Degradation of Aqueous CONFIDOR® Pesticide by Simultaneous TiO2 Photocatalysis and Fe-Zeolite Catalytic Ozonation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
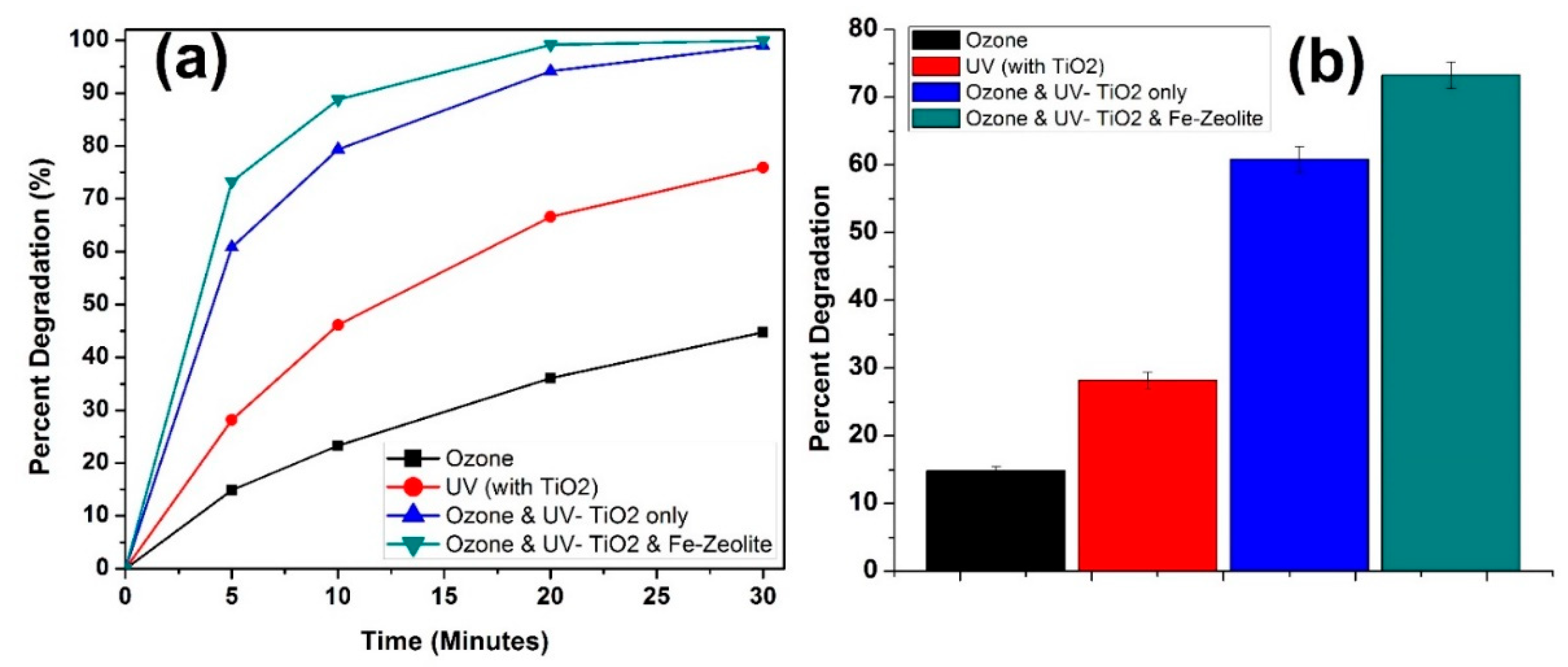
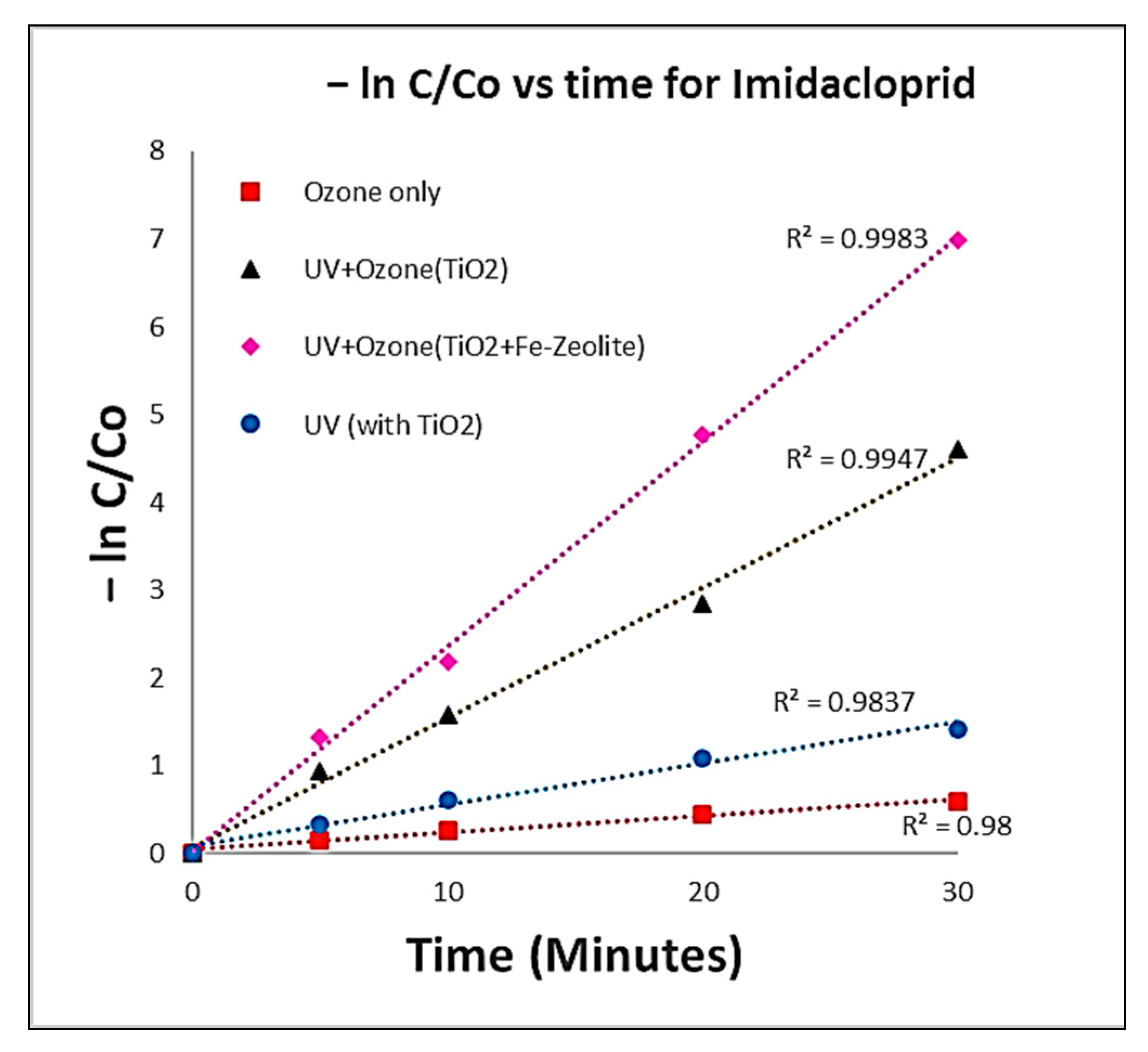
3.1. Degradation Studies
3.2. Effect of Initial Concentration
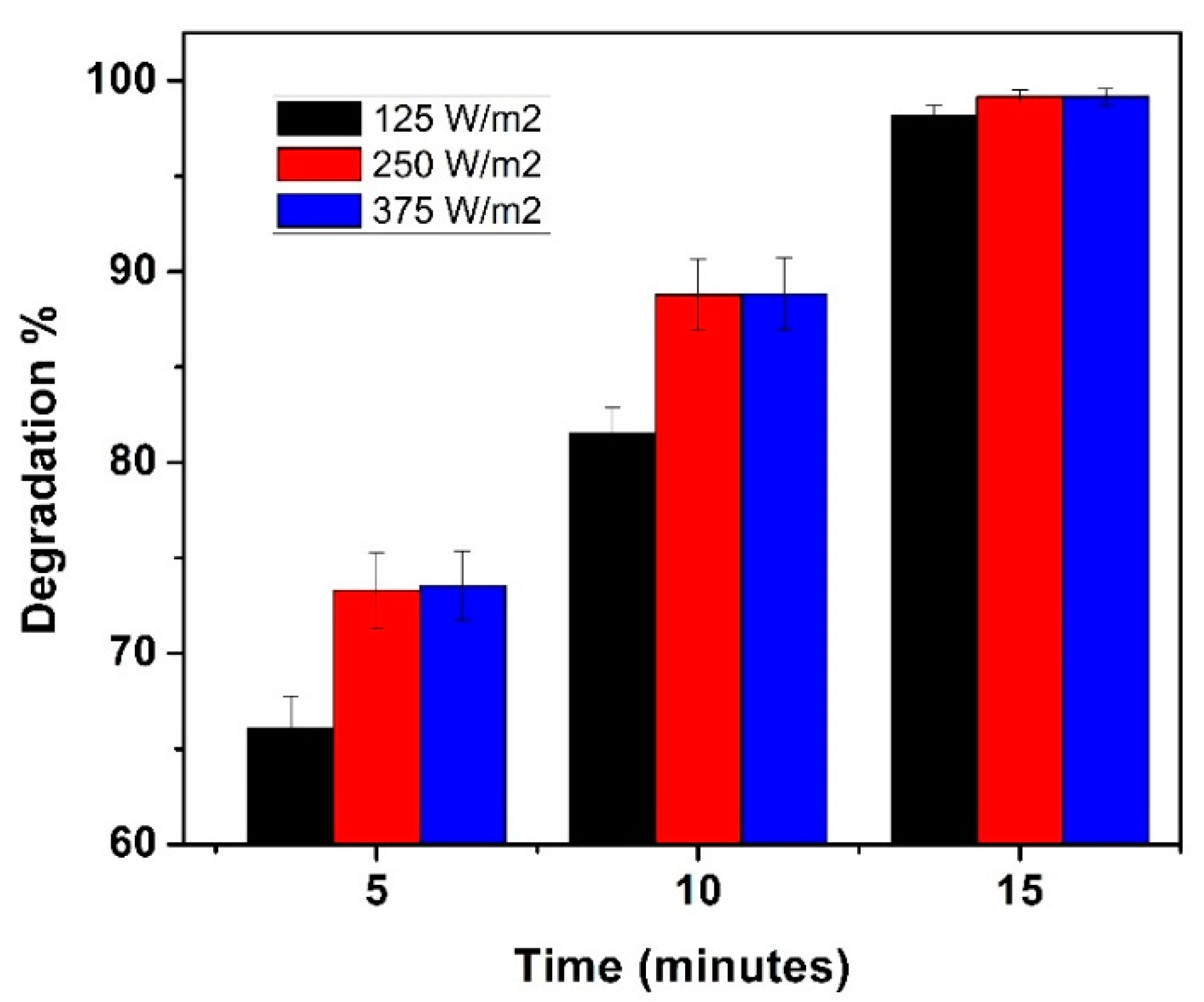
3.3. Variation of UV Intensity
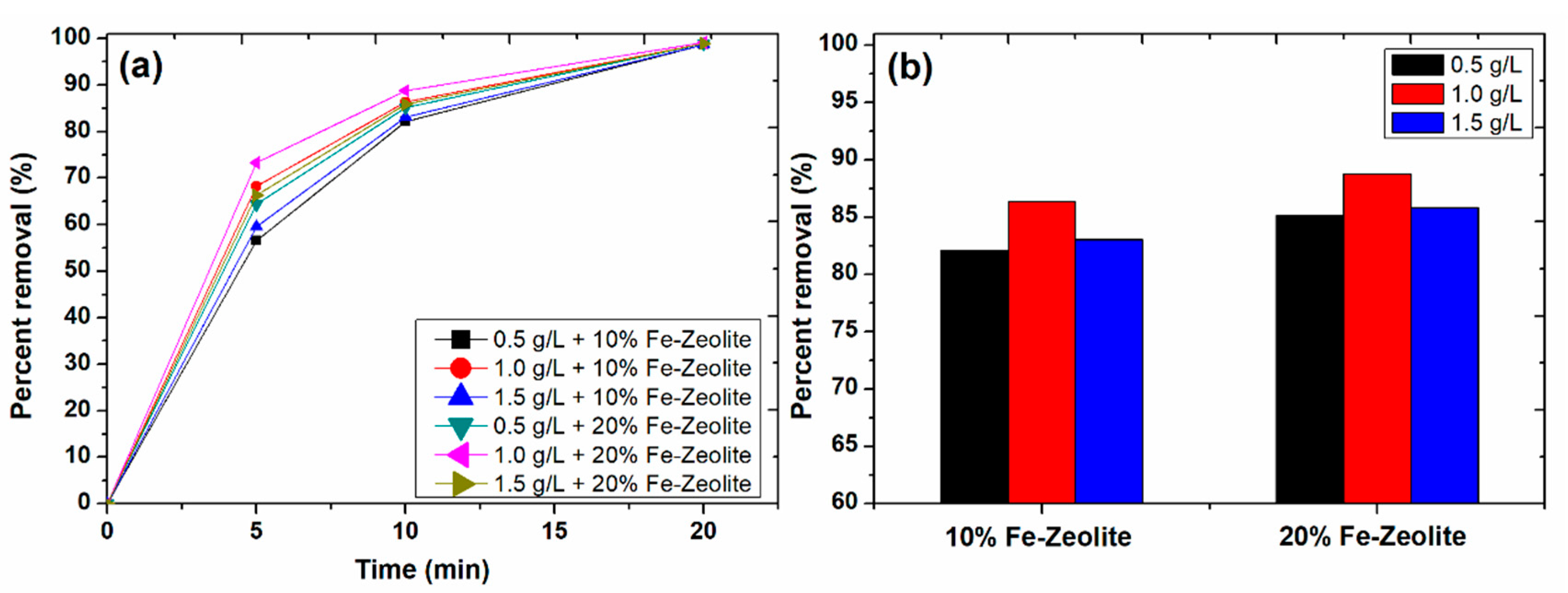
3.4. Effect of Catalyst Dose and Fe-Zeolite Percentage
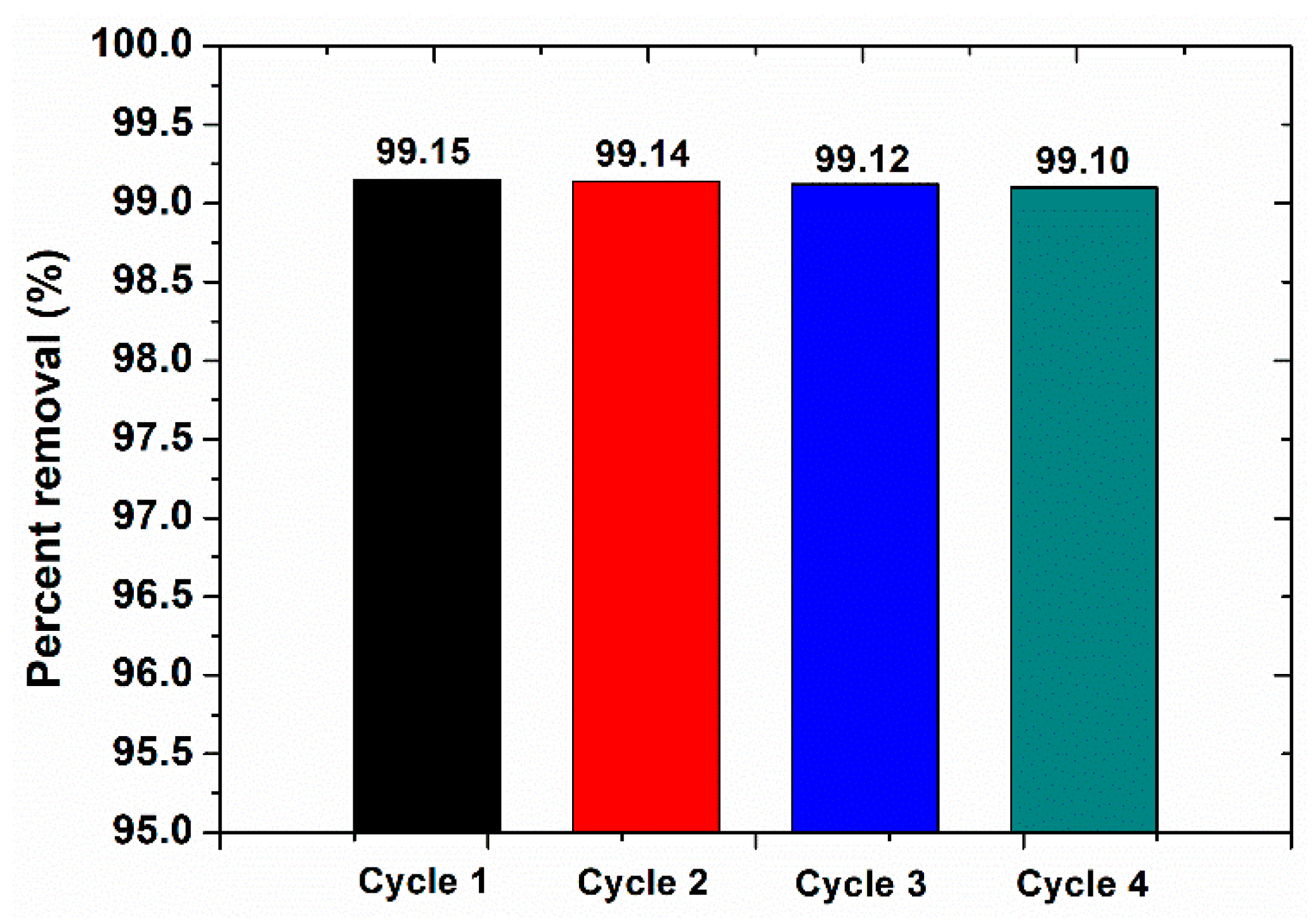
3.5. Catalyst Reuse
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qasim, S.R.; Zhu, G. Wastewater Treatment and Reuse: Theory and Design Examples: Volume 1: Principles and Basic Treatment; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781351402026. [Google Scholar]
- Baste, I.A.; Watson, R.T.; Brauman, K.I.; Samper, C.; Walzer, C. Making Peace with Nature: A Scientific Blueprint to Tackle the Climate, Biodiversity and Pollution Emergencies; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Bui, X.-T.; Chiemchaisri, C.; Fujioka, T.; Varjani, S. Water and Wastewater Treatment Technologies; Springer: Singapore, 2019. [Google Scholar]
- Finance Division Government of Pakistan. Pakistan Economic Survey 2020–21; Finance Division Government of Pakistan: Islamabad, Pakistan, 2021. [Google Scholar]
- Borsuah, J.F.; Messer, T.L.; Snow, D.D.; Comfort, S.D.; Mittelstet, A.R. Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water 2020, 12, 3388. [Google Scholar] [CrossRef]
- Bermúdez, L.A.; Pascual, J.M.; Martínez, M.d.M.M.; Poyatos Capilla, J.M. Effectiveness of Advanced Oxidation Processes in Wastewater Treatment: State of the Art. Water 2021, 13, 2094. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Gholami, M.; Shirzad-Siboni, M.; Farzadkia, M.; Yang, J.-K. Synthesis, characterization, and application of ZnO/TiO2 nanocomposite for photocatalysis of a herbicide (Bentazon). Desalin. Water Treat. 2016, 57, 13632–13644. [Google Scholar] [CrossRef]
- Sraw, A.; Toor, A.P.; Wanchoo, R.K. Adsorption kinetics and degradation mechanism study of water persistent insecticide quinalphos: For heterogeneous photocatalysis onto TiO2. Desalin. Water Treat. 2016, 57, 16831–16842. [Google Scholar] [CrossRef]
- Juan, J.L.X.; Maldonado, C.S.; Sánchez, R.A.L.; Díaz, O.J.E.; Rojas Ronquillo, M.R.; Sandoval-Rangel, L.; Pineda Aguilar, N.; Ramos Delgado, N.A.; Martínez-Vargas, D.X. TiO2 doped with europium (Eu): Synthesis, characterization and catalytic performance on pesticide degradation under solar irradiation. Catal. Today 2021. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Waheed, S.; Joya, K.S.; Kazmi, M. Catalytic ozonation of paracetamol on zeolite A: Non-radical mechanism. Catal. Commun. 2018, 112, 15–20. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Munir, H.M.S.; Khan, A.; Javed, F.; Joya, K.S. Comparative study of catalytic ozonation and Fenton-like processes using iron-loaded rice husk ash as catalyst for the removal of methylene blue in wastewater. Ozone Sci. Eng. 2019, 41, 250–260. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Zafar, M.; Javed, F.; Yasar, A.; Akram, A.; Shabbir, S.; Qi, F. Catalytic ozonation for the removal of reactive black 5 (RB-5) dye using zeolites modified with CuMn2O4/gC3N4 in a synergic electro flocculation-catalytic ozonation process. Water Sci. Technol. 2021, 84, 1943–1953. [Google Scholar] [CrossRef]
- Gomes, J.; Roccamante, M.; Contreras, S.; Medina, F.; Oller, I.; Martins, R.C. Scale-up impact over solar photocatalytic ozonation with benchmark-P25 and N-TiO2 for insecticides abatement in water. J. Environ. Chem. Eng. 2021, 9, 104915. [Google Scholar] [CrossRef]
- Bougarrani, S.; Baicha, Z.; Latrach, L.; Mahi, M.E.; Hernandez Fernandez, F.J. Improving the Imazapyr Degradation by Photocatalytic Ozonation: A Comparative Study with Different Oxidative Chemical Processes. Processes 2020, 8, 1446. [Google Scholar] [CrossRef]
- An, W.; Tian, L.; Hu, J.; Liu, L.; Cui, W.; Liang, Y. Efficient degradation of organic pollutants by catalytic ozonation and photocatalysis synergy system using double-functional MgO/g-C3N4 catalyst. Appl. Surf. Sci. 2020, 534, 147518. [Google Scholar] [CrossRef]
- Nageswara Rao, T.; Prashanthi, Y.; Ahmed, F.; Kumar, S.; Arshi, N.; Rajasekhar Reddy, G.; Manohra Naidu, T. Photocatalytic Applications of Fe–Ag Co-Doped TiO2 Nanoparticles in Removal of Flumioxazin Pesticide Residues in Water. Front. Nanotechnol. 2021, 3, 14. [Google Scholar] [CrossRef]
- Cheng, S.-W.; Li, Y.-H.; Yuan, C.-S.; Tsai, P.-Y.; Shen, H.-Z.; Hung, C.-H. An Innovative Advanced Oxidation Technology for Effective Decomposition of Formaldehyde by Combining Iron Modified Nano-TiO2 (Fe/TiO2) Photocatalytic Degradation with Ozone Oxidation. Aerosol Air Qual. Res. 2018, 18, 3220–3233. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Raashid, M.; Akram, A.; Kazmi, M.; Farman, S. Removal of methylene blue dye from aqueous solutions by adsorption in combination with ozonation on iron loaded sodium zeolite: Role of adsorption. Desalin. Water Treat. 2021, 49, 376–383. [Google Scholar]
- Baghirzade, B.S.; Yetis, U.; Dilek, F.B. Imidacloprid elimination by O3 and O3/UV: Kinetics study, matrix effect, and mechanism insight. Environ. Sci. Pollut. Res. 2021, 28, 24535–24551. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Alizadeh, R.; Ebadi, A.; Pelalak, R.; Oturan, N.; Oturan, M.A. Degradation of furosemide using photocatalytic ozonation in the presence of ZnO/ICLT nanocomposite particles: Experimental, modeling, optimization and mechanism evaluation. J. Mol. Liq. 2020, 319, 114193. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, R.; Bansal, A. Photocatalytic degradation of imidacloprid using semiconductor hybrid nano-catalyst: Kinetics, surface reactions and degradation pathways. Int. J. Environ. Sci. Technol. 2021, 18, 1425–1442. [Google Scholar] [CrossRef]
- Chen, S.; Deng, J.; Deng, Y.; Gao, N. Influencing factors and kinetic studies of imidacloprid degradation by ozonation. Environ. Technol. 2019, 40, 2127–2134. [Google Scholar] [CrossRef]
- Sun, J.; Yan, X.; Lv, K.; Sun, S.; Deng, K.; Du, D. Photocatalytic degradation pathway for azo dye in TiO2/UV/O3 system: Hydroxyl radical versus hole. J. Mol. Catal. A Chem. 2013, 367, 31–37. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F. Mechanism and kinetics of sulfamethoxazole photocatalytic ozonation in water. Water Res. 2009, 43, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Zhu, D.; Li, L.; Lan, B. Enhanced photocatalytic ozonation of organics by g-C3N4 under visible light irradiation. J. Hazard. Mater. 2014, 280, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Fourie, C.J.S.; Momba, M.N.B. Synergistic effect of UV—vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: A comparative study. J. Catal. 2016, 341, 116–125. [Google Scholar] [CrossRef]


| Pore Size (Å) | Composition (Dry) | Thermal Decomposition (°C) | Surface Area (m2/g) | Point of Zero Charge (pHpzc) |
|---|---|---|---|---|
| 4 | 2Na2O-Al2O3-1.75SiO2-6H2O | 700 | 91.3 | 6.2 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raashid, M.; Kazmi, M.; Ikhlaq, A.; Iqbal, T.; Sulaiman, M.; Shakeel, A. Degradation of Aqueous CONFIDOR® Pesticide by Simultaneous TiO2 Photocatalysis and Fe-Zeolite Catalytic Ozonation. Water 2021, 13, 3327. https://doi.org/10.3390/w13233327
Raashid M, Kazmi M, Ikhlaq A, Iqbal T, Sulaiman M, Shakeel A. Degradation of Aqueous CONFIDOR® Pesticide by Simultaneous TiO2 Photocatalysis and Fe-Zeolite Catalytic Ozonation. Water. 2021; 13(23):3327. https://doi.org/10.3390/w13233327
Chicago/Turabian StyleRaashid, Muhammad, Mohsin Kazmi, Amir Ikhlaq, Tanveer Iqbal, Muhammad Sulaiman, and Ahmad Shakeel. 2021. "Degradation of Aqueous CONFIDOR® Pesticide by Simultaneous TiO2 Photocatalysis and Fe-Zeolite Catalytic Ozonation" Water 13, no. 23: 3327. https://doi.org/10.3390/w13233327
APA StyleRaashid, M., Kazmi, M., Ikhlaq, A., Iqbal, T., Sulaiman, M., & Shakeel, A. (2021). Degradation of Aqueous CONFIDOR® Pesticide by Simultaneous TiO2 Photocatalysis and Fe-Zeolite Catalytic Ozonation. Water, 13(23), 3327. https://doi.org/10.3390/w13233327






