Tingxi Zhang, Shengyan Jin, Siyu Chen, and Yinlong Zhang. 2021. "Effects of Escherichia coli Alkaline Phosphatase PhoA on the Mineralization of Dissolved Organic Phosphorus" Water 13, no. 23: 3315. https://doi.org/10.3390/w13233315
APA StyleZhou, Y., Zhang, T., Jin, S., Chen, S., & Zhang, Y. (2021). Effects of Escherichia coli Alkaline Phosphatase PhoA on the Mineralization of Dissolved Organic Phosphorus. Water, 13(23), 3315. https://doi.org/10.3390/w13233315






