A Novel Combined Treatment Process of Hybrid Biosorbent–Nanofiltration for Effective Pb(II) Removal from Wastewater
Abstract
:1. Introduction
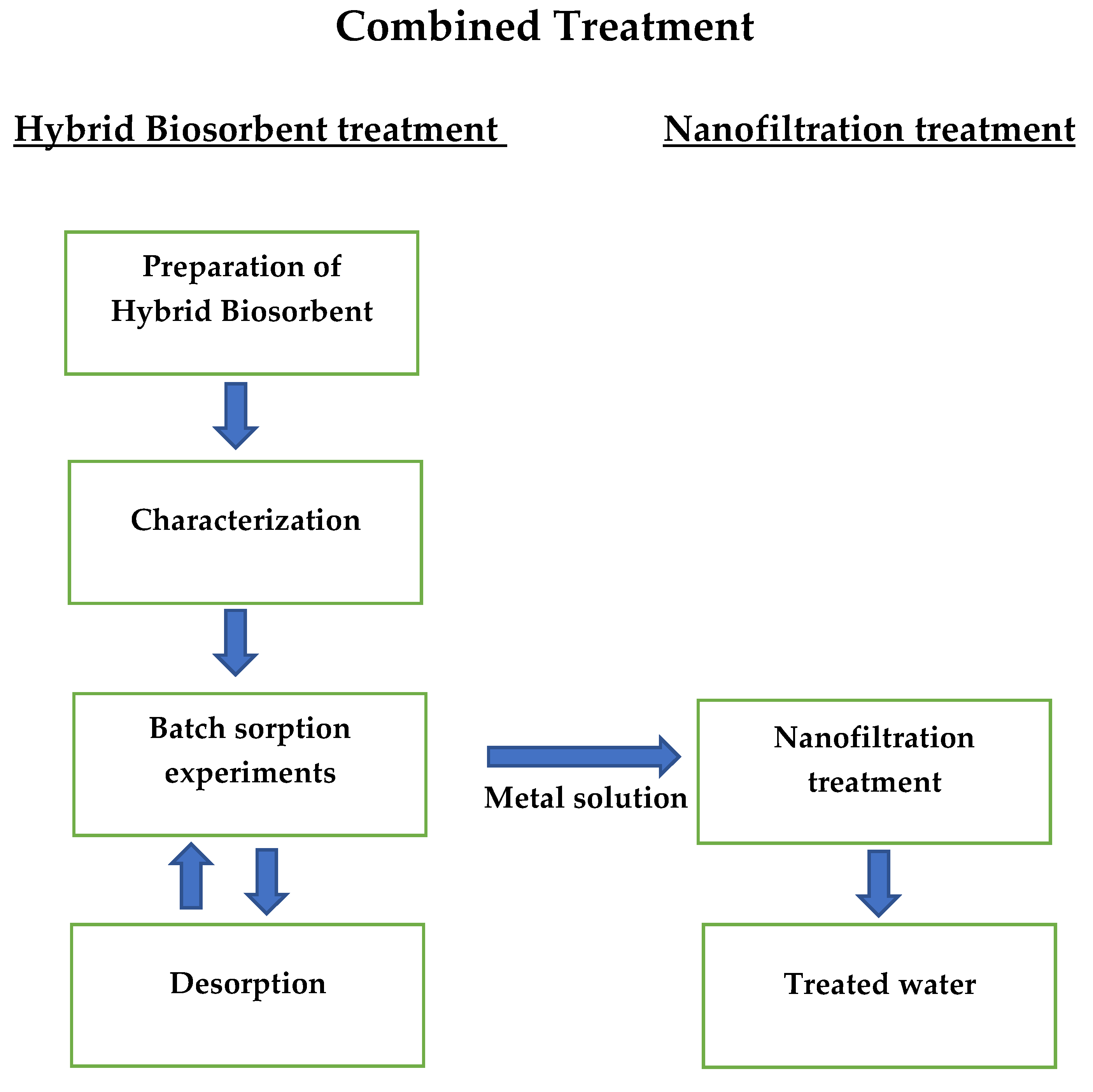
2. Materials and Methods
2.1. Chemicals
2.2. Hybrid Biosorbent Preparation and Characterization
2.3. Biosorption Studies
2.4. Biosorption Isotherm Models
2.5. Biosorption Thermodynamics
2.6. Desorption
2.7. Nanofiltration
Membrane Filtration Experiments
2.8. Statistical Analysis
3. Results and Discussion
3.1. Biosorption Study
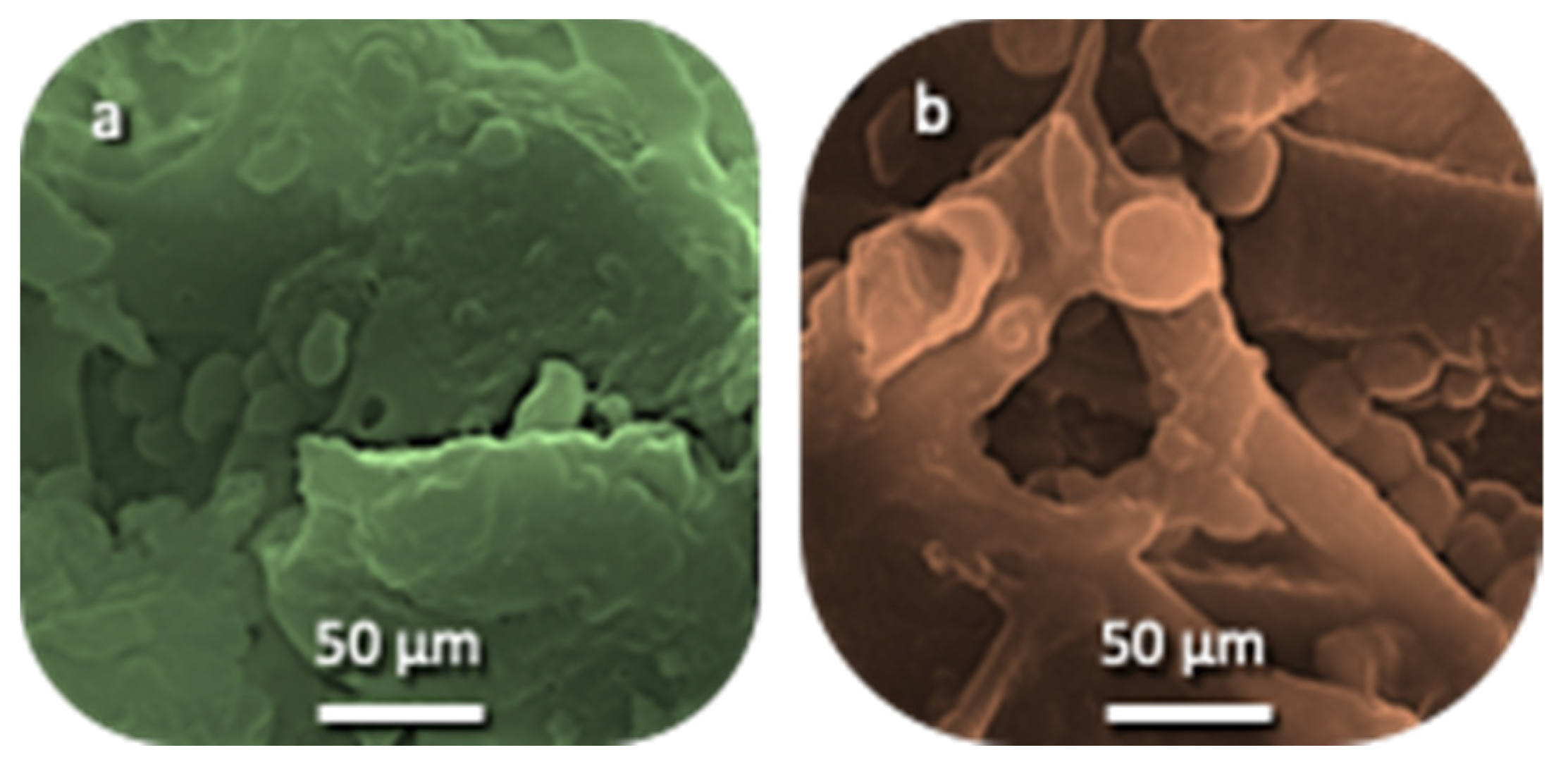
3.1.1. Characterization
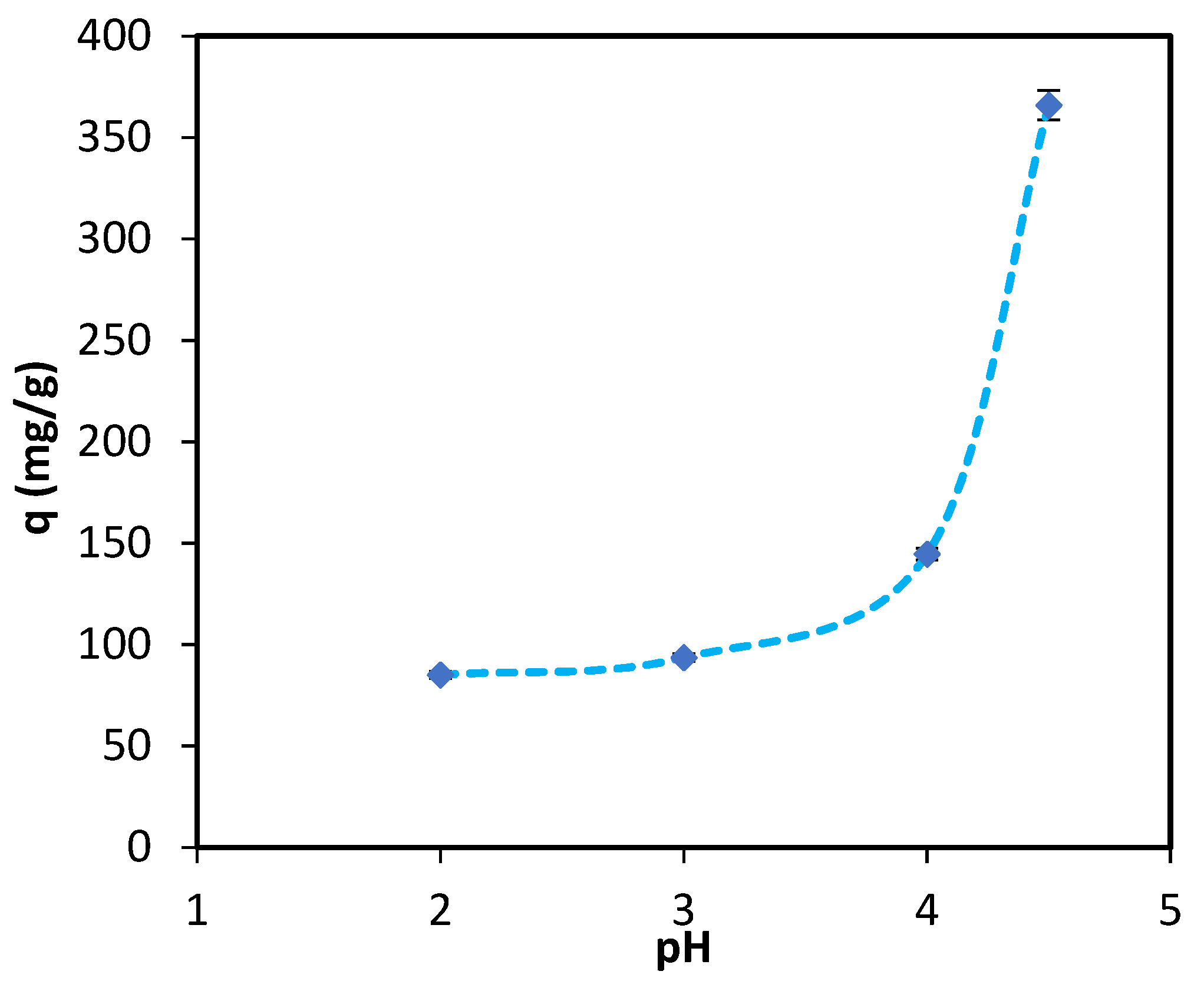
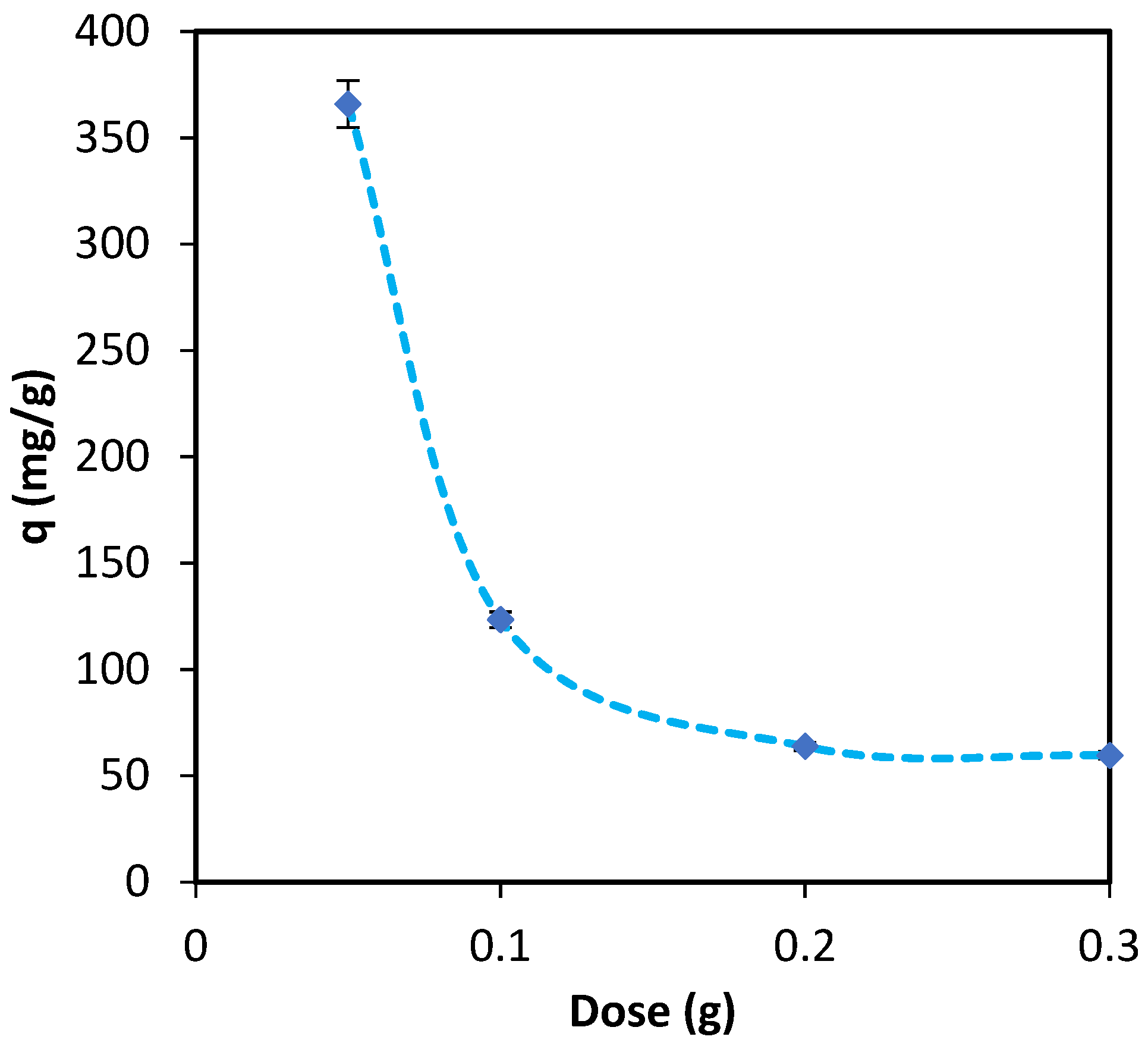
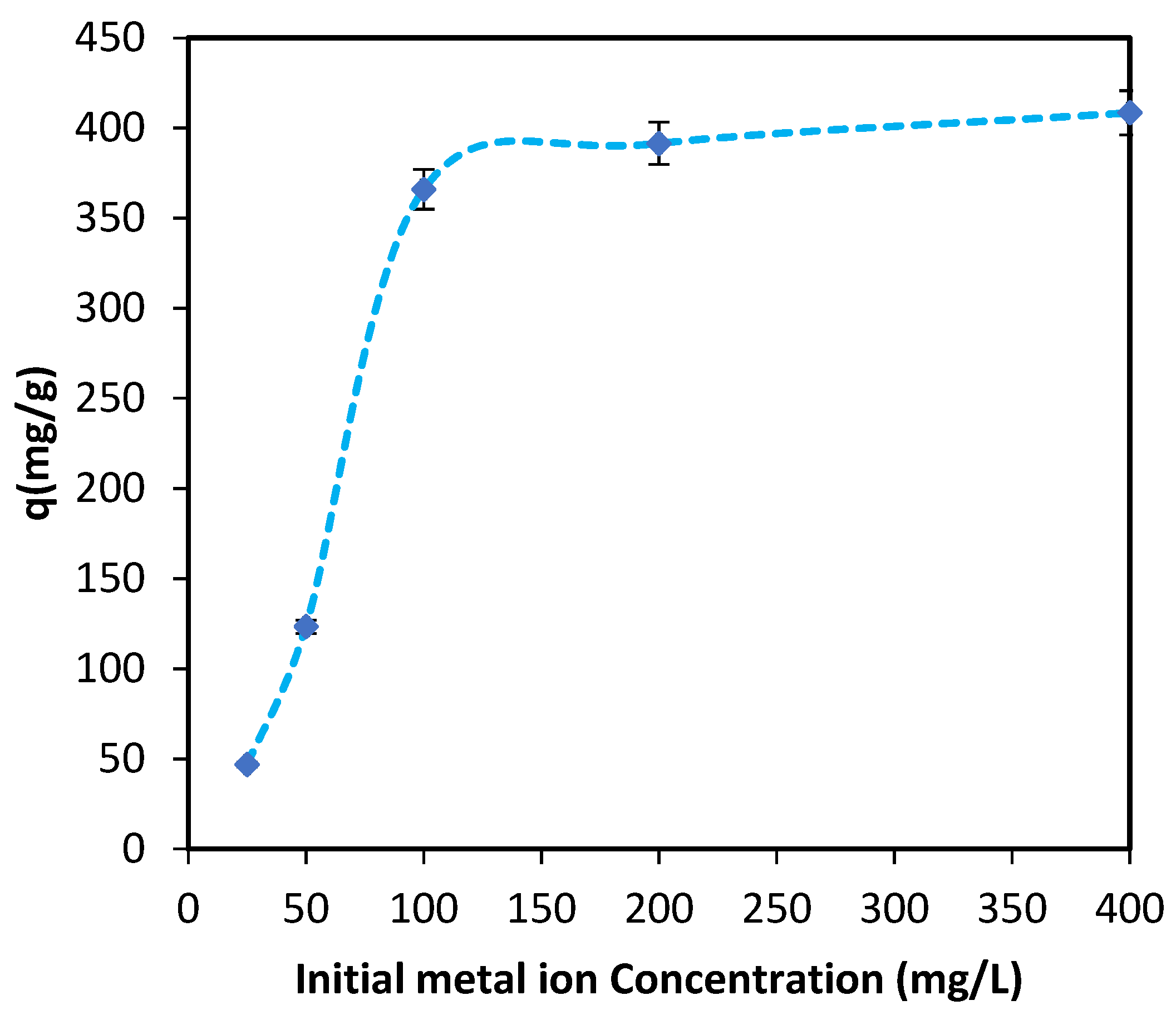
3.1.2. Batch Study
3.1.3. Biosorption Isotherms
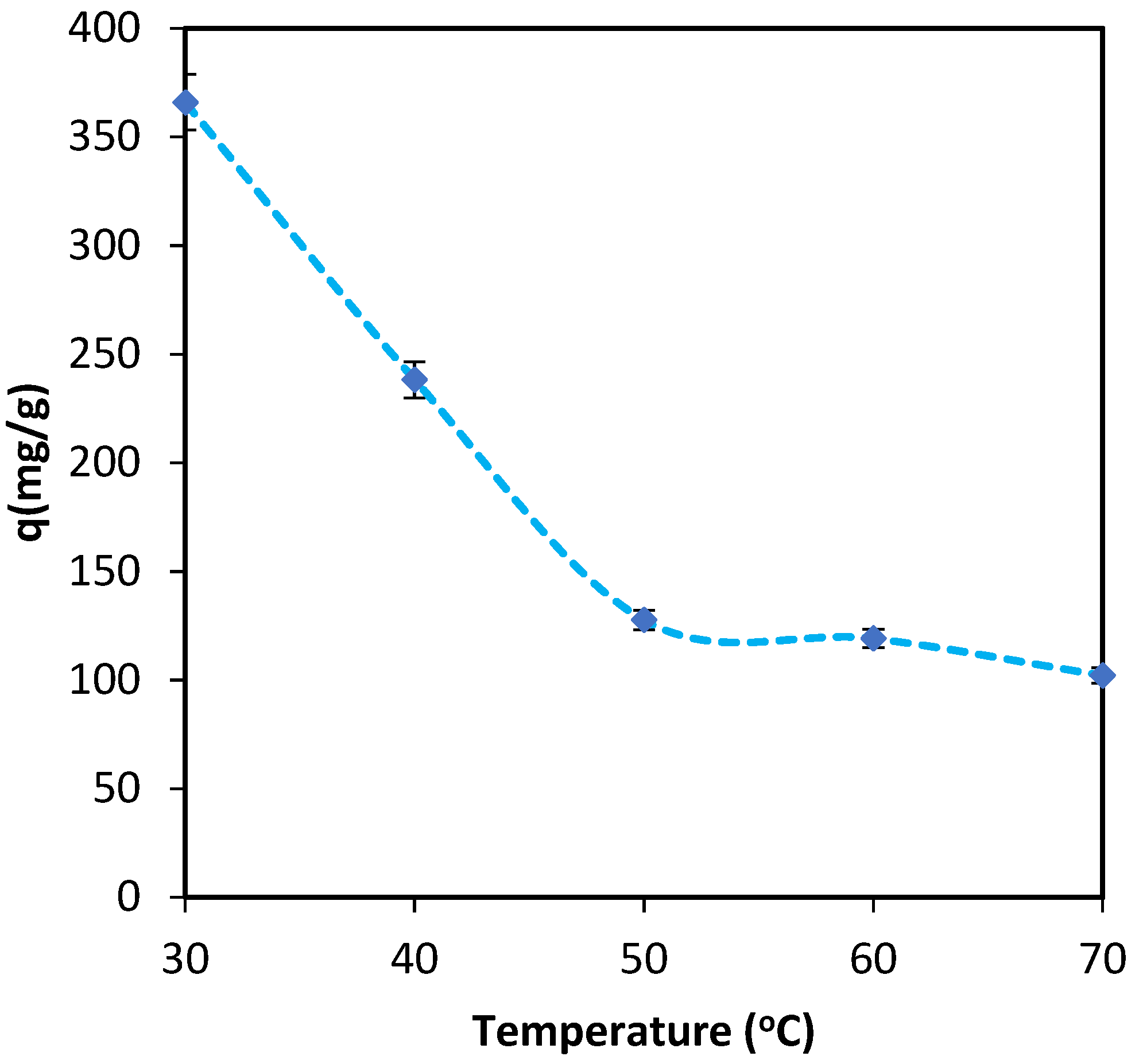
3.1.4. Adsorption Thermodynamics
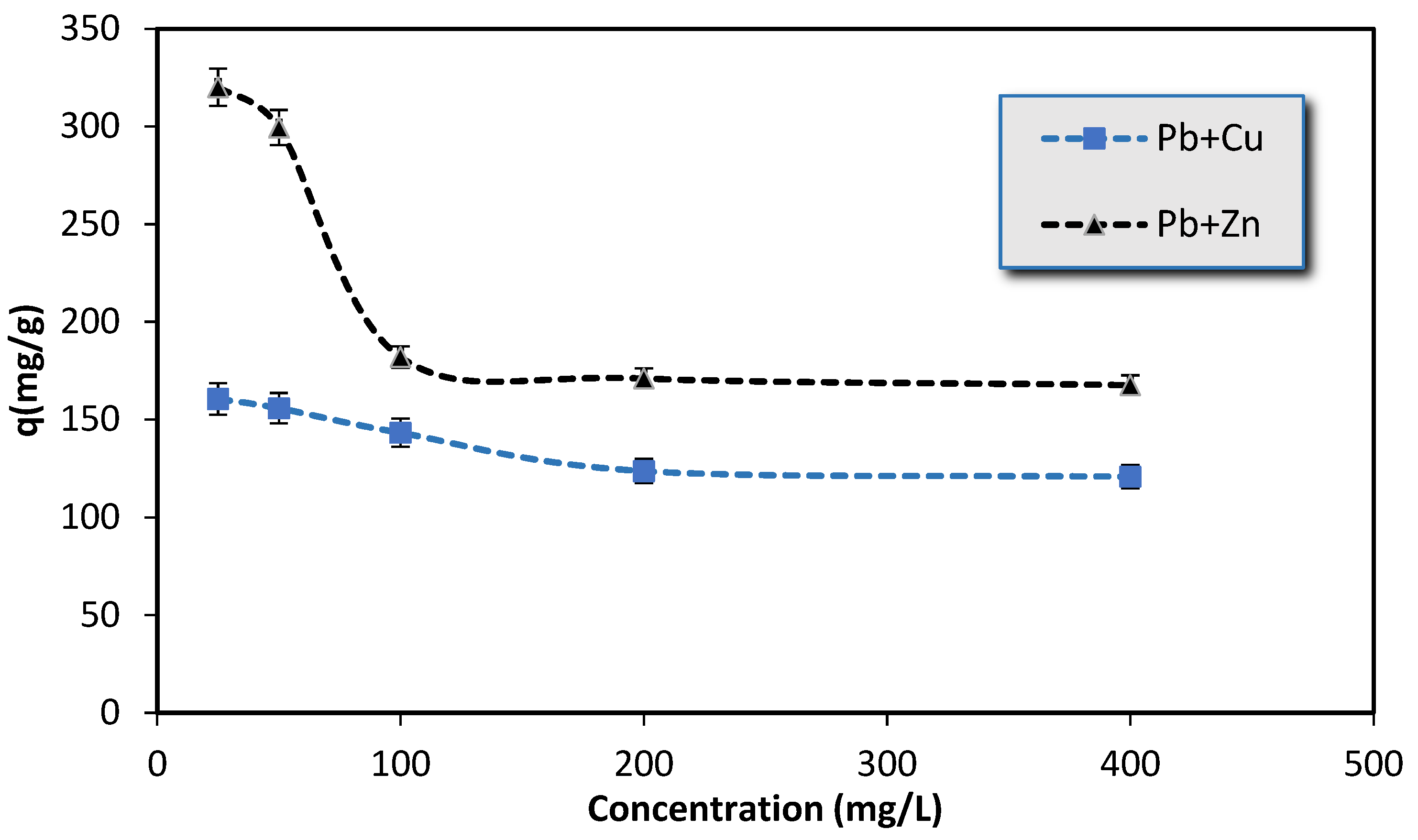
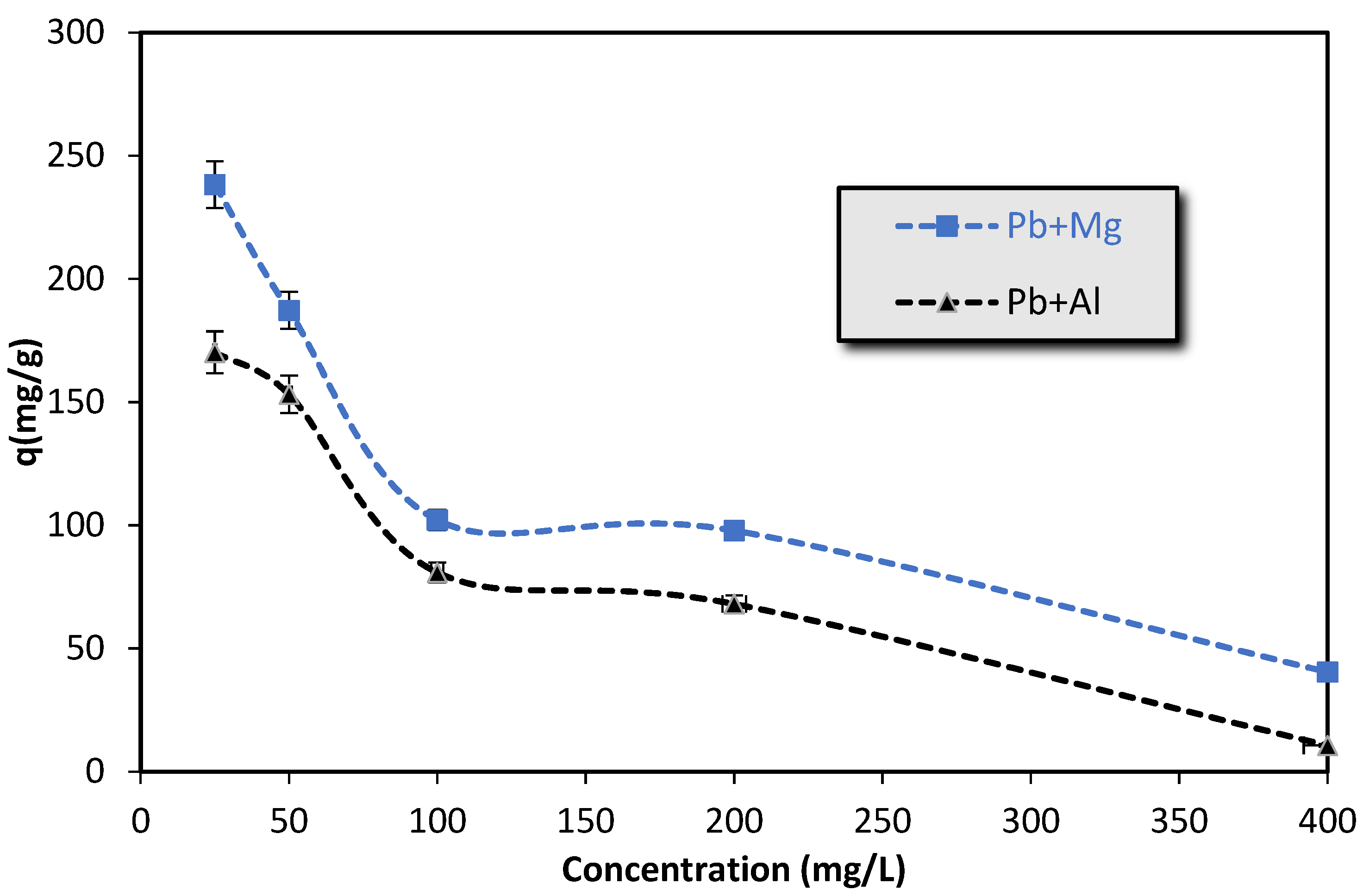
3.1.5. Competitive Adsorption
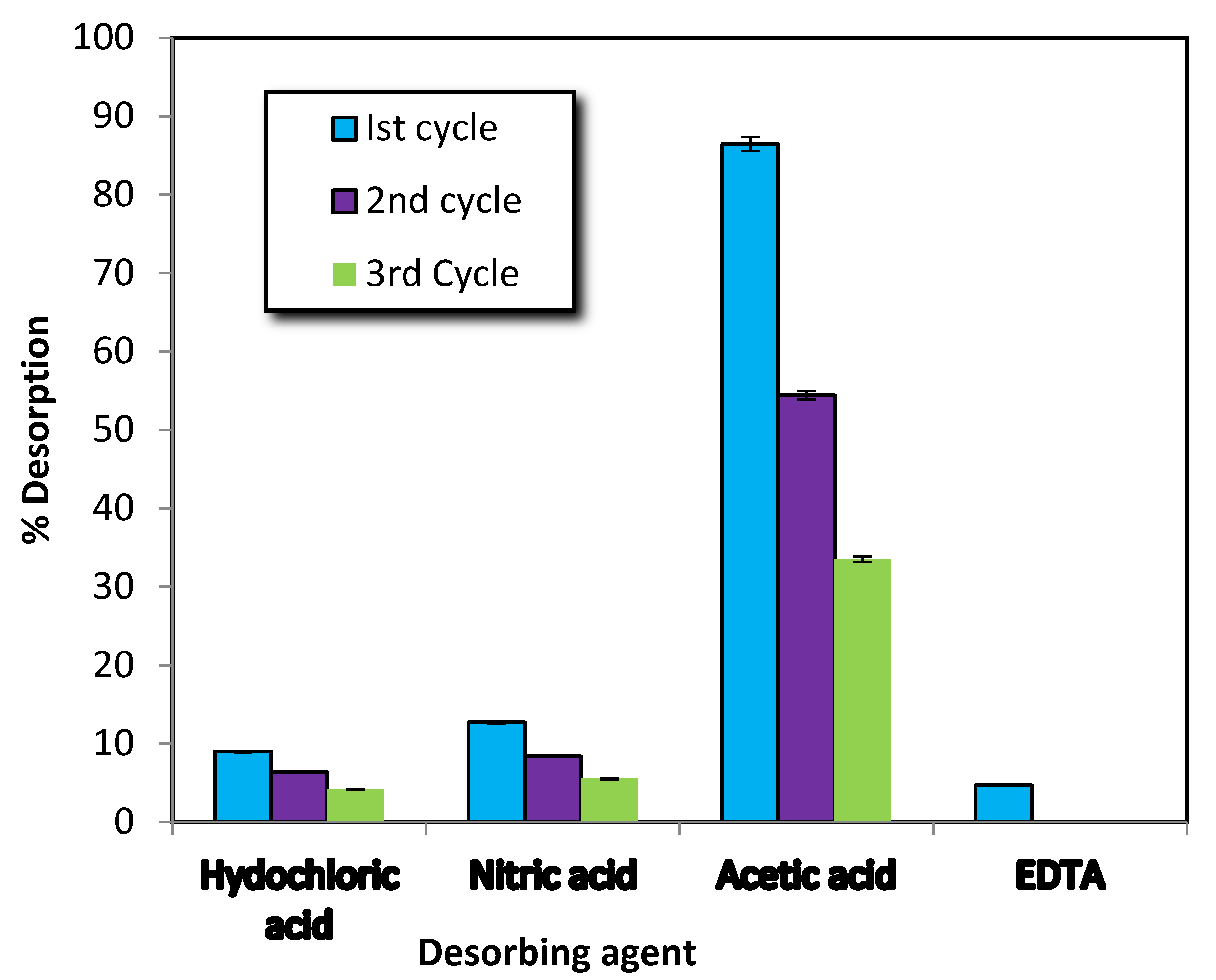
3.1.6. Desorption
3.2. Nanofiltration Study
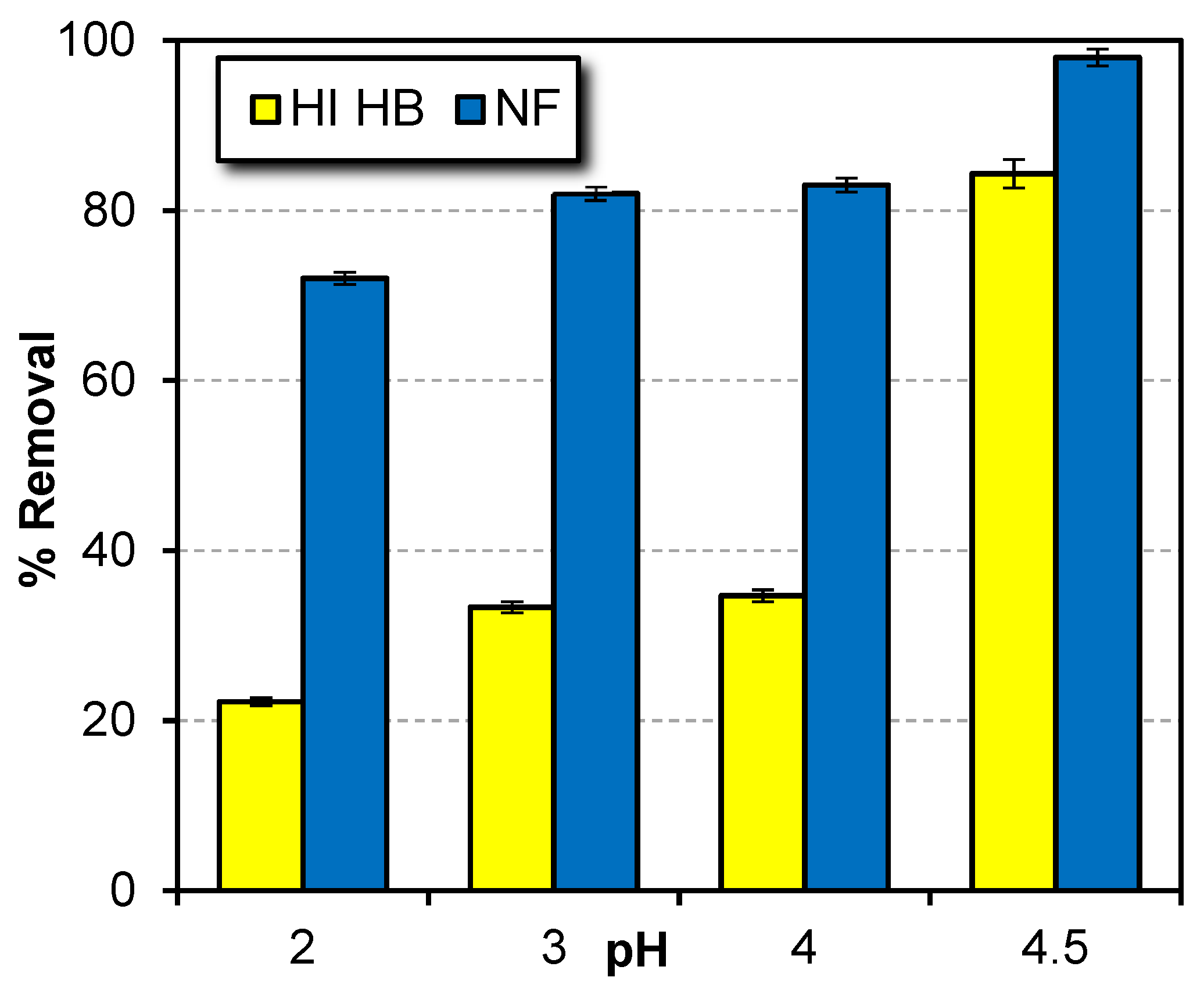
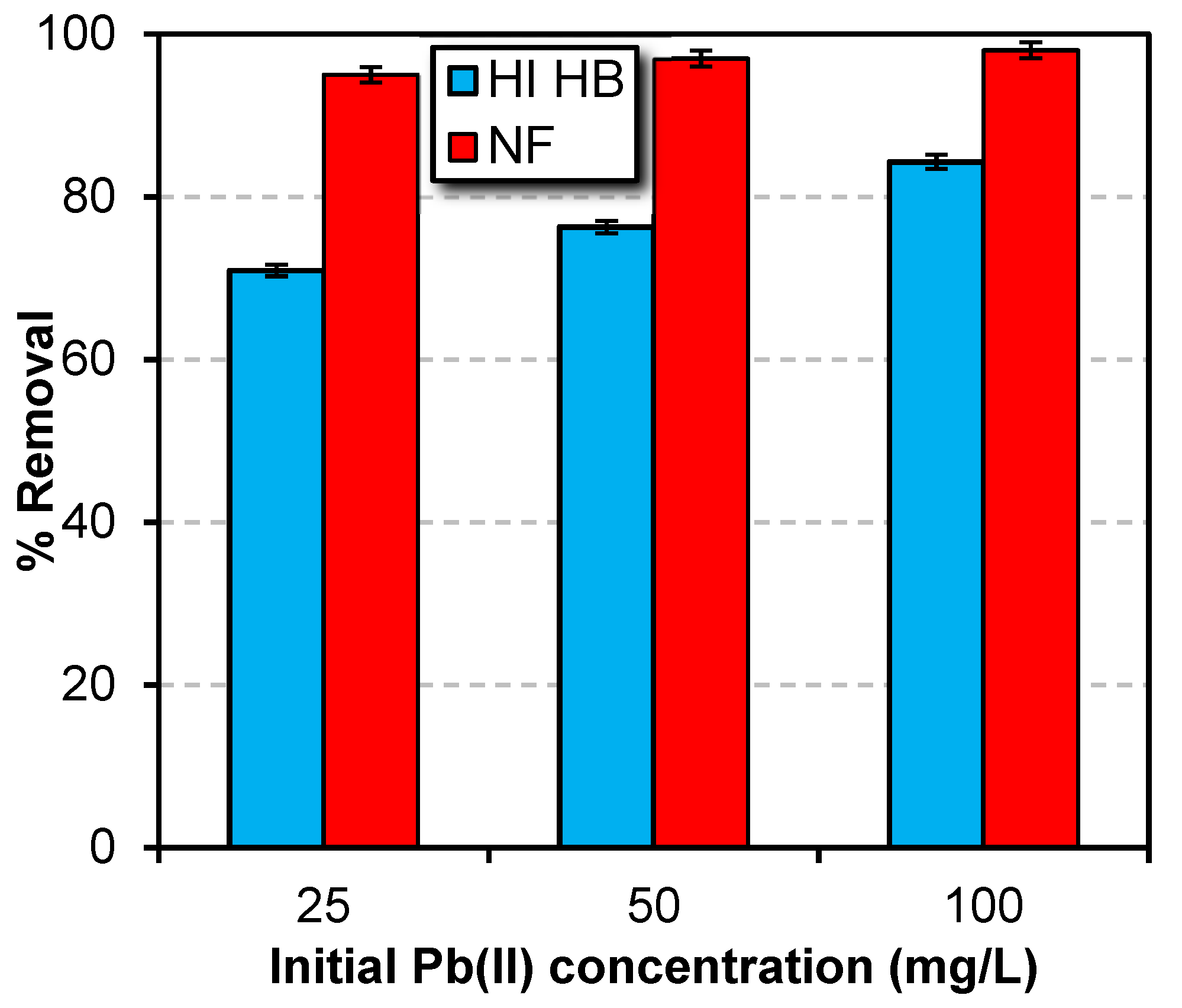
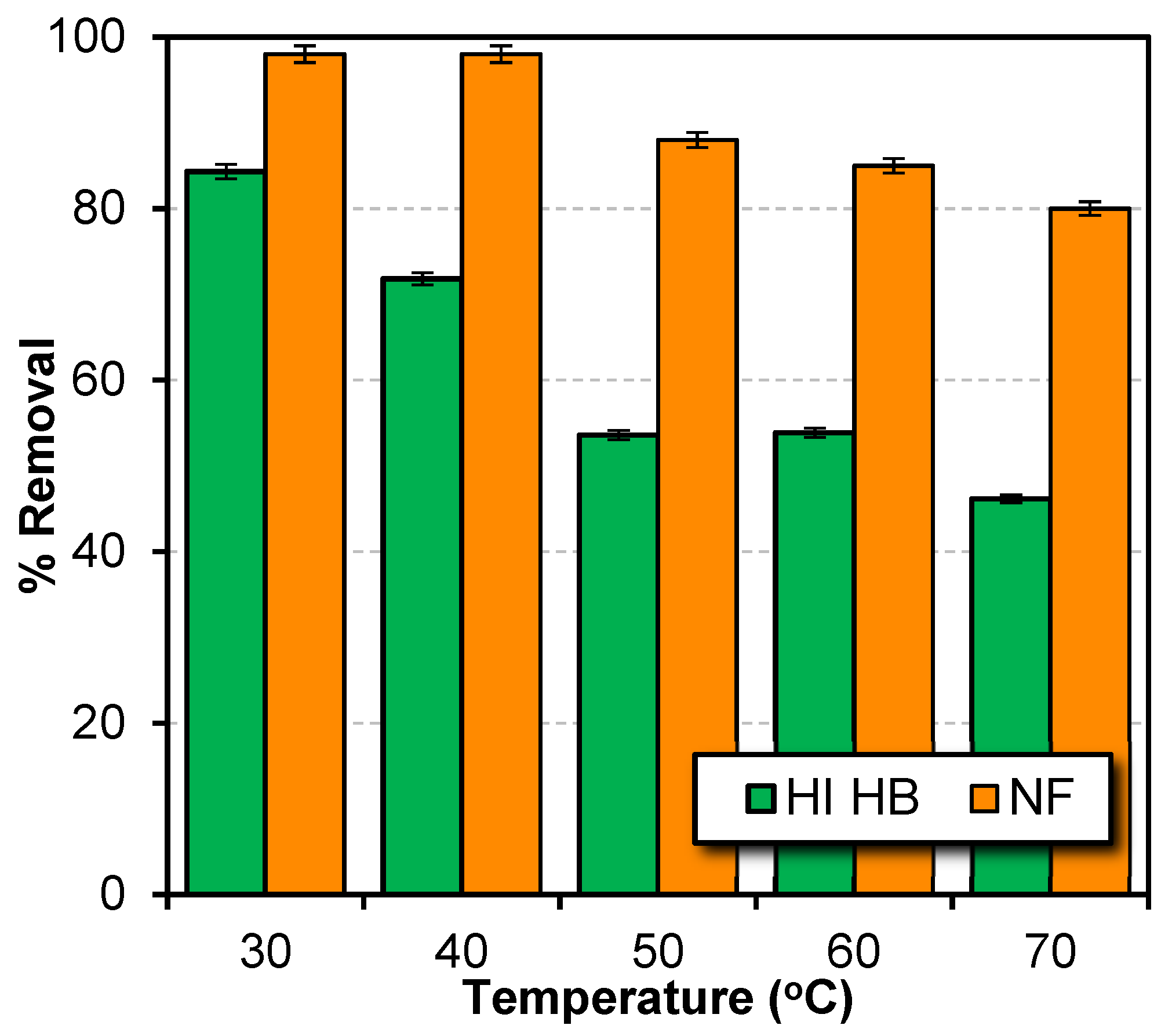
Parameters Affecting the Performance of NF Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Seliem, M.K.; Mobarak, M.; Selim, A.; Mohamed, E.; Halfaya, R.A.; Gomaa, H.K.; Anastopoulos, I.; Giannakoudakis, D.A.; Lima, E.C.; Bonilla-Petriciolet, A. A novel multifunctional adsorbent of pomegranate peel extract and activated anthracite for Mn (VII) and Cr (VI) uptake from solutions: Experiments and theoretical treatment. J. Mol. Liq. 2020, 311, 113169. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M.; Antoniou, Ε.; Terpiłowski, K.; Mohammadi, E.; Shams, M.; Coy, E.; Bakandritsos, A.; Katsoyiannis, I.A. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: Surface chemistry matters the most. J. Hazard. Mater. 2021, 413, 125279. [Google Scholar] [CrossRef] [PubMed]
- Yargıç, A.Ş.; Şahin, R.Y.; Özbay, N.; Önal, E. Assessment of toxic copper (II) biosorption from aqueous solution by chemically-treated tomato waste. J. Clean. Prod. 2015, 88, 152–159. [Google Scholar] [CrossRef]
- Black, R.; Sartaj, M.; Mohammadian, A.; Qiblawey, H.A. Biosorption of Pb and Cu using fixed and suspended bacteria. J. Environ. Chem. Eng. 2014, 2, 1663–1671. [Google Scholar] [CrossRef]
- Abatal, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Olguin, M. Carbonaceous material obtained from bark biomass as adsorbent of phenolic compounds from aqueous solutions. J. Environ. Chem. Eng. 2020, 8, 103784. [Google Scholar] [CrossRef]
- Liakos, E.V.; Rekos, K.; Giannakoudakis, D.A.; Mitropoulos, A.C.; Fu, J.; Kyzas, G.Z. Activated porous carbon derived from tea and plane tree leaves biomass for the removal of pharmaceutical compounds from wastewaters. Antibiotics 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Riera-Torres, M.; Gutiérrez-Bouzán, C.; Crespi, M. Combination of coagulation–flocculation and nanofiltration techniques for dye removal and water reuse in textile effluents. Desalination 2010, 252, 53–59. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Mikulášek, P. Influence of operating variables on the removal of heavy metal ions from aqueous solutions by nanofiltration. Desalination 2014, 343, 67–74. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Hanif, A.; Bhatti, H.N.; Hanif, M.A. Removal of zirconium from aqueous solution by Ganoderma lucidum: Biosorption and bioremediation studies. Desalination Water Treat. 2015, 53, 195–205. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Yaashikaa, P.; Karishma, S.; Jeevanantham, S.; Swetha, S. Mixed biosorbent of agro waste and bacterial biomass for the separation of Pb (II) ions from water system. Chemosphere 2021, 277, 130236. [Google Scholar] [CrossRef] [PubMed]
- Sayin, F.; Akar, S.T.; Akar, T.; Celik, S.; Gedikbey, T. Chitosan immobilization and Fe3O4 functionalization of olive pomace: An eco–friendly and recyclable Pb2+ biosorbent. Carbohydr. Polym. 2021, 269, 118266. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Batch adsorption studies on surface tailored chitosan/orange peel hydrogel composite for the removal of Cr (VI) and Cu (II) ions from synthetic wastewater. Chemosphere 2021, 271, 129415. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Harkins, W.D.; Jura, G. An adsorption method for the determination of the area of a solid without the assumption of a molecular area, and the area occupied by nitrogen molecules on the surfaces of solids. J. Chem. Phys. 1943, 11, 431–432. [Google Scholar] [CrossRef]
- Torab-Mostaedi, M.; Ghassabzadeh, H.; Ghannadi-Maragheh, M.; Ahmadi, S.; Taheri, H. Removal of cadmium and nickel from aqueous solution using expanded perlite. Braz. J. Chem. Eng. 2010, 27, 299–308. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Sánchez, E.; Stael, C.; Cumbal, L. Andean Sacha inchi (Plukenetia volubilis L.) shell biomass as new biosorbents for Pb 2+ and Cu 2+ ions. Ecol. Eng. 2016, 93, 152–158. [Google Scholar] [CrossRef]
- Alencar, W.S.; Acayanka, E.; Lima, E.C.; Royer, B.; de Souza, F.E.; Lameira, J.; Alves, C.N. Application of Mangifera indica (mango) seeds as a biosorbent for removal of Victazol Orange 3R dye from aqueous solution and study of the biosorption mechanism. Chem. Eng. J. 2012, 209, 577–588. [Google Scholar] [CrossRef]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Milojković, J.; Mihajlović, M.; Stanojević, M.; Stanković, S. Removal of Pb 2+ ions by raw corn silk (Zea mays L.) as a novel biosorbent. J. Taiwan Inst. Chem. Eng. 2016, 58, 407–416. [Google Scholar] [CrossRef]
- Amini, M.; Younesi, H.; Bahramifar, N. Biosorption of U (VI) from aqueous solution by Chlorella vulgaris: Equilibrium, kinetic, and thermodynamic studies. J. Environ. Eng. 2013, 139, 410–421. [Google Scholar] [CrossRef]
- Galedar, M.; Younesi, H. Biosorption of ternary cadmium, nickel and cobalt ions from aqueous solution onto Saccharomyces cerevisiae cells: Batch and column studies. Am. J. Biochem. Biotechnol. 2013, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Yang, Z.; Liu, Y.; Kong, X. Kinetics and equilibrium studies on biosorption of Pb (II) from aqueous solution by a novel biosorbent: Cyclosorus interruptus. J. Environ. Chem. Eng. 2015, 3, 2219–2228. [Google Scholar] [CrossRef]
- Hajahmadi, Z.; Younesi, H.; Bahramifar, N.; Khakpour, H.; Pirzadeh, K. Multicomponent isotherm for biosorption of Zn (II), CO (II) and Cd (II) from ternary mixture onto pretreated dried Aspergillus niger biomass. Water Resour. Ind. 2015, 11, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Pirbazari, A.E.; Saberikhah, E.; Kozani, S.H. Fe3O4–wheat straw: Preparation, characterization and its application for methylene blue adsorption. Water Resour. Ind. 2014, 7, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Hanif, M.A.; Nadeem, R.; Bhatti, H.N.; Ahmad, N.R.; Ansari, T.M. Ni (II) biosorption by Cassia fistula (Golden Shower) biomass. J. Hazard. Mater. 2007, 139, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, M.N.; Kumaran, P. Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron. J. Biotechnol. 2005, 8, 43–53. [Google Scholar]
- Aksu, Z.; Isoglu, I.A. Use of agricultural waste sugar beet pulp for the removal of Gemazol turquoise blue-G reactive dye from aqueous solution. J. Hazard. Mater. 2006, 137, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Toor, M.; Jin, B. Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem. Eng. J. 2012, 187, 79–88. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2009, 165, 52–62. [Google Scholar] [CrossRef]
- Akar, S.T.; Özcan, A.S.; Akar, T.; Özcan, A.; Kaynak, Z. Biosorption of a reactive textile dye from aqueous solutions utilizing an agro-waste. Desalination 2009, 249, 757–761. [Google Scholar] [CrossRef]
- Sağ, Y.; Akcael, B.; Kutsal, T. Ternary biosorption equilibria of chromium (VI), copper (II), and cadmium (II) on Rhizopus arrhizus. Sep. Sci. Technol. 2002, 37, 279–309. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Verma, B.; Balomajumder, C.; Sabapathy, M.; Gumfekar, S.P. Pressure-Driven Membrane Process: A Review of Advanced Technique for Heavy Metals Remediation. Processes 2021, 9, 752. [Google Scholar] [CrossRef]
- Askari, N.; Farhadian, M.; Razmjou, A.; Hashtroodi, H. Nanofiltration performance in the removal of dye from binary mixtures containing anthraquinone dyes. Desalination Water Treat. 2016, 57, 18194–18201. [Google Scholar] [CrossRef]
- Abhang, R.; Wani, K.; Patil, V.; Pangarkar, B.; Parjane, S. Nanofiltration for recovery of heavy metal ions from waste water-a review. Int. J. Res. Environ. Sci. Technol. 2013, 3, 29–34. [Google Scholar]
- Chai, X.; Chen, G.; Po-Lock, Y.; Mi, Y. Pilot scale membrane separation of electroplating waste water by reverse osmosis. J. Membr. Sci. 1997, 123, 235–242. [Google Scholar] [CrossRef]

| Parameters | Nanofiltration |
|---|---|
| Membrane type | Flat sheet |
| Material | PA (polyamide) |
| Membrane area | 0.006 m2 |
| Pressure | 5 bar |
| Parameters Derived from Isotherm Models | Values |
|---|---|
| Langmuir | |
| qm Calculated (mg/g) | 500 |
| qm Experimental (mg/g) | 408.9 |
| b | 0.02 |
| R2 | 0.993 |
| Freundlich | |
| KF | 38.90 |
| n | 4.20 |
| R2 | 0.821 |
| Temkin | |
| A | 5.25 |
| B | 0.009 |
| R2 | 0.717 |
| Harkins–Jura | |
| A | 25.64 |
| B | 3.84 |
| R2 | 0.590 |
| Temperature (K) | ∆G° (kJ/mol) | ∆H° (kJ/mol) | ∆S° (Jmol−1 K−1) |
|---|---|---|---|
| 303 | 33.4 | −38.9 | −110.3 |
| 313 | 34.5 | ||
| 323 | 35.6 | ||
| 333 | 36.7 | ||
| 343 | 37.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, A.; Ali, S.; Hanif, M.A.; Rashid, U.; Bhatti, H.N.; Asghar, M.; Alsalme, A.; Giannakoudakis, D.A. A Novel Combined Treatment Process of Hybrid Biosorbent–Nanofiltration for Effective Pb(II) Removal from Wastewater. Water 2021, 13, 3316. https://doi.org/10.3390/w13233316
Hanif A, Ali S, Hanif MA, Rashid U, Bhatti HN, Asghar M, Alsalme A, Giannakoudakis DA. A Novel Combined Treatment Process of Hybrid Biosorbent–Nanofiltration for Effective Pb(II) Removal from Wastewater. Water. 2021; 13(23):3316. https://doi.org/10.3390/w13233316
Chicago/Turabian StyleHanif, Asma, Shaukat Ali, Muhammad Asif Hanif, Umer Rashid, Haq Nawaz Bhatti, Muhammad Asghar, Ali Alsalme, and Dimitrios A. Giannakoudakis. 2021. "A Novel Combined Treatment Process of Hybrid Biosorbent–Nanofiltration for Effective Pb(II) Removal from Wastewater" Water 13, no. 23: 3316. https://doi.org/10.3390/w13233316
APA StyleHanif, A., Ali, S., Hanif, M. A., Rashid, U., Bhatti, H. N., Asghar, M., Alsalme, A., & Giannakoudakis, D. A. (2021). A Novel Combined Treatment Process of Hybrid Biosorbent–Nanofiltration for Effective Pb(II) Removal from Wastewater. Water, 13(23), 3316. https://doi.org/10.3390/w13233316









