Abstract
The O3/PMS system has appeared as an effective wastewater treatment method because of the simultaneous generation of hydroxyl radicals (•OH) and sulfate radicals (SO4•−). Many research achievements have been made on the degradation of micropollutants and the reaction mechanism of the O3/PMS system. However, an integral understanding of the O3/PMS system is lacking, which limits the development of safe and effective AOP-based water treatment schemes. Therefore, in this review, the degradation effects, toxicity changes, and reaction mechanisms of various micropollutants in the O3/PMS system are reviewed. The formation of oxidation by-products (OBPs) is an important issue that affects the practical application of O3/PMS systems. The formation mechanism and control methods of OBPs in the O3/PMS system are overviewed. In addition, the influence of different reaction conditions on the O3/PMS system are comprehensively evaluated. Finally, future research needs are proposed based on the limited understanding of O3/PMS systems in the degradation of micropollutants and formation of OBPs. Specifically, the formation rules of several kinds of OBPs during the O3/PMS system are not completely clear yet. Furthermore, pilot-scale research, the operational costs, sustainability, and general feasibility of the O3/PMS system also need to be studied. This review can offer a comprehensive assessment on the O3/PMS system to fill the knowledge gap and provide guidance for the future research and engineering applications of the O3/PMS system. Through this effort, the O3/PMS system can be better developed and turned towards practical applications.
1. Introduction
At present, the emergence of some pollutants (such as drugs, personal care products, endocrine disruptors, and other refractory organics) pose a threat to water quality and safety, which has aroused widespread concern [1,2,3]. Sulfate radicals (SO4•−)-based advanced oxidation processes (AOPs) have received widespread attention owing to their strong oxidation ability, fast reaction rate, and wide applicability to contaminants in wastewater [4,5,6]. SO4•− can be obtained by activation of PMS through various methods, which include ultraviolet light (UV) irradiation, heating, or addition of transition metals, carbon materials and ozone (O3) [4,7,8,9]. As a strong oxidant, O3 can activate PMS to produce SO4•−, while at the same time it will decompose to produce a large amount of hydroxyl radicals (•OH) [10,11,12]. In addition, singlet oxygen (1O2) and superoxide radicals (O2•−) also can be generated in the process of O3 activating PMS [13,14]. Under the combined action of these reactive oxygen species (ROS), different types of micropollutants can be efficiently degraded. Specifically, the results of studies showed that O3/PMS achieved 81% removal of ATZ in 10 min [10], and PMT was eliminated by 99.27% approximately in O3/PMS system within 10 min [15], while pCBA was fully degraded by O3/PMS in less than 5 min [16]. Furthermore, Gholikandi et al. reported that in terms of sludge stabilization and dewatering, O3/PMS was a better choice than other processes (i.e., O3, O3/H2O2, O3/PS) [17]. The study of Andrés et al. indicated that the O3/PMS combination produced a synergistic effect in the inactivation of microorganisms [18]. All these studies have shown that the O3/PMS system has a very great application potential in water treatment.
However, ROS will unavoidably react with co-existing substances in aqueous solution, leading to the generation of large amounts of oxidation by-products (OBPs). The formation and control of OBPs is an important issue that has been relatively neglected in the study of AOPs. The common OBPs in AOPs-treated water include: (1) low-molecular-weight carbonyls (LMWCs) (e.g., carboxylic acids, benzoic compounds, aldehydes, ketones, keto-acids), (2) organic halogenated OBPs (X-OBPs) (e.g., trihalomethane (THM), haloacetic acids (HAA), chloral hydrate (CH)), and (3) inorganic OBPs (e.g., nitrite (NO2−), chlorite (ClO2−), chlorate (ClO3−), and bromate (BrO3−)) [19,20,21,22]. Many of these low-molecular-weight carbonyls constitute assimilable organic carbon (AOC) easily, which can be rapidly utilized by microorganisms, leading to an increase in biomass [23,24]. In the United States, it is stipulated that chloroform (TCM), bromodichloromethane (BDCM), dibromochloromethane (DBCM), and bromoform (TBM) should be controlled below 80 μg·L−1, and chloroacetic acid (CAA), bromoacetic acid (BAA), dichloroacetic acid (DCAA), dibromoacetic acid (DBAA), and trichloroacetic acid (TCAA)) should be controlled below 60 μg·L−1 [25]. Both the World Health Organization (WHO) and China have issued individual guidelines for ClO2− and ClO3−, each of which should be below 700 μg·L−1 in drinking water [26,27]. BrO3− is a class 2B carcinogen stipulated by the WHO and the U.S. Environmental Protection Agency (USEPA), and 10 μg/L is set to be the maximum contaminant level of BrO3− in drinking water [27]. It should be noted that the difference between OBPs and disinfection by-products (DBPs) is only that the former is a kind of by-product of AOPs, and the latter is a kind of product of conventional disinfection (i.e., adding chlorine (Cl2), monochloramine (NH2Cl), chlorine dioxide (ClO2)) [28,29]. As an AOP, the O3/PMS system will produce many kinds of OBPs during the process of treating micropollutants, which greatly limits the application of an O3/PMS system in the actual water treatment.
In addition, the influence of different reaction parameters (e.g., concentration of reactive substances), reaction conditions (e.g., pH, temperature), and water quality (e.g., concentration of inorganic and organic substances) on the reaction system is also one of the key points that needs to be studied urgently in AOPs. The results of several studies have shown that the concentration of O3 and PMS have an appropriate range. Excessive dosage of O3 and PMS would have a negative impact on the degradation of micropollutants in the O3/PMS system [30,31,32]. Temperature affects the decomposition of O3 and the activation of PMS [33,34,35]. In addition, pH also shows influence on the conversion of free radicals [30,31,36]. Inorganic ions (e.g., Cl−, NO2−, CO32−, HCO3−, phosphate) usually inhibit the degradation of micropollutants in the O3/PMS system by scavenging free radicals [37,38,39,40]. Natural organic matter (NOM) in water acts as a promoter or inhibitor for the generation of free radicals [41,42,43]. Therefore, the influence of these external conditions on the O3/PMS system needs to be comprehensively considered in both the analysis of the degradation effect of O3/PMS system on micropollutants and the study of the generation and control of OBPs in O3/PMS system.
To the best of our knowledge, the current research on the O3/PMS system mainly focuses on the theoretical exploration of a single direction. There has been no specific review on the O3/PMS system so far, which stimulated us to write this review article on this fast-growing research area with emphasis on the introduction, influence factors, degradation of micropollutants and formation and control of OBPs of O3/PMS system. The aim of this work is to develop an integrated understanding of the O3/PMS system through a critical evaluation of the relevant publications. As a result, the knowledge gaps in related research and future research directions are explored, so that the O3/PMS system can be better developed and used for practical applications.
2. Background of the O3/PMS System
2.1. Proposal of the O3/PMS System
As a strong oxidant, O3 can effectively degrade many organic substances which are refractory to traditional oxidation processes [44,45]. However, O3 has strong selectivity and tends to attack the double bonds, activated aromatic groups and non-protonated amines of organic substances [21,41]. On the other hand, •OH produced in the process of O3 decomposition is a non-selective strong oxidant (Equations (1) and (2)), which can rapidly react with various micropollutants at nearly diffusion-controlled rates, and the diffusion-controlled rate of •OH is ~108–1010 M−1s−1 [46,47]. Typically, the degradation of micropollutants by O3 is achieved by the combined activities of molecular O3 and •OH. However, the oxidation efficiency of O3 alone is very low for the refractory micropollutants in water due to the smaller amount of •OH produced by O3 decomposition and the selectivity of molecular O3.
O3 + OH−→HO2− + O2 70 M−1s−1
O3 + HO2−→•OH + O2•− + O2 2.8 × 106 M−1s−1
The •OH-based AOPs have attracted widespread attention. The reaction between O3 and hydrogen peroxide (H2O2) is one of the most common AOPs to produce •OH for contaminant degradation [48]. The O3/H2O2 system was firstly proposed in a study by Staehelin and Hoigne [49]. Subsequently, the underlying mechanism of the O3/H2O2 system through quantum–chemical and thermokinetic analysis was revised [46,50]. O3 and H2O2 firstly react to form the adduct HO5− (Equation (3)), which subsequently decomposes in two ways (Equations (4) and (5)). Eventually, •OH is generated through Equations (6)–(8).
HO2− + O3→HO5−
HO5−→O3•− + HO2•
HO5−→2O2 + OH−
O3•−⇌O• + O2
O•− + H2O⇌•OH + OH−
HO2•⇌O2•− + H+
O2•− + O3→O3•− + O2
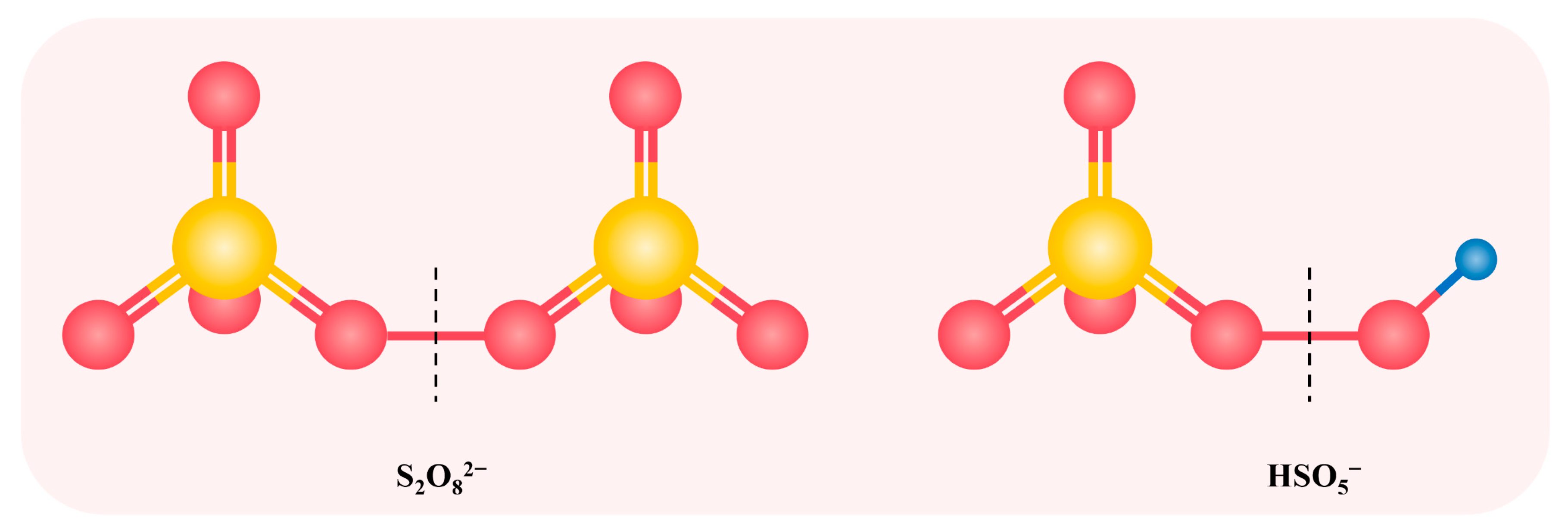
SO4•−, as a strong oxidant, has a higher selectivity and higher redox potential (E0 = 2.5–3.1 V) than •OH, and can react with many micropollutants at nearly diffusion-controlled rates [51]. Additionally, compared with •OH, the reactions between SO4•− and micropollutants are less affected by alkalinity and NOM [51,52,53]. In many studies, the formation of SO4•− was achieved through activating persulfate (i.e., peroxodisulfate (PDS) and peroxymonosulfate (PMS)). The activation strategies include ultraviolet light (UV) irradiation, heating, or addition of transition metals and carbon materials [54,55,56,57]. The structure of PDS and PMS are shown in Figure 1. PMS has an asymmetric structure and a parallel peroxy bond (O–O) with H2O2, indicating that it is likely to substitute H2O2 by PMS in the O3/H2O2 system to achieve a synergistic effect [58]. Wen et al. reported that O3-activated PMS enhanced the degradation of pCBA, proving that PMS had a similar effect as H2O2 in promoting the generation of free radicals during ozonation [16]. Furthermore, the study of Li et al. theoretically demonstrated that high chemical reactivity and low kinetic stability of PMS prompted its reaction with O3 [13]. However, according to Figure 1, PDS exists in the form of symmetric structure, where the peroxy group of it is stable and can hardly react with O3 [46]. The research by Yuan et al. also indicated that no radical signal was detected in the O3/PDS system [36]. Wen et al. reported that O3 decomposition was only slightly enhanced in the presence of PDS [16]. Therefore, extensive research has used O3 to activate PMS to generate •OH and SO4•− simultaneously, which could quickly and effectively degrade a variety of micropollutants.
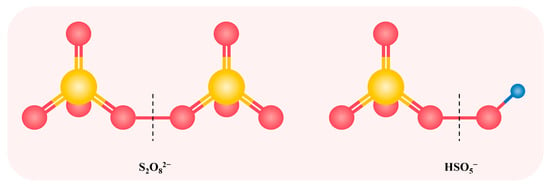
Figure 1.
The structure of PDS and PMS. Yellow color is the sulfur atom and the red color is the oxygen atom. Dashed line represents the fission position of O–O bond for the formation of sulfate radicals.
2.2. Mechanism and Influencing Factors
2.2.1. Mechanism
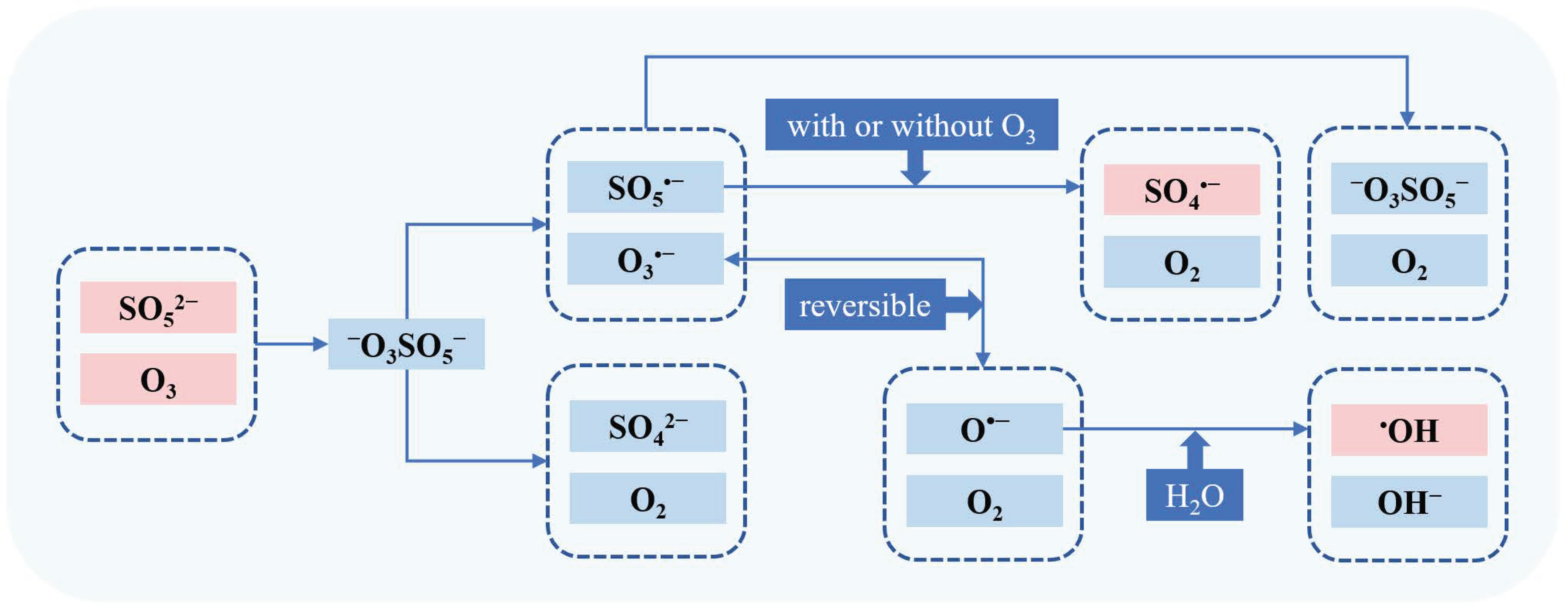
Yang et al. reported the mechanism of the simultaneous production of •OH and SO4•− in the O3/PMS system [10]. The TBA assay and competition kinetics were used to determine the yields of •OH and SO4•−, respectively. As shown in Figure 2, O3 firstly reacted with SO52− (PMS) to produce −O3SO5− (Equation (10)), which is decomposed in two ways (Equations (11) and (12)). Next, SO5•− would further transform into SO4•− by reacting with O3 or decaying bimolecularly (Equations (13) and (14)), and O3•− would convert into •OH (Equations (16) and (17)). Equations (12) and (15) are termination reactions with formation of SO42− and S2O82−.
−O3SOO− + O3→−O3SO5− 2.12 × 104 M−1s−1
−O3SO5−→SO5•− + O3•−
−O3SO5−→SO42− + 2O2
SO5•− + O3→SO4•− + 2O2 1.6 × 105 M−1s−1
2SO5•−→2SO4•− + O2 2.1 × 108 M−1s−1
2SO5•−→S2O82− + O2 2.2 × 108 M−1s−1
O3•−⇌O•− + O2 2.1 × 103 M−1s−1
O•− + H2O→•OH + OH− 108 s−1
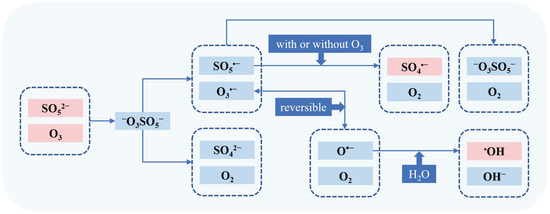
Figure 2.
The mechanism of the simultaneous production of •OH and SO4•− in the O3/PMS system.
In addition, some studies reported that both HSO5− and SO52− could also react with H2O to produce H2O2, thus enhancing •OH generation during the O3/PMS system (Equations (18)–(20)). Moreover, SO4•− could react with H2O or OH− to produce •OH according to Equations (21) and (22) [13,15,59]. On the other hand, 1O2 and O2•− would be produced in ozonation system. The self-decomposition of PMS would also produce 1O2 according to Equation (23) [14].
HSO5− + H2O→H2O2 + HSO4−
SO52− + H2O→H2O2 + SO42−
2O3 + H2O2→2•OH + 3O2
SO4•− + OH−→SO42− + •OH (6.5 ± 1.0) × 107 M−1s−1
SO4•− + H2O→H+ + SO4− + •OH <3 × 103 s−1
SO52− + HSO5−→HSO4− + SO42− + 1O2
2.2.2. Influencing Factors
The influence of reaction conditions on the O3/PMS system is shown in Table 1. Related research mainly focuses on the influence of pH, concentration and molar ratio of O3 and PMS, temperature, inorganic ions, and NOM on the O3/PMS system. These studies have explored the internal mechanism by analyzing the impact of the changes in external conditions on the O3/PMS system. These factors mainly influence the O3/PMS system by affecting the decomposition of O3, the activation of PMS, and the generation and conversion of free radicals.

Table 1.
The influence of reaction conditions on the O3/PMS system.
pH is an important factor in the O3/PMS system because of its remarkable effect on the decomposition of O3, the speciation of PMS, and the conversation of free radicals. In acidic conditions, the presence of excessive proton (H+) could scavenge •OH and SO4•− based on Equations (24) and (25) [31]. As the pH increases up to alkaline, the decomposition of O3 accelerates, resulting in the formation of more •OH [71]. In addition, since pKa2 of PMS is 9.4, the dominant species of PMS would change from HSO5− to SO52− under alkaline conditions, which could induce more SO4•− generation [36,72]. Besides, according to Equations (1), (2), (26), and (27), O3 and PMS could react with OH− to produce HO2−, which then reacts with O3 and PMS to generate •OH and SO4•−, respectively [32,41,73]. At the same time, the presence of OH− leads to the transformation of SO4•− to •OH based on Equation (21) [13,15,30].
•OH + H+ + e−→H2O
SO4•− + H+ + e−→HSO4−
HSO5− + OH−→HO2− + SO42− + H+
HSO5− + HO2−→SO4•− + O2•− + H2O (6.5 ± 1.0) × 107 M−1s−1
The dosage and the molar ratio of O3 and PMS are also very important influencing factors in the O3/PMS system. The increase of O3 and PMS dosage leads to the generation of more free radicals in a proper range [10,36], while self-consumption between free radicals also occurs when there are too many •OH and SO4•− in the solution, according to Equations (28)–(30) [13,15,47,51,74]. On the other hand, excessive O3 and PMS would exhibit an inhibitory effect on the reaction. Specifically, excessive O3 could influence the amount of free radicals and act as scavenger based on Equations (31) and (32) [32,63,75]. Excessive PMS could act as a scavenger of •OH and SO4•− and facilitate the transformation of abundant SO4•− into SO42−, as described in Equations (33) and (34) [10,13,14,15,36,72]. In addition, the high concentration of PMS would reduce the pH value and excessive H+ could scavenge free radicals [31,32]. When the molar ratio of PMS: O3 was 1:1, the amount of PMS that could be activated by O3 tended to stabilize [60]. By contrast, H2O2:O3 = 0.5 was the optimal molar ratio for the O3/H2O2 system [76,77].
SO4•− + SO4•−→S2O82− 7.0 × 108 M−1s−1
•OH + •OH→H2O2
SO4•− + •OH→HSO5−
O3 + •OH→HO2• + O2 1.0 × 108 M−1s−1
O3 + SO4•−→SO5•− + O2
HSO5− + •OH→SO5•− + H2O 5.0 × 106 M−1s−1
HSO5− + SO4•−→SO5•− + HSO4− 1.0 × 106 M−1s−1
Temperature is a very important influencing factor in all reaction systems. Although the study by Shao et al. indicated that the O3/PMS system was not controlled by thermodynamics in the temperature range of 5–40 °C [32], other studies have shown that the amount of free radicals in the O3/PMS system increased with the increase of temperature [33,78,79]. Specifically, the O–O bond of PMS was easily broken at a higher temperature while PMS activation was reduced at a lower temperature, leading to the reduction of SO4•−. Furthermore, the solubility and availability of O3 to produce free radicals in aqueous solution were reduced at a higher temperature [80,81,82].
The presence of some kinds of inorganic ions has a significant impact on the O3/PMS system [14], while the impact of different ionic strengths on the O3/PMS system is very limited. According to Equations (35)–(37), Cl− had limited effect on •OH because the reaction between Cl− and •OH was reversible and the generation of Cl• occurred only at low pH conditions [21]. On the other hand, Cl− could scavenge SO4•− to produce less reactive Cl• (Equations (38) and (39)) [32,37,38]. The reaction between Cl− and SO4•− could lead to the generation of •OH [40,83]. Br− affected the O3/PMS system through rapid and irreversible reacting with •OH and SO4•− (Equations (40) and (41)) [84,85]. Equations (42)–(45) describe the reaction of free radicals with CO32− and HCO3− [32]. CO32− and HCO3− could quench the free radicals effectively to generate CO3•−, with lower redox potential (E0 = 1.78 V) than •OH and SO4•− [14]. NO2− influenced oxidants and free radicals due to its reducibility (Equations (46) and (47)) [14,86]. Phosphate ions showed a strong inhibitory effect on O3 decomposition [87]. Therefore, the use of phosphate buffer solution in the O3/PMS system should control the concentration of phosphate ions.
•OH + Cl−→ClOH•− 4.3 × 109 M−1s−1
ClOH•−→•OH + Cl− 6.1 × 109 M−1s−1
ClOH•− + H+→H2O + Cl• 2.1 × 1010 M−1s−1
SO4•− + Cl−→SO42− + Cl• 3.0 × 108 M−1s−1
SO42− + Cl•→SO4•− + Cl− 2.5 × 108 M−1s−1
•OH + Br−→BrOH•− 1.1 × 1010 M−1s−1
SO4•− + Br−→SO42− + Br• 3.5 × 109 M−1s−1
•OH + CO32−→CO3•− + OH− 3.9 × 108 M−1s−1
•OH + HCO3−→CO3•− + H2O 8.6 × 106 M−1s−1
SO4•− + CO32−→CO3•− + SO42− 6.1 × 106 M−1s−1
SO4•− + HCO3−→CO3•− + HSO4− 2.8 × 106 M−1s−1
NO2− + •OH or SO4•−→NO2• + HO− or SO42−
NO2− + HSO5− or O3→NO3− + HSO4− or O2
NOM plays a dual role in the O3/PMS system [41,42]. The low concentration of NOM enhanced the decomposition of O3 to produce •OH [43]. However, NOM acted as a scavenger for •OH and SO4•− at relatively high concentrations [70]. HA, as an important component of NOM, also played an obvious dual role in the O3/PMS system [15].
3. Degradation of Micropollutants Using the O3/PMS System
3.1. Degradation Effect and Energy Efficiency
The O3/PMS system can quickly and effectively generate •OH and SO4•−, so it is widely used in the research of micropollutant degradation. As shown in Table 2, the O3/PMS system exhibits a good degradation effect when treating sewage-containing general chemicals, agricultural chemicals, and medical chemicals. The SO4•− formed by PMS activation exists in the system for a long time, so it can oxidize micropollutants more effectively. Specifically, the O3/PMS system has high efficiency in degrading typical micropollutants in agricultural and medical industries, so it can be used for soil remediation and medical wastewater treatment. There are many factors that affect the degradation effect of O3/PMS on micropollutants, such as the type and concentration of micropollutants, the concentration and molar ratio of O3 and PMS, pH, and temperature. Tang et al. studied the effect of the O3/PMS system on the degradation of micropollutants with different molecular weights (MW). The MW distributions were divided into five fractions: F1 (<3 kDa), F2 (3–10 kDa), F3 (10–100 kDa), F4 (100 kDa–0.45 µm), and F5 (>0.45 µm) (low: F1; lower: F2; higher: F3, F4; high: F5). The results indicated that O3/PMS oxidation degraded higher MW fractions more efficiently than low MW fractions in DOM [88].

Table 2.
Degradation effect of O3/PMS process on micropollutants.
The reaction rate constants between different micropollutants with O3 and free radicals are shown in Table 3. The reaction rate constants determine which ROS plays a key role in the degradation of target micropollutants in the O3/PMS system. For example, when the solution pH shifted from neutral to alkaline, the proportion of O3 that directly reacted with ACE decreased, resulting in an enhanced formation of SO4•− and suppressed formation of •OH. Considering that SO4•− degraded ACE more slowly than •OH did, the oxidation capacity of the system was weakened due to the decrease of •OH formation [32]. On the other hand, the synergy between the various ROS (i.e., O3, •OH, SO4•−, O2•−, 1O2) produced by the O3/PMS system results in the degradation efficiency of micropollutants faster than other O3-based oxidation processes (i.e., O3, O3/H2O2, O3/PDS). Wen et al. reported that the degradation efficiency of pCBA by O3 alone and O3/H2O2 was only 48.9% and 54.7% after 5 min, respectively. On the contrary, pCBA was fully degraded by O3/PMS in less than 5 min [16]. In the study by Yang et al., it was found that the removal rate of ATZ by O3/PMS reached 81% in 10 min, while the removal rate of O3 alone in 20 min was only 27% [10]. Besides, the removal rate of PMT within 10 min in the O3/PMS system was about 99.27%, while the removal rate of PMT by O3 alone and O3/PDS was 46.16% and 53.45%, respectively [15].

Table 3.
The reaction rate constant between the substance and O3, •OH and SO4•−.
Yu et al. studied the electrical energy per order (EE/O) of ATL in several AOPs. Specifically, the EE/O of UV/O3/PMS, UV/O3, O3/PMS, UV/PMS and O3 was 4.48 × 10−4, 2.37 × 10−4, 5.37 × 10−4, 4.40 × 10−4, and 2.80 × 10−4 kW·h/L, respectively, which followed the order: O3/PMS > UV/O3/PMS ≈ UV/PMS > O3 > UV/O3. The results indicated that the O3/PMS system was the most energy-intensive process for ATL degradation [89]. Besides, Miklos et al. also reported the higher energy efficiency for the SO4•−-based AOPs [90]. This is mainly due to the selectivity of SO4•−, which will consume more energy when degrading the target micropollutants at a low reaction rate with SO4•−.
3.2. Toxicity Changes and Degradation Pathway
The O3/PMS system can significantly reduce the toxicity of micropollutants. Specifically, the biodegradability of activated sludge containing 2,4-D was increased from 8.3% to 58.9%, and the toxicity was reduced from 76.5% to 3.8% after treatment by the PMS/MCFNs/O3 system [14]. With the oxidation of O3/PMS, the toxic equivalent (TE) and the relative inhibition light ratio (RILR) of BCPMW were significantly lowered from 0.08 mg/L to 0.02 mg/L and 36% to 9%, respectively [88]. Tan et al. studied the degradation effect of O3/PMS system on micropollutants containing a variety of anti-inflammatory drugs. Toxicity was calculated based on the toxicity parameter 50% lethal concentration (LC50) of each DBP. The results indicated that the toxicity of the system was decreased after O3/PMS pre-oxidation. Specifically, the toxicity of disinfection by-products (DBPs) reduced from 6.63 × 10−2 min−1 to 5.27 × 10−2 min−1 under neutral conditions [93].
Among the ROS generated in the O3/PMS system, •OH and SO4•− have the strongest oxidizing ability. Therefore, the priority attack sites of these two free radicals should be firstly considered when analyzing the degradation path of micropollutants. SO4•− has electrophilicity and tends to react with electron-donating groups such as hydroxyl (–OH), alkoxy (–RO) and amino (–NH2) groups, but does not easily react with the nitro (–NO2), carbonyl (C=O), or other electron-withdrawing groups [115,116]. On the other hand, •OH is nonselective toward organic pollutants in the oxidation reaction. For some examples, the aromatic ring or the side chains (isopropylamino and alkoxy) of PMT are likely to be attacked by •OH and SO4•− mainly through addition to unsaturated carbon, H-abstraction, and electron abstraction [15,117,118,119]. In addition, •OH and SO4•− participated in the degradation of ACE and the attack sites were C=C, C–O, and C–N bonds [32].
4. Formation and Control of OBPs during the O3/PMS System
4.1. Formation Pathway and Influencing Factors
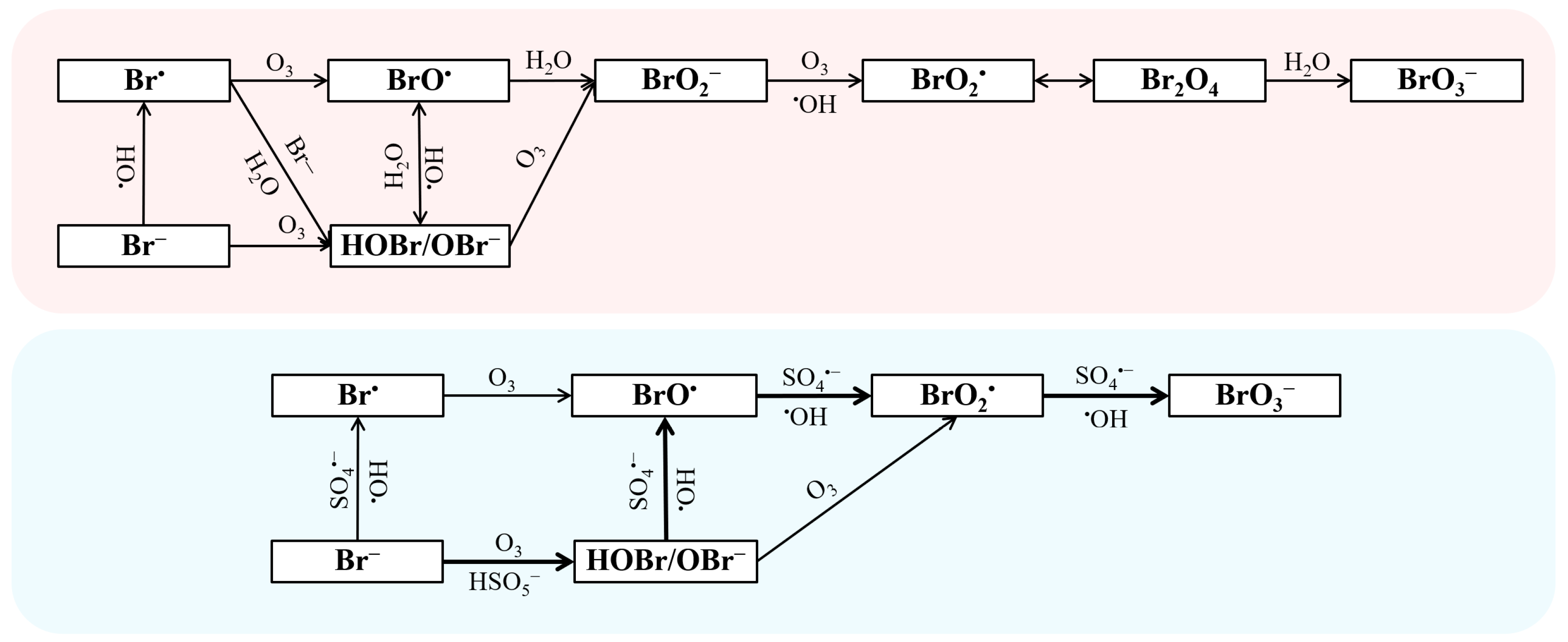
The OBPs formed in the O3-based oxidation process are mainly low-molecular-weight carbonyls, organic halogenated OBPs, and inorganic OBPs. Among them, the inorganic OBPs generally includes chlorinated OBPs, brominated OBPs, and iodinated OBPs. Compared to brominated OBPs, the production of chlorinated and iodinated OBPs during the O3/PMS system is negligible [65]. On the other hand, Frederik et al. reported the formation rule of AOC in O3 alone, but there is no relevant research on the O3/PMS system [120,121]. As typical brominated OBPs, the formation mechanism of bromate (BrO3−) in the treatment of bromide-containing water by O3/PMS has been reported in detail by Wen et al., as shown in Figure 3 [64]. The interaction between bromide (Br−) and molecular O3, •OH, and SO4•− in the O3/PMS system leads to the formation of BrO3− [64,122,123]. The Br− would be oxidized into Br• by •OH and SO4•−, then Br• would transform into BrO• by reacting with O3 and finally convert into BrO3−. Furthermore, Br− would react with O3 to produce hypobromous acid (HOBr/OBr−), which would also convert into BrO• by reacting with •OH and SO4•− [64]. Compared with the BrO3− generation path of the traditional ozone oxidation process, the SO4•− path is added in the O3/PMS system. Therefore, the O3/PMS system will generate more BrO3− than O3 alone. In addition, the research by Liu et al. indicated that some brominated OBPs including dibromoacetaldehyde and tribromoacetaldehyde may possess much higher cytotoxicity than BrO3− [65]. Thus, more attention should be paid to the formation and control of organic halogenated OBPs during O3-based processes [124].
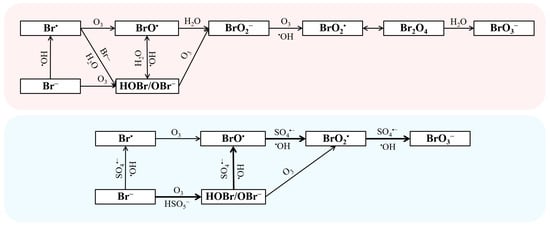
Figure 3.
The mechanism of bromate formation (red: ozonation; blue: O3/PMS). Reprinted with permission of refs. [64,124].
The influence of reaction conditions on the OBP formation is shown in Table 4. The amount of BrO3− produced increases with the increase of Br− concentration within a certain range. However, too much Br− exhibits an inhibition effect [64]. The pH value of the solution comprehensively affects the formation of OBPs in the O3/PMS system by affecting O3 decomposition, Rct,•OH and Rct,SO4•−, and PMS speciation [64,125]. According to the research results, BrO3− formation would increase as O3 and PMS dosage increases [64,125,126]. However, according to the reaction mechanism of the O3/PMS system, this promotion effect may be reduced with the addition of excessive O3 and PMS. The HCO3− in the inorganic ions inhibits the formation of BrO3− by scavenging free radicals. On the other hand, NH4+ prevented the conversion of Br− into BrO3− by masking important intermediate products (HOBr/OBr−) [64]. HA, as an important constituent of NOM, could scavenge ROS and thus reduce the formation of BrO3− [78,127]. In addition, HA could readily capture the intermediates, providing an additional inhibitory effect [122].

Table 4.
The influence of reaction conditions on OBP formation.
4.2. Control Strategy
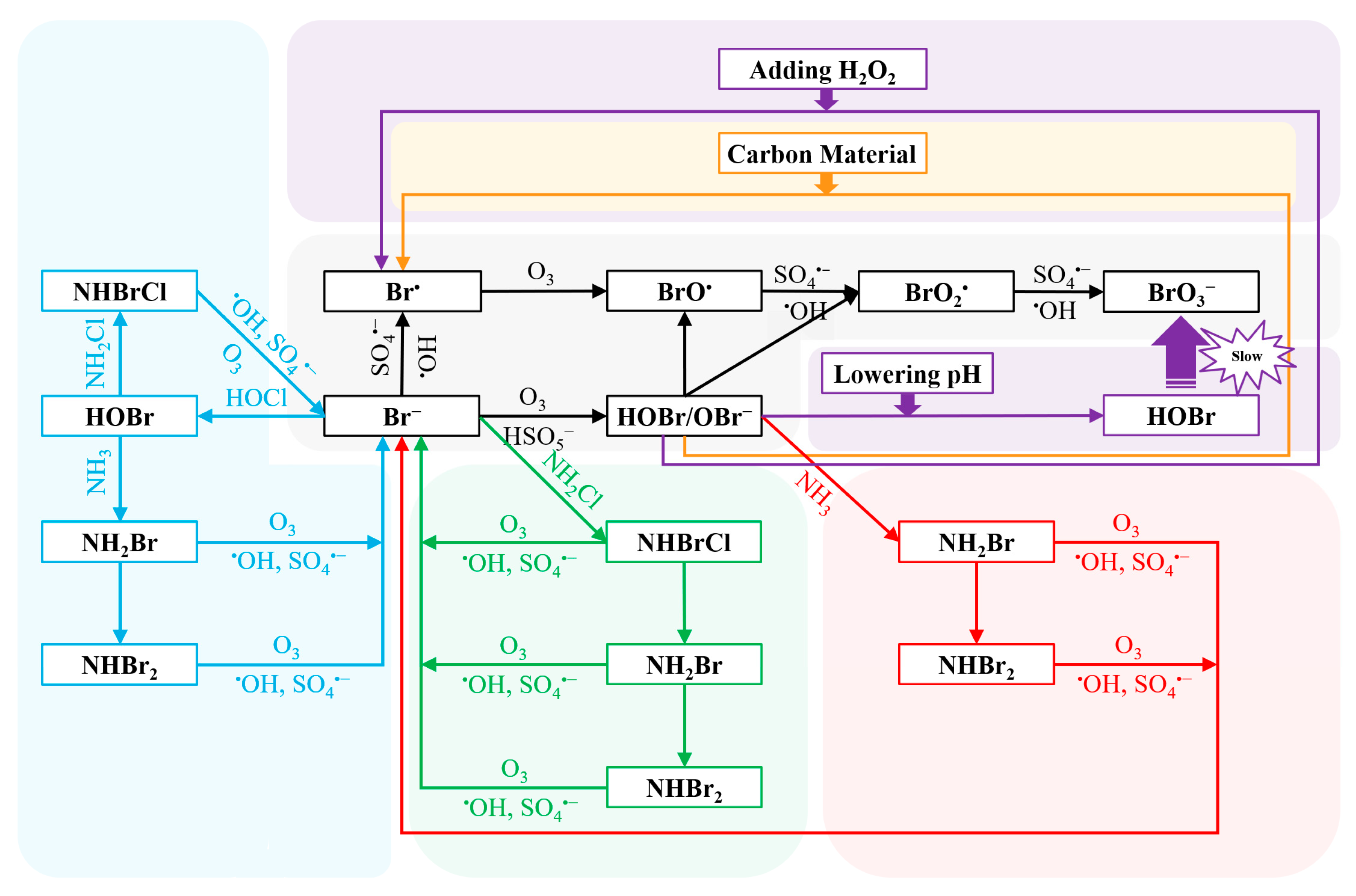
The current research on the control methods of OBP formation in the O3/PMS system focuses on inhibiting the formation of BrO3−. Several methods were used to control the formation of BrO3− in O3 alone: reducing pH [125], adding carbon materials [128,129], H2O2 [130], and ammonia (NH3) and chlorine (Cl2) [123,131]. pH depression shifts the equilibrium of HOBr/OBr− into HOBr (pKa = 8.8), thus slowing down the reaction between HOBr/OBr− and O3 (k(O3,HOBr) = 0.01 M−1s−1, k(O3,OBr−) = 100 M−1s−1), and finally reducing BrO3− formation. Besides, pH depression can lower the •OH exposure, and thus inhibits the BrO3− formation from the oxidation pathways by •OH [125]. Carbon materials suppress the BrO3− formation by reducing HOBr/OBr−, which is crucial to the formation of BrO3−. [132]. H2O2 can inhibit BrO3− formation during ozonation because H2O2 can also reduce HOBr/OBr− into Br− (k = 7.6 × 108 M−1s−1) [21,130]. Therefore, BrO3− formation is negligible in O3/H2O2 system with excess H2O2 [90]. In the pretreatment strategies of NH3-Cl2 and Cl2-NH3, Br− is mainly masked as bromine-containing haloamines (i.e., NH2Br, NHBr2 and NHBrCl) to inhibit the formation of BrO3− [123,131].
At present, only Wen et al. have reported the control of BrO3− formation in the O3/PMS system [92]. The research results indicated that the addition of carbon materials significantly inhibited the BrO3− formation, and the order of the inhibition efficiency was as follows: graphene (GO) > carbon nano tube (CNT) > powdered activated carbon (PAC). According to the study, the carbon materials could block the BrO3− formation by reducing HOBr/OBr− in the reaction system [92]. Besides, Wen et al. synthesized a catalyst (CuCo2O4-GO), which could simultaneously inhibit the formation of BrO3− and enhance the degradation of micropollutants in the O3/PMS system. Specifically, when 100 mg/L CuCo2O4-GO was added, the BrO3− inhibition efficiency reached 96.17% and the degradation efficiency of SMX increased from 0.163 min−1 to 0.422 min−1 [133]. The pretreatment strategy (i.e., NH3, Cl2-NH3 and NH3-Cl2) was also used to inhibit BrO3− generated in the O3/PMS system. All the pretreatment strategies reduced 90% or more of the overall BrO3− formation, while the NH3-Cl2 pretreatment strategy was prior to that of the NH3 and Cl2-NH3 [134]. The inhibitory effects of the common BrO3− control strategies, lowering pH and adding H2O2, in the O3/PMS system have not been studied yet. Many studies have reported that lowering pH could effectively inhibit the formation of BrO3− in an O3-only system. This is because the intermediate substance HOBr/OBr− (pKa = 8.8–9.0) exists in the form of OBr− under alkaline conditions, which is more likely to react with O3 to form BrO3− [125,135,136]. On the other hand, adding excess H2O2 could suppress the formation of BrO3− in O3 alone system by reducing HOBr/OBr− to Br− [137,138,139,140]. These two kinds of BrO3− inhibition strategies may be able to inhibit the formation of BrO3− in the O3/PMS system through similar mechanisms. In general, as shown in Figure 4, the control strategies are used to inhibit the formation of BrO3− by affecting the initial Br− or HOBr/OBr−.
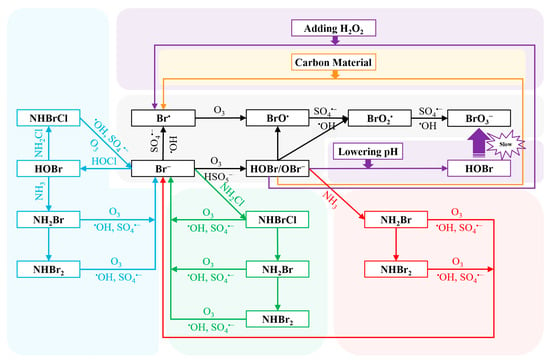
Figure 4.
The mechanism of the inhibition of bromate formation (gray: bromate formation; orange: carbon materials; red: NH3; blue: Cl2-NH3; green: NH3-Cl2; violet: The bromate control strategies that have not been verified in the O3/PMS system). Reprinted with permission [64,92,133,134].
5. Recommendations and Future Prospects
In terms of micropollutant degradation, the research on the O3/PMS system is still at the laboratory level. The investigation using real water should be strengthened to reflect the feasibility of O3/PMS system in practical applications, because many substances contained in actual water will affect the O3/PMS system. Besides, the degradation efficiency under different actual water conditions (i.e., surface water and groundwater) should be studied and comparable to explore the water quality condition which is suitable for the application of the O3/PMS system. At the same time, more pilot-scale research is needed to promote the conversion of O3/PMS system to practical applications.
Due to the generation of SO4•−, the O3/PMS system has higher selectivity than the O3/H2O2 system. Therefore, the degradation rules of different types of micropollutants in the O3/PMS system should be extensively researched. The toxicity changes of the treated micropollutants also need to be studied, which are important indicators for evaluating the practical application potential of the O3/PMS system. In order to evaluate the advantages and disadvantages of the O3/PMS system and the suitable application conditions, the O3/PMS system should be compared with the O3-alone and O3/H2O2 systems when conducting the above research.
The formation rules of several kinds of OBPs under different conditions during the O3/PMS system are not completely clear yet. Notable are the structure change of NOM and the formation rule of small molecular organic matter after treatment by O3/PMS system. At the same time, the effectiveness of various OBP control methods in the O3/PMS system has not been widely studied. Since the O3/PMS system can generate several kinds of ROS, the formation and control of the OBPs need to be compared with the O3 alone and O3/H2O2 systems to explore the mechanism. In addition, micropollutants are not fully mineralized by the O3/PMS system but degraded into transformation products (TPs), which arouse a growing concern because of the unknown structures and potential biological effects. Therefore, more research needs to pay attention to the TPs formed during the degradation of micropollutants in the O3/PMS system.
The operational costs (e.g., energy consumption, chemical input), sustainability (e.g., resource use, carbon footprint), and general feasibility (e.g., physical footprint and oxidation by-product formation) of the O3/PMS system need to be studied to enable to compare their efficiency with other AOPs and alternative treatment processes (i.e., O3/H2O2, O3/UV). In addition, the combination of O3 and biological activated carbon (BAC) is a very common water treatment process in practical applications, which can enhance the degradation efficiency of organic matter while reducing OBPs in the effluent. Therefore, the combined effect of O3/PMS and BAC is also worth studying.
6. Conclusions
As a new advanced oxidation process, O3/PMS degrades many refractory micropollutants rapidly and effectively by generating many strong oxidizing ROS simultaneously. Compared with the widely used O3 and O3/H2O2 systems, the O3/PMS system produces more types of free radicals and has higher selectivity. Based on the current research, the O3/PMS system has a good degradation efficiency on general chemicals, agrochemicals and medical chemicals, and the degradation effect is affected by a variety of influencing factors (e.g., pH, the concentration of O3 and PMS, temperature, and inorganic ions). These factors mainly influence the O3/PMS system by affecting the decomposition of O3, the activation of PMS, and the generation and conversion of free radicals. The generation and control of OBPs during the degradation of micropollutants in the O3/PMS system is another current research focus. According to the research results, the BrO3− produced in the O3/PMS system is mainly due to the interaction between Br− and molecular O3, •OH and SO4•−, and the BrO3− formation can be effectively inhibited by addition of carbon materials, or NH3 and Cl2 combined pretreatment strategy. However, it is not practical enough to apply the O3/PMS system to actual water treatment processes, and there are still many key problems that need to be addressed. Specifically, the degradation rule and toxicity change of different types of micropollutants in the O3/PMS system should be extensively studied. The formation rules of several kinds of OBPs during the O3/PMS system are not completely clear yet. Furthermore, pilot-scale research, the operational costs, sustainability, and general feasibility of the O3/PMS system also need to be studied. Currently, there is no integrated understanding of the O3/PMS system. It is expected that the findings of this review may advance future research and application of O3/PMS system. Specifically, the continuous exploration in the research directions proposed by this article will not only make the O3/PMS system perform better in the degradation of micropollutants, but also enhance the potential of applications of the O3/PMS process in other areas such as sludge stabilization, dewatering, and inactivation of microorganisms.
Author Contributions
Z.L. (Zhao Liu): Investigation, Visualization, Writing—original draft; Z.L. (Zhiting Liang): Writing—Review and Editing; K.L.: Writing—Review and Editing; T.H.: Resources; J.M.: Formal analysis; G.W.: Conceptualization, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 51978557, 51678472), Shaanxi Science Fund for Distinguished Young Scholars (No. 2018JC-026), The Youth Innovation Team of Shaanxi Universities, and Shaanxi Provincial Key Research and Development Project (2020ZDLSF06-05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclatures
| Abbreviation | Full Name |
| pCBA | 4-chlorobenzoic acid |
| KET | Ketoprofen |
| ATZ | Atrazine |
| METR | Metronidazole |
| HA | Humic acid |
| PMT | Prometon |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| MCFNs | CuFe2O4 magnetic nanoparticles |
| SMT | sulfamethazine |
| BCPMW | Biotreated Chinese patent medicine wastewater (e.g., cellulose, lignin, etc.) |
| ACE | Acesulfame |
| DEP | Diethyl phthalate |
| CN | Cyanide |
| BTA | Benzotriazole |
| ASA | Aspirin |
| CAP | Chloramphenicol |
| METO | Metoprolol |
| VEN | Venlafaxine |
| CBZ | Carbamazepine |
| MOX | Moxifloxacin |
| NB | Nitrobenzene |
| MeOH | Methanol |
| TBA | Tert-Butanol |
| BA | Benzoic acid |
| IPM | Iopamidol |
| MCFN | Magnetic copper ferrite nano-particle (CuFe2O4) |
| IBP | Ibuprofen |
| RBV | Ribavirin |
| OA | Oxalic acid |
| ATL | Atenolol |
| PNT | Phenacetin |
| SMX | Sulfamethoxazole |
References
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M. Emerging contaminants of high concern and their enzyme-assisted biodegradation—A review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Huang, H.; Chen, Y. Effects of emerging pollutants on the occurrence and transfer of antibiotic resistance genes: A review. J. Hazard. Mater. 2021, 420, 126602. [Google Scholar] [CrossRef]
- Patel, N.; Khan, Z.A.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging Pollutants in Aquatic Environment: Source, Effect, and Challenges in Biomonitoring and Bioremediation—A Review. Pollution 2020, 6, 99–113. [Google Scholar]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y. Sulfate radicals-based advanced oxidation technology in various en-vironmental remediation: A state-of-the–art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Xia, Y.C.; Wan, Q.Q.; Xu, X.Q.; Cao, R.H.; Li, Y.F.; Wang, J.Y.; Xu, H.N.; Huang, T.L.; Wen, G. Solar disinfection of fungal spores in water: Kinetics, influencing factors, mechanisms and regrowth. Chem. Eng. J. 2022, 428, 132065. [Google Scholar] [CrossRef]
- Hou, J.; He, X.; Zhang, S.; Yu, J.; Feng, M.; Li, X. Recent advances in cobalt-activated sulfate radical-based advanced oxidation processes for water remediation: A review. Sci. Total Environ. 2021, 770, 145311. [Google Scholar] [CrossRef]
- Addison, F.; Offiong, N.-A.; Han, Q.; Wang, R.; Liu, N. Nitrogen-doped mesoporous carbon material (NCMK-3) as a catalyst for the removal of 4-chlorophenol during persulfate oxidation and its efficiency after reuse. Environ. Technol. 2020, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Per-oxymonosulfate: A Novel Advanced Oxidation Process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Deniere, E.; Alagappan, R.P.; Van Langenhove, H.; Van Hulle, S.; Demeestere, K. The ozone-activated peroxymonosulfate process (O3/PMS) for removal of trace organic contaminants in natural and wastewater: Effect of the (in)organic matrix composition. Chem. Eng. J. 2021, 133000. [Google Scholar] [CrossRef]
- Huang, Y.; He, Z.; Liao, X.; Cheng, Y.; Qi, H. NDMA reduction mechanism of UDMH by O3/PMS technology. Sci. Total Environ. 2022, 805, 150418. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Li, X.; Li, L. The relation of interface electron transfer and PMS activation by the H-bonding interaction between composite metal and MCM-48 during sulfamethazine ozonation. Chem. Eng. J. 2020, 398, 125529. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosul-fate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Wu, G.; Qin, W.; Sun, L.; Yuan, X.; Xia, D. Role of peroxymonosulfate on enhancing ozonation for micropollutant degradation: Performance evaluation, mechanism insight and kinetics study. Chem. Eng. J. 2019, 360, 115–123. [Google Scholar] [CrossRef]
- Cong, J.; Wen, G.; Huang, T.; Deng, L.; Ma, J. Study on enhanced ozonation degradation of para-chlorobenzoic acid by per-oxymonosulfate in aqueous solution. Chem. Eng. J. 2015, 264, 399–403. [Google Scholar] [CrossRef]
- Gholikandi, G.B.; Zakizadeh, N.; Masihi, H. Application of peroxymonosulfate-ozone advanced oxidation process for sim-ultaneous waste-activated sludge stabilization and dewatering purposes: A comparative study. J. Environ. Manag. 2018, 206, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Andres, J.; Morillo-Ponce, J.; Ibáñez-López, M.E.; Acevedo-Merino, A.; García-Morales, J.L. Disinfection enhance-ment of single ozonation by combination with peroxymonosulfate salt. J. Environ. Chem. Eng. 2020, 8, 104335. [Google Scholar] [CrossRef]
- Ike, I.A.; Karanfil, T.; Cho, J.; Hur, J. Oxidation byproducts from the degradation of dissolved organic matter by advanced oxidation processes—A critical review. Water Res. 2019, 164, 114929. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Zhou, Y.-T.; Li, W.; Zhang, X.; Du, Y.; Hu, H.-Y. Underestimated risk from ozonation of wastewater containing bromide: Both organic byproducts and bromate contributed to the toxicity increase. Water Res. 2019, 162, 43–52. [Google Scholar] [CrossRef] [PubMed]
- von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Ji, Y.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, D. Assimilable organic carbon as an indicator of bacterial regrowth. J. Am. Water Works Assoc. 1992, 84, 57–65. [Google Scholar] [CrossRef]
- Escobar, I.C.; Randall, A.A.; Taylor, J.S. Bacterial Growth in Distribution Systems: Effect of Assimilable Organic Carbon and Biodegradable Dissolved Organic Carbon. Environ. Sci. Technol. 2001, 35, 3442–3447. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule: Final Rule. Fed. Regist. 2006, 71, 388–493. [Google Scholar]
- Jin, Y.; Chen, E.; Chen, C.; Zhang, X.; Chen, L. Standards for Drinking Water Quality (GB-5749-2006); Beijing Ministry of Health of the People’s Republic of China: Bejing, China, 2006.
- Edition, F. Guidelines for drinking-water quality. WHO Chrzonicle 2011, 38, 104–108. [Google Scholar]
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Changani, F. Chlorination disinfection by-products in Mu-nicipal drinking water–A review. J. Clean. Prod. 2020, 273, 123159. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Yang, M.; Tan, C.; Chu, W. The occurrence, characteristics, transformation and control of aromatic disinfection by-products: A review. Water Res. 2020, 184, 116076. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Khatebasreh, M.; Mahdavianpour, M.; Lin, K.-Y.A. Oxidative removal of benzotriazole using peroxymono-sulfate/ozone/ultrasound: Synergy, optimization, degradation intermediates and utilizing for real wastewater. Chemosphere 2020, 244, 125326. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Shao, Y.; Pang, Z.; Wang, L.; Liu, X. Efficient Degradation of Acesulfame by Ozone/Peroxymonosulfate Advanced Oxidation Process. Molecules 2019, 24, 2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yuan, X.; Wang, D.; Wang, H.; Wu, Z.; Jiang, L.; Mo, D.; Yang, G.; Guan, R.; Zeng, G. Recyclable zero-valent iron acti-vating peroxymonosulfate synchronously combined with thermal treatment enhances sludge dewaterability by altering physicochemical and biological properties. Bioresour. Technol. 2018, 262, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Chegini, Z.G.; Hassani, H.; Torabian, A.; Borghei, S.M. Enhancement of PMS activation in an UV/ozone process for cyanide degradation: A comprehensive study. Pigment Resin Technol. 2020, 49, 409–414. [Google Scholar] [CrossRef]
- Jung, Y.; Hong, E.; Kwon, M.; Kang, J.-W. A kinetic study of ozone decay and bromine formation in saltwater ozonation: Effect of O3 dose, salinity, pH, and temperature. Chem. Eng. J. 2017, 312, 30–38. [Google Scholar] [CrossRef]
- Yuan, Z.; Sui, M.; Yuan, B.; Li, P.; Wang, J.; Qin, J.; Xu, G. Degradation of ibuprofen using ozone combined with peroxymo-nosulfate. Environ. Sci. Water Res. 2017, 3, 960–969. [Google Scholar]
- Wang, Y.; Chu, W. Degradation of a xanthene dye by Fe(II)-mediated activation of Oxone process. J. Hazard. Mater. 2011, 186, 1455–1461. [Google Scholar] [CrossRef]
- Sharma, J.; Mishra, I.; Dionysiou, D.; Kumar, V. Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chem. Eng. J. 2015, 276, 193–204. [Google Scholar] [CrossRef]
- Muthukumar, M.; Selvakumar, N. Studies on the effect of inorganic salts on decolouration of acid dye effluents by ozonation. Dye. Pigment. 2004, 62, 221–228. [Google Scholar] [CrossRef]
- Lutze, H.V.; Kerlin, N.; Schmidt, T.C. Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res. 2015, 72, 349–360. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Okawa, K.; Nakano, Y.; Nishijima, W.; Okada, M. Effects of humic substances on the decomposition of 2,4-dichlorophenol by ozone after extraction from water into acetic acid through activated carbon. Chemosphere 2004, 57, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Buffle, M.O.; von Gunten, U. Phenols and amine induced HO• generation during the initial phase of natural water ozonation. Environ. Sci. Technol. 2006, 40, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J. Degradation and mineralization of ofloxacin by ozonation and peroxone (O3/H2O2) process. Chemosphere 2021, 269, 128775. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, E.; Wang, J.; Zhang, X.; Huang, H.; Yu, G.; Wang, Y. Comparison of emerging contaminant abatement by conventional ozonation, catalytic ozonation, O3/H2O2 and electro-peroxone processes. J. Hazard. Mater. 2020, 389, 121829. [Google Scholar] [CrossRef] [PubMed]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing: London, UK, 2012. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O—In Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Piras, F.; Santoro, O.; Pastore, T.; Pio, I.; De Dominicis, E.; Gritti, E.; Caricato, R.; Lionetto, M.; Mele, G. Controlling micropollutants in tertiary municipal wastewater by O3/H2O2, granular biofiltration and UV254/H2O2 for potable reuse applications. Chemosphere 2020, 239, 124635. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water: Rate of initiation by hydroxide ions and hydrogen peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Merényi, G.; Lind, J.; Naumov, S.; von Sonntag, C. Reaction of Ozone with Hydrogen Peroxide (Peroxone Process): A Revision of Current Mechanistic Concepts Based on Thermokinetic and Quantum-Chemical Considerations. Environ. Sci. Technol. 2010, 44, 3505–3507. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Lutze, H.V.; Bircher, S.; Rapp, I.; Kerlin, N.; Bakkour, R.; Geisler, M.; von Sonntag, C.; Schmidt, T.C. Degradation of chlo-rotriazine pesticides by sulfate radicals and the influence of organic matter. Environ. Sci. Technol. 2015, 49, 1673–1680. [Google Scholar] [CrossRef]
- Gara, P.M.D.; Bosio, G.N.; Gonzalez, M.; Martire, D. Kinetics of the sulfate radical-mediated photo-oxidation of humic substances. Int. J. Chem. Kinet. 2007, 40, 19–24. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, H.; Croué, J.-P. Production of Sulfate Radical from Peroxymonosulfate Induced by a Magnetically Separable CuFe2O4 Spinel in Water: Efficiency, Stability, and Mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- He, X.; de la Cruz, A.A.; Dionysiou, D.D. Destruction of cyanobacterial toxin cylindrospermopsin by hydroxyl radicals and sulfate radicals using UV-254nm activation of hydrogen peroxide, persulfate and peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2013, 251, 160–166. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Mao, Y.; Dong, H.; Liu, S.; Zhang, L.; Qiang, Z. Accelerated oxidation of iopamidol by ozone/peroxymonosulfate (O3/PMS) process: Kinetics, mechanism, and simultaneous reduction of iodinated disinfection by-product formation potential. Water Res. 2020, 173, 115615. [Google Scholar] [CrossRef]
- Deniere, E.; Van Hulle, S.; Van Langenhove, H.; Demeestere, K. Advanced oxidation of pharmaceuticals by the ozone-activated peroxymonosulfate process: The role of different oxidative species. J. Hazard. Mater. 2018, 360, 204–213. [Google Scholar] [CrossRef]
- Yang, N.; Cui, J.; Zhang, L.; Xiao, W.; Alshawabkeh, A.N.; Mao, X. Iron electrolysis-assisted peroxymonosulfate chemical oxidation for the remediation of chlorophenol-contaminated groundwater. J. Chem. Technol. Biotechnol. 2016, 91, 938–947. [Google Scholar] [CrossRef]
- Chen, Q.; Ji, F.; Liu, T.; Yan, P.; Guan, W.; Xu, X. Synergistic effect of bifunctional Co-TiO2 catalyst on degradation of Rho-damine B: Fenton-photo hybrid process. Chem. Eng. J. 2013, 229, 57–65. [Google Scholar] [CrossRef]
- Akbari, S.; Ghanbari, F.; Moradi, M. Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: Applying low current density for oxidation mechanism. Chem. Eng. J. 2016, 294, 298–307. [Google Scholar] [CrossRef]
- Wen, G.; Qiang, C.; Feng, Y.; Huang, T.; Ma, J. Bromate formation during the oxidation of bromide-containing water by ozone/peroxymonosulfate process: Influencing factors and mechanisms. Chem. Eng. J. 2018, 352, 316–324. [Google Scholar] [CrossRef]
- Liu, X.; Hong, Y.; Ding, S.; Jin, W.; Dong, S.; Xiao, R.; Chu, W. Transformation of antiviral ribavirin during ozone/PMS in-tensified disinfection amid COVID-19 pandemic. Sci. Total Environ. 2021, 790, 148030. [Google Scholar] [CrossRef]
- Das, T.N. Reactivity and Role of SO5•- Radical in Aqueous Medium Chain Oxidation of Sulfite to Sulfate and Atmospheric Sulfuric Acid Generation. J. Phys. Chem. A 2001, 105, 9142–9155. [Google Scholar] [CrossRef]
- Matthew, B.M.; Anastasio, C. A chemical probe technique for the determination of reactive halogen species in aqueous solution: Part 1—Bromide solutions. Atmos. Chem. Phys. Discuss. 2006, 6, 2423–2437. [Google Scholar] [CrossRef] [Green Version]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of Halide Ions and Carbonates on Organic Contaminant Degradation by Hydroxyl Radical-Based Advanced Oxidation Processes in Saline Waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef]
- Hoigné, J. Chemistry of Aqueous Ozone and Transformation of Pollutants by Ozonation and Advanced Oxidation Processes. In Quality and Treatment of Drinking Water II. The Handbook of Environmental Chemistry (Part C: Water Pollution); Hrubec, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 5, pp. 83–141. [Google Scholar]
- Cao, Y.; Qiu, W.; Zhao, Y.; Li, J.; Jiang, J.; Yang, Y.; Pang, S.-Y.; Liu, G. The degradation of chloramphenicol by O3/PMS and the impact of O3-based AOPs pre-oxidation on dichloroacetamide generation in post-chlorination. Chem. Eng. J. 2020, 401, 126146. [Google Scholar] [CrossRef]
- Sehested, K.; Holcman, J.; Bjergbakke, E.; Hart, E.J. Formation of ozone in the reaction of hydroxyl with O3− and the decay of the ozonide ion radical at pH 10–13. J. Phys. Chem. 1984, 88, 269–273. [Google Scholar] [CrossRef]
- Maruthamuthu, P.; Neta, P. Radiolytic chain decomposition of peroxomonophosphoric and peroxomonosulfuric acids. J. Phys. Chem. 1977, 81, 937–940. [Google Scholar] [CrossRef]
- Furman, O.S.; Teel, A.L.; Watts, R.J. Mechanism of Base Activation of Persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef] [PubMed]
- Klaning, U.K.; Sehested, K.; Appelman, E.H. Laser flash photolysis and pulse radiolysis of aqueous solutions of the fluoroxysulfate ion, SO4F-. Inorg. Chem. 1991, 30, 3582–3584. [Google Scholar] [CrossRef]
- Guo, L.; Zhong, Q.; Ding, J.; Lv, Z.; Zhao, W.; Deng, Z. Low-temperature NOx (x = 1, 2) removal with •OH radicals from catalytic ozonation over a RGO-CeO2 nanocomposite: The highly promotional effect of oxygen vacancies. RSC Adv. 2016, 6, 87869–87877. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic mi-cropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef] [PubMed]
- Pisarenko, A.N.; Stanford, B.D.; Yan, D.; Gerrity, D.; Snyder, S.A. Effects of ozone and ozone/peroxide on trace organic contaminants and NDMA in drinking water and water reuse applications. Water Res. 2012, 46, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.S.; Von Gunten, U.; Kaiser, H.-P. Hydroxyl Radical/Ozone Ratios during Ozonation Processes. II. The Effect of Temperature, pH, Alkalinity, and DOM Properties. Ozone Sci. Eng. 2000, 22, 123–150. [Google Scholar] [CrossRef]
- Ikehata, K.; El-Din, M.G. Aqueous Pesticide Degradation by Ozonation and Ozone-Based Advanced Oxidation Processes: A Review (Part I). Ozone Sci. Eng. 2005, 27, 83–114. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, C.; Qin, J.; Wei, F.; Rao, M.; Wang, S. Synthesis of Magnetic Cobalt Nanoparticles Anchored on Graphene Nanosheets and Catalytic Decomposition of Orange II. Ind. Eng. Chem. Res. 2013, 52, 17341–17350. [Google Scholar] [CrossRef]
- Shi, P.; Su, R.; Zhu, S.; Zhu, M.; Li, D.; Xu, S. Supported cobalt oxide on graphene oxide: Highly efficient catalysts for the removal of Orange II from water. J. Hazard. Mater. 2012, 229–230, 331–339. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, J.; Montero-De-Espinosa, R. Catalytic ozonation of oxalic acid in an aqueous TiO2 slurry reactor. Appl. Catal. B Environ. 2002, 39, 221–231. [Google Scholar] [CrossRef]
- McElroy, W.J. A laser photolysis study of the reaction of sulfate(1-) with chloride and the subsequent decay of chlorine(1-) in aqueous solution. J. Phys. Chem. 1990, 94, 2435–2441. [Google Scholar] [CrossRef]
- Wang, Z.; An, N.; Shao, Y.; Gao, N.; Du, E.; Xu, B. Experimental and simulation investigations of UV/persulfate treatment in presence of bromide: Effects on degradation kinetics, formation of brominated disinfection byproducts and bromate. Sep. Purif. Technol. 2020, 242, 116767. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W. Comparison of Halide Impacts on the Efficiency of Contaminant Degradation by Sulfate and Hydroxyl Radical-Based Advanced Oxidation Processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Naumov, S.; Mark, G.; Jarocki, A.; von Sonntag, C. The reactions of nitrite ion with ozone in aqueous solution–new exper-imental data and quantum-chemical considerations. Ozone Sci. Eng. 2010, 32, 430–434. [Google Scholar] [CrossRef]
- Morozov, P.A.; Ershov, B.G. The influence of phosphates on the decomposition of ozone in water: Chain process inhibition. Russ. J. Phys. Chem. A 2010, 84, 1136–1140. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, Y.; Wei, Y.; Wang, S.; Liu, P.; Jia, Z.; Yu, X.; Ma, F. Advanced treatment of bio-treated Chinese patent medicine wastewater using ozone/peroxymonosulfate-upflow biological aerated filter. Chem. Eng. J. 2020, 390, 124527. [Google Scholar] [CrossRef]
- Yu, X.; Qin, W.; Yuan, X.; Sun, L.; Pan, F.; Xia, D. Synergistic mechanism and degradation kinetics for atenolol elimination via integrated UV/ozone/peroxymonosulfate process. J. Hazard. Mater. 2021, 407, 124393. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Sun, H.; Xiao, J.; Cao, H.; Wang, S. 2D/2D nano-hybrids of γ-MnO2 on reduced graphene oxide for catalytic ozonation and coupling peroxymonosulfate activation. J. Hazard. Mater. 2016, 301, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Wang, S.; Wang, T.; Feng, Y.; Chen, Z.; Lin, W.; Huang, T.; Ma, J. Inhibition of bromate formation in the O3/PMS process by adding low dosage of carbon materials: Efficiency and mechanism. Chem. Eng. J. 2020, 402, 126207. [Google Scholar] [CrossRef]
- Tan, C.; Cui, X.; Sun, K.; Xiang, H.; Du, E.; Deng, L.; Gao, H. Kinetic mechanism of ozone activated peroxymonosulfate system for enhanced removal of anti-inflammatory drugs. Sci. Total Environ. 2020, 733, 139250. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Successive non-radical and radical process of peroxymonosulfate-based oxidation using various activation methods for enhancing mineralization of sulfamethoxazole. Chemosphere 2021, 263, 127964. [Google Scholar] [CrossRef]
- Jung, H.; Choi, H. Catalytic decomposition of ozone and para-Chlorobenzoic acid (pCBA) in the presence of nanosized ZnO. Appl. Catal. B Environ. 2006, 66, 288–294. [Google Scholar] [CrossRef]
- Real, F.J.; Benitez, F.J.; Acero, J.L.; Sagasti, J.J.P.; Casas, F. Kinetics of the Chemical Oxidation of the Pharmaceuticals Primidone, Ketoprofen, and Diatrizoate in Ultrapure and Natural Waters. Ind. Eng. Chem. Res. 2009, 48, 3380–3388. [Google Scholar] [CrossRef]
- Acero, J.L.; Stemmler, K.; von Gunten, U. Degradation Kinetics of Atrazine and Its Degradation Products with Ozone and OH Radicals: A Predictive Tool for Drinking Water Treatment. Environ. Sci. Technol. 2000, 34, 591–597. [Google Scholar] [CrossRef]
- Sanchez-Polo, M.; Rivera-Utrilla, J.; Prados-Joya, G.; García, M.; Ángeles, F.; Bautista-Toledo, I. Removal of pharmaceutical compounds, nitroimidazoles, from waters by using the ozone/carbon system. Water Res. 2008, 42, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Yao, B.; Hou, S.; Fang, J.; Yan, S.; Song, W. Kinetic Study of Hydroxyl and Sulfate Radical-Mediated Oxidation of Pharmaceuticals in Wastewater Effluents. Environ. Sci. Technol. 2017, 51, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Benner, J.; Ternes, T.A. Ozonation of Metoprolol: Elucidation of Oxidation Pathways and Major Oxidation Products. Environ. Sci. Technol. 2009, 43, 5472–5480. [Google Scholar] [CrossRef] [PubMed]
- Benitez, F.J.; Acero, J.L.; Real, F.J.; Roldán, G. Ozonation of pharmaceutical compounds: Rate constants and elimination in various water matrices. Chemosphere 2009, 77, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Santoke, H.; Song, W.; Cooper, W.J.; Peake, B.M. Advanced oxidation treatment and photochemical fate of selected anti-depressant pharmaceuticals in solutions of Suwannee River humic acid. J. Hazard. Mater. 2012, 217, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kovalova, L.; McArdell, C.S.; von Gunten, U. Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent. Water Res. 2014, 64, 134–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, D.C.; Huber, M.M.; Wagner, M.; von Gunten, U.; Ternes, T.A. Ozonation of Carbamazepine in Drinking Water: Identification and Kinetic Study of Major Oxidation Products. Environ. Sci. Technol. 2005, 39, 8014–8022. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.; Tlili, S.; Chiron, S.; Barbati, S. Removal of carbamazepine from urban wastewater by sulfate radical oxidation. Environ. Chem. Lett. 2010, 9, 347–353. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Rate constants of reactions of ozone with organic and inorganic compounds in water—II: Dissociating organic compounds. Water Res. 1983, 17, 185–194. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Liang, C.; Su, H.-W. Identification of Sulfate and Hydroxyl Radicals in Thermally Activated Persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Toth, J.E.; Rickman, K.A.; Venter, A.R.; Kiddle, J.J.; Mezyk, S.P. Reaction Kinetics and Efficiencies for the Hydroxyl and Sulfate Radical Based Oxidation of Artificial Sweeteners in Water. J. Phys. Chem. A 2012, 116, 9819–9824. [Google Scholar] [CrossRef]
- Shu, Z.; Bolton, J.R.; Belosevic, M.; El Din, M.G. Photodegradation of emerging micropollutants using the medium-pressure UV/H2O2 Advanced Oxidation Process. Water Res. 2013, 47, 2881–2889. [Google Scholar] [CrossRef]
- Ma, J.; Graham, N.J. Degradation of atrazine by manganese-catalysed ozonation: Influence of humic substances. Water Res. 1999, 33, 785–793. [Google Scholar] [CrossRef]
- Lau, T.K.; Chu, W.; Graham, N.J.D. The Aqueous Degradation of Butylated Hydroxyanisole by UV/S2O82−: Study of Reaction Mechanisms via Dimerization and Mineralization. Environ. Sci. Technol. 2007, 41, 613–619. [Google Scholar] [CrossRef]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S.; Li, X.; Wiesner, M.R.; Fang, J. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar] [CrossRef]
- Zuo, Z.; Cai, Z.; Katsumura, Y.; Chitose, N.; Muroya, Y. Reinvestigation of the acid–base equilibrium of the (bi)carbonate radical and pH dependence of its reactivity with inorganic reactants. Radiat. Phys. Chem. 1999, 55, 15–23. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef]
- Wen, G.; Wang, S.-J.; Ma, J.; Huang, T.-L.; Liu, Z.-Q.; Zhao, L.; Su, J.-F. Enhanced ozonation degradation of di-n-butyl phthalate by zero-valent zinc in aqueous solution: Performance and mechanism. J. Hazard. Mater. 2014, 265, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Intermediates and reaction pathways from the degradation of micro-cystin-LR with sulfate radicals. Environ. Sci. Technol. 2010, 44, 7238–7244. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Díaz, J.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Canonica, S.; von Gunten, U. Advanced oxidation of the surfactant SDBS by means of hydroxyl and sulphate radicals. Chem. Eng. J. 2010, 163, 300–306. [Google Scholar] [CrossRef]
- Song, W.; Yan, S.; Cooper, W.J.; Dionysiou, D.D.; O’Shea, K.E. Hydroxyl radical oxidation of cylindrospermopsin (cyano-bacterial toxin) and its role in the photochemical transformation. Environ. Sci. Technol. 2012, 46, 12608–12615. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Salhi, E.; Köster, O.; Kaiser, H.P.; Egli, T.; Von Gunten, U. Mechanistic and kinetic evaluation of organic disin-fection by-product and assimilable organic carbon (AOC) formation during the ozonation of drinking water. Water Res. 2006, 40, 2275–2286. [Google Scholar] [CrossRef]
- Hammes, F.; Meylan, S.; Salhi, E.; Köster, O.; Egli, T.; Von Gunten, U. Formation of assimilable organic carbon (AOC) and specific natural organic matter (NOM) fractions during ozonation of phytoplankton. Water Res. 2007, 41, 1447–1454. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Shang, C. Bromate Formation from Bromide Oxidation by the UV/Persulfate Process. Environ. Sci. Technol. 2012, 46, 8976–8983. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U.; Hoigne, J. Bromate Formation during Ozonization of Bromide-Containing Waters: Interaction of Ozone and Hydroxyl Radical Reactions. Environ. Sci. Technol. 1994, 28, 1234–1242. [Google Scholar] [CrossRef]
- Fischbacher, A.; Löppenberg, K.; von Sonntag, C.; Schmidt, T.C. A New Reaction Pathway for Bromite to Bromate in the Ozonation of Bromide. Environ. Sci. Technol. 2015, 49, 11714–11720. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Dong, W.; Ma, J.; Yang, Y.; Li, J.; Yang, Z.; Zhang, X.; Gu, J.; Xie, W.; et al. Enhancement of bromate formation by pH depression during ozonation of bromide-containing water in the presence of hydroxylamine. Water Res. 2017, 109, 135–143. [Google Scholar] [CrossRef]
- Legube, B.; Parinet, B.; Gelinet, K.; Berne, F.; Croue, J.-P. Modeling of bromate formation by ozonation of surface waters in drinking water treatment. Water Res. 2004, 38, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Naumov, S.; von Sonntag, C. Standard Gibbs free energies of reactions of ozone with free radicals in aqueous solution: Quantum-chemical calculations. Environ. Sci. Technol. 2011, 45, 9195–9204. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.L.; Griffini, O.; Santianni, D.; Barbieri, K.; Burrini, D.; Pantani, F. Removal of bromate ion from water using granular activated carbon. Water Res. 1999, 33, 2959–2970. [Google Scholar] [CrossRef]
- Huang, W.-J.; Cheng, Y.-L. Effect of characteristics of activated carbon on removal of bromate. Sep. Purif. Technol. 2008, 59, 101–107. [Google Scholar] [CrossRef]
- Yang, J.; Dong, Z.; Jiang, C.; Wang, C.; Liu, H. An overview of bromate formation in chemical oxidation processes: Occurrence, mechanism, influencing factors, risk assessment, and control strategies. Chemosphere 2019, 237, 124521. [Google Scholar] [CrossRef]
- Ling, L.; Deng, Z.; Fang, J.; Shang, C. Bromate control during ozonation by ammonia-chlorine and chlorine-ammonia pre-treatment: Roles of bromine-containing haloamines. Chem. Eng. J. 2020, 389, 123447. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Zhou, J.; Huang, Z.; Liu, S.; Qian, G.; Gao, N. Bromate inhibition by reduced graphene oxide in ther-mal/PMS process. Water Res. 2017, 122, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, S.; Ma, W.; Wang, J.; Xu, H.; Li, K.; Huang, T.; Ma, J.; Wen, G. Adding CuCo2O4-GO to inhibit bromate formation and enhance sulfamethoxazole degradation during the ozone/peroxymonosulfate process: Efficiency and mechanism. Chemosphere 2022, 286, 131829. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wen, G.; Ni, Y.; Wang, S.; Wang, S.; Yu, Y.; Huang, T.; Ma, J. Inhibition of bromate formation in the ozone/peroxymonosulfate process by ammonia, ammonia-chlorine and chlorine-ammonia pretreatment: Comparisons with ozone alone. Sep. Purif. Technol. 2022, 278, 119600. [Google Scholar] [CrossRef]
- Zehavi, D.; Rabani, J. Oxidation of aqueous bromide ions by hydroxyl radicals. Pulse radiolytic investigation. J. Phys. Chem. 1972, 76, 312–319. [Google Scholar] [CrossRef]
- Zuo, J.; Xu, X.; Wan, Q.; Cao, R.; Liang, Z.; Xu, H.; Li, K.; Huang, T.; Wen, G.; Ma, J. Inactivation of fungal spores in water with peracetic acid: Efficiency and mechanism. Chem. Eng. J. 2022, 427, 131753. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. The role of hydroxyl radical reactions in ozonation processes in aqueous solutions. Water Res. 1976, 10, 377–386. [Google Scholar] [CrossRef]
- Von Gunten, U.; Bruchet, A.; Costentin, E. Bromate formation in advanced oxidation processes. J. Am. Water Work. Assoc. 1996, 88, 53–65. [Google Scholar] [CrossRef]
- Wang, L.; Jing, K.; Hu, B.; Lu, J. Hydrogen peroxide suppresses the formation of brominated oxidation by-products in heat-activated peroxydisulfate oxidation process. Chem. Eng. J. 2021, 417, 129138. [Google Scholar] [CrossRef]
- Wan, Q.Q.; Cao, R.H.; Wen, G.; Xu, X.Q.; Xia, Y.C.; Wu, G.H.; Li, Y.F.; Wang, J.Y.; Lin, Y.Z.; Huang, T.L. Sequential use of UV-LEDs irradiation and chlorine to disinfect waterborne fungal spores: Efficiency, mechanism and photoreactivation. J. Hazard. Mater. 2022, 423, 127102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).