Association between Aqueous Atrazine and Pediatric Cancer in Nebraska
Abstract
:1. Introduction
2. Materials and Methods
3. Results
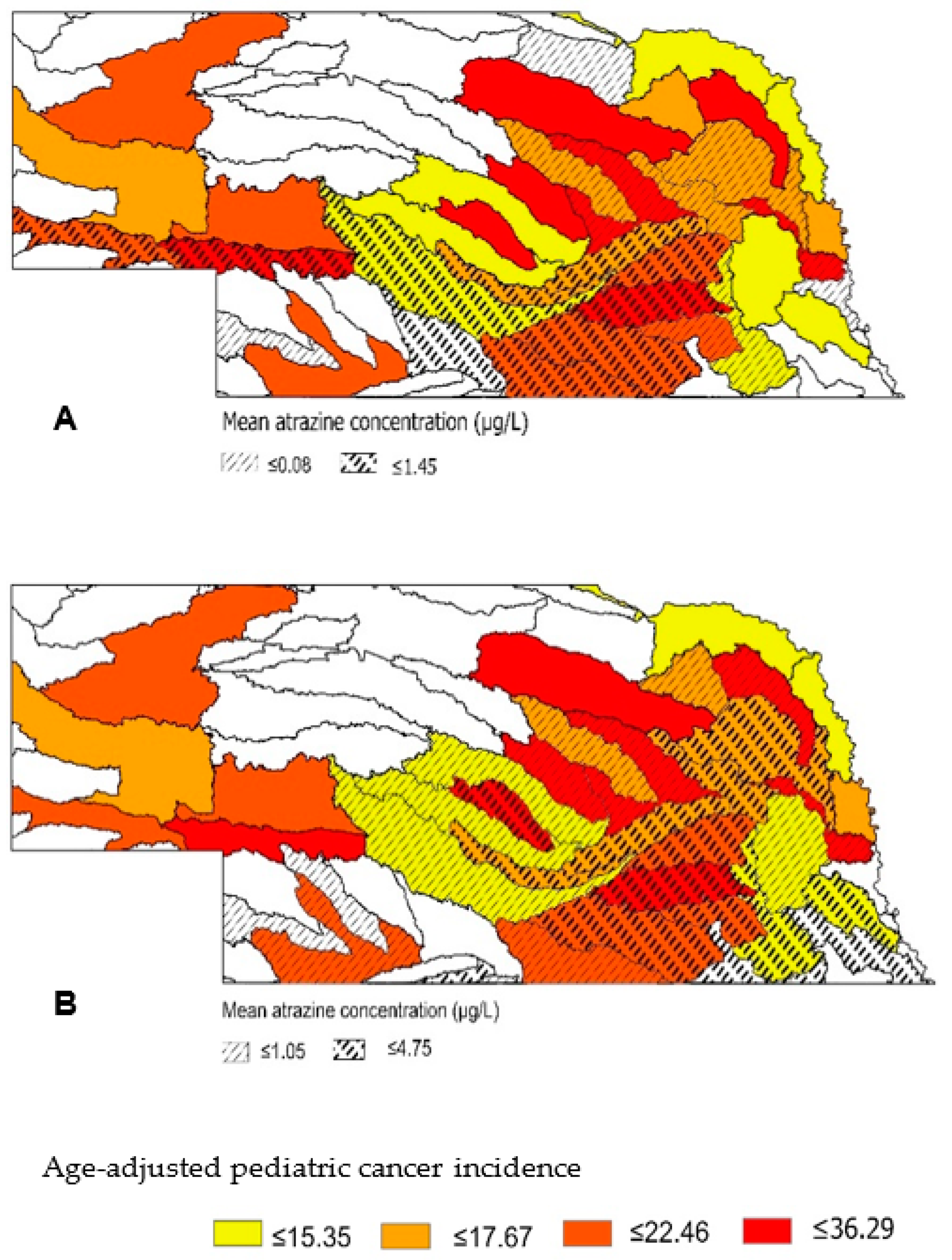
3.1. Surface and Groundwater Atrazine Concentrations
3.2. Pediatric Cancer
3.3. Association between Atrazine and Pediatric Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture (USDA). 2017 Census of Agriculture. United States Summary and State Data. Volume 1, Geographic Area Series; Part 51. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Full_Report/Volume_1,_Chapter_1_US/usv1.pdf (accessed on 9 February 2021).
- Fernandez-Cornejo, J.; Nehring, R.; Osteen, C.; Wechsler, S.J.; Martin, A.; Vialou, A. Pesticide Use in U.S. Agriculture: 21 Selected Crops, 1960–2008. 2014. Available online: https://doi.org/10.22004/ag.econ.178462 (accessed on 21 March 2021).
- Ma, Q.; Hook, J.E.; Wauchope, R.D.; Dowler, C.C.; Johnson, A.W.; Davis, J.G.; Gascho, G.J.; Truman, C.C.; Sumner, H.R.; Chandler, L.D. GLEAMS, opus, PRZM2β, and PRZM3 simulations compared with measured Atrazine Runoff. Soil Sci. Soc. Am. J. 2000, 64, 2070–2079. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhang, J.E.; Lin, D.; Liu, G.D. A STELLA model for the estimation of atrazine runoff, leaching, adsorption, and degradation from an agricultural land. J. Soils Sediments 2010, 10, 263–271. [Google Scholar] [CrossRef]
- Nebraska Department of Environmental Quality (NE-DEQ). Nebraska Groundwater Quality Monitoring Report (LB329-2001). 2018, 11. Available online: http://deq.ne.gov/Publica.nsf/PubsForm.xsp?documentId=B8F8D47840E9665886258360005B61CF&action=openDocument (accessed on 22 July 2019).
- Rhoades, M.G.; Meza, J.L.; Beseler, C.L.; Shea, P.J.; Kahle, A.; Vose, J.M.; Eskridge, K.M.; Spalding, R.F. Atrazine and nitrate in public drinking water supplies and non-hodgkin lymphoma in Nebraska, USA. Environ. Health Insights 2013, 7, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Yanase, T.; Morinaga, H.; Gondo, S.; Okabe, T.; Nomura, M.; Komatsu, T.; Morohashi, K.-I.; Hayes, T.B.; Takayanagi, R.; et al. Atrazine-induced aromatase expression is SF-1 dependent: Implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ. Health Perspect. 2007, 115, 720–727. [Google Scholar] [CrossRef]
- Sathiakumar, N.; MacLennan, P.A.; Mandel, J.; Delzell, E. A review of epidemiologic studies of triazine herbicides and cancer. Crit. Rev. Toxicol. 2011, 41, 1–34. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Lyon Int. Agency Res. Cancer 1999, 73, 59–114. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono73.pdf (accessed on 21 January 2018).
- United States Environmental Protection Agency (USEPA). Atrazine Human Health Risk Assessment for Registration Review. 2018. Available online: https://www.regulations.gov/document?D=EPA-HQ-OPP-2013-0266-1159 (accessed on 21 April 2019).
- Pathak, R.K.; Dikshit, A.K. Atrazine and human health. Int. J. Ecosyst. 2011, 1, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Rohr, J.R.; McCoy, K.A. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ. Health Perspect. 2010, 118, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farazi, P.; Watanabe-Galloway, S.; Westman, L.; Rettig, B.; Hunt, P.; Cammack, R.; Sparks, J.; Coulter, D. Temporal and geospatial trends of pediatric cancer incidence in Nebraska over a 24-year period. Cancer Epidemiol. 2018, 52, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kolok, A.S.; Beseler, C.L.; Chen, X.-H.; Shea, P.J. The watershed as a conceptual framework for the study of environmental and human health. Environ. Health Insights 2009, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Omernik, J.M.; Bailey, R.G. Distinguishing between watersheds and ecoregions. JAWRA J. Am. Water Resour. Assoc. 1997, 33, 935–949. [Google Scholar] [CrossRef]
- Ritter, W.F.; Shirmohammadi, A. Agricultural Nonpoint Source Pollution: Watershed Management and Hydrology; Lewis Publishers: Boca Raton, FL, USA, 2001. [Google Scholar]
- Corley, B.; Bartelt-Hunt, S.; Rogan, E.; Coulter, D.; Sparks, J.; Baccaglini, L.; Howell, M.; Liaquat, S.; Commack, R.; Kolok, A.S. Using watershed boundaries to map adverse health outcomes: Examples from Nebraska, USA. Environ. Health Insights 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine the Unequal Burden of Cancer. An Assessment of NIH Research and Programs for Ethnic Minorities and the Medically Underserved; The National Academies Press: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- United States Geological Survey (USGS). Water-Quality Historical Instantaneous Data for the Nation. 2020. Available online: https://waterdata.usgs.gov/nwis/sw (accessed on 2 March 2018).
- University of Nebraska-Lincoln (UNL). Quality-Assessed Agrichemical Contaminant Database for Nebraska Ground Water. A Cooperative Project of the Nebraska Departments of Agriculture, Environmental Quality, and Natural Resources and the University of Nebraska-Lincoln. 2019. Available online: https://clearinghouse.nebraska.gov/Clearinghouse.aspx (accessed on 2 February 2018).
- United States Environmental Protection Agency (US EPA). Storage and Retrieval. 2018. Available online: https://www3.epa.gov/storet/bck/dw_home.html (accessed on 10 February 2018).
- United States Environmental Protection Agency (US EPA). Triazine Herbicides as Atrazine in Water by Quantitative Immunoassay. 2007. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/4670.pdf (accessed on 24 September 2021).
- Yokley, R.A.; Cheung, M.W. Analytical method for the determination of atrazine and its dealkylated chlorotriazine metabolites in water using gas chromatography/mass selective detection. J. Agric. Food Chem. 2000, 48, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- United States Geological Survey (USGS). National Map Downloader. 2018. Available online: https://apps.nationalmap.gov/downloader/#/ (accessed on 15 January 2018).
- Nebraska Department of Health and Human Services (NE DHHS). Cancer registry. 2019. Available online: https://dhhs.ne.gov/Pages/Cancer-Registry.aspx (accessed on 10 January 2018).
- Environmental Systems Research Institute (ESRI). Geocode Addresses (Geocoding). 2020. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/geocoding/geocode-addresses.htm (accessed on 10 May 2019).
- United States Census-Bureau (USCB). Centers of Population by Block Group. 2010. Available online: https://www.census.gov/geographies/reference-files/time-series/geo/centers-population.htm (accessed on 5 March 2018).
- Centers for Disease Control and Prevention/ Agency for Toxic Substances and Disease Registry/Geospatial Research, Analysis and Services Program. CDC/ATSDR Social Vulnerability Index [2010] Database [Nebraska]. Available online: https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html (accessed on 15 March 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar] [CrossRef]
- Siegel, D.A.; Li, J.; Henley, S.J.; Wilson, R.J.; Lunsford, N.B.; Tai, E.; Van Dyne, E.A. Geographic variation in pediatric cancer incidence—United States, 2003–2014. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 707–713. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). CropScape-Cropland Data Layer. 2020. Available online: https://nassgeodata.gmu.edu/CropScape/ (accessed on 13 May 2020).
- Carozza, S.E.; Li, B.; Elgethun, K.; Whitworth, R.; Cooper, S. Agricultural pesticides and risk of childhood cancers associated with residence in agriculturally intense areas in the United States. Environ. Health Perspect. 2008, 116, 559–565. [Google Scholar] [CrossRef]
- Nielsen, S.S.; McKean-Cowdin, R.; Farin, F.M.; Holly, E.A.; Preston-Martin, S.; Mueller, B.A. Childhood brain tumors, residential insecticide exposure, and pesticide metabolism genes. (Children’s Health). Environ. Health Perspect. 2010, 118, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Booth, B.J.; Ward, M.H.; Turyk, M.E.; Stayner, L.T. Agricultural crop density and risk of childhood cancer in the midwestern United States: An ecologic study. Environ. Health 2015, 14, 82. [Google Scholar] [CrossRef] [Green Version]
- Malagoli, C.; Costanzini, S.; Heck, J.E.; Malavolti, M.; De Girolamo, G.; Oleari, P.; Palazzi, G.; Teggi, S.; Vinceti, M. Passive exposure to agricultural pesticides and risk of childhood leukemia in an Italian community. Int. J. Hyg. Environ. Health 2016, 219, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Goodman, M.; Mandel, J.S.; DeSesso, J.; Scialli, A.R. Atrazine and pregnancy outcomes: A systematic review of epidemiologic evidence. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2014, 101, 215–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stayner, L.T.; Almberg, K.; Jones, R.M.; Graber, J.; Pedersen, M.; Turyk, M. Atrazine and nitrate in drinking water and the risk of preterm delivery and low birth weight in four Midwestern states. Environ. Res. 2017, 152, 294–303. [Google Scholar] [CrossRef] [PubMed]

| HUC-8 Watershed | Corresponding Counties | Atrazine Measuremnts | Atrazine Concentration | Incidence a | ||
|---|---|---|---|---|---|---|
| Surface | Ground | Surface | Ground | |||
| Mud | Custer, Sherman | 89 | 33 | 1.857 | 0 | 36.29 |
| Lower Platte | Cass, Sarpy, Dodge | 1172 | 1106 | 0.044 | 0.789 | 35.14 |
| Logan | Wayne, Cedar, Thurston, Cuming, Burt | 148 | 220 | 0.001 | 0.360 | 34.64 |
| Lower North Loup | Valley, Greeley, Howard, Loup, Sherman, Custer | 430 | 83 | 0.001 | 0.354 | 30.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puvvula, J.; Bartelt-Hunt, S.L.; Ouattara, B.S.; Kolok, A.S.; Bell, J.E.; Rogan, E.G. Association between Aqueous Atrazine and Pediatric Cancer in Nebraska. Water 2021, 13, 2727. https://doi.org/10.3390/w13192727
Puvvula J, Bartelt-Hunt SL, Ouattara BS, Kolok AS, Bell JE, Rogan EG. Association between Aqueous Atrazine and Pediatric Cancer in Nebraska. Water. 2021; 13(19):2727. https://doi.org/10.3390/w13192727
Chicago/Turabian StylePuvvula, Jagadeesh, Shannon L. Bartelt-Hunt, Balkissa S. Ouattara, Alan S. Kolok, Jesse E. Bell, and Eleanor G. Rogan. 2021. "Association between Aqueous Atrazine and Pediatric Cancer in Nebraska" Water 13, no. 19: 2727. https://doi.org/10.3390/w13192727
APA StylePuvvula, J., Bartelt-Hunt, S. L., Ouattara, B. S., Kolok, A. S., Bell, J. E., & Rogan, E. G. (2021). Association between Aqueous Atrazine and Pediatric Cancer in Nebraska. Water, 13(19), 2727. https://doi.org/10.3390/w13192727







