Abstract
Improving the microbial quality of agricultural water through filtration can benefit small farms globally. The incorporation of zero-valent iron (ZVI) into sand filters (ZVI–sand) has been effective in reducing E. coli, Listeria spp., and viruses from agricultural water. This study evaluated ZVI–sand filtration in reducing E. coli levels based on influent water type and the percentage of ZVI in sand filters. A ZVI–sand filter (50% ZVI/50% sand) significantly (p < 0.001) reduced E. coli levels in deionized water by more than 1.5 log CFU/mL compared to pond water over six separate trials, indicating that water type impacts E. coli removal. Overall reductions in E. coli in deionized water and pond water were 98.8 ± 1.7% and 63 ± 24.0% (mean ± standard deviation), respectively. Filters constructed from 50% ZVI/50% sand showed slightly more reduction in E. coli in pond water than filters made from a composition of 35% ZVI/65% sand; however, the difference was not statistically significant (p = 0.48). Principal component analysis identified that the turbidity and conductivity of influent water affected E. coli reductions in filtered water in this study. ZVI–sand filtration reduces Escherichia coli levels more effectively in waters that contain low turbidity values.
1. Introduction
Rapid urbanization, climate change, population growth, and water scarcity have focused more attention on agricultural water quality, specifically irrigation water intended for fresh fruits and vegetables that are to be consumed raw [1]. Growers are interested in the use of non-traditional irrigation water, including untreated surface waters, to supplement groundwater in order to reduce water scarcity [2,3]. However, multiple studies have shown that irrigation water from rivers, creeks, and ponds in the U.S. can contain varying levels of foodborne bacterial pathogens such as Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes [4,5,6,7]. Cooler seasons (lower water temperatures) were associated with a higher prevalence of L. monocytogenes compared to warmer seasons [4,6], and specific non-traditional water sources (ponds) had a lower prevalence of Salmonella enterica compared to rivers or creeks [6,7].
To prevent foodborne illness from the consumption of microbiologically contaminated produce, multiple criteria and standards for the microbiological quality of irrigation water have been established or recommended by multiple international, governmental, and industry organizations. Some of these are shown in Table 1.

Table 1.
Selected current and proposed regulations and guidelines for the use of irrigation water in the growing and handling of edible produce.
To meet these criteria, a variety of methods and techniques have been developed and updated to remove and inactivate pathogenic microorganisms in irrigation water intended for produce that is to be consumed raw or with minimal processing [9,14,15,16,17]. The use of chemical disinfection (sodium hypochlorite, peroxyacetic-acid-based sanitizers) remains a cost-effective method to disinfect surface irrigation water less than 21 days before harvest for a large portion of the leafy green crops grown in the U.S. [9]. However, concerns have emerged about the long-term effects of the accumulation of chlorine (hypochlorite) disinfectant byproducts (DBPs) in soils. DBPs such as chlorates, which can have negative human health effects, have been shown to accumulate and bioaccumulate in edible crops [14,15]. Membrane filtration technology is used effectively to reduce microorganisms in various settings, but this method can result in the development of bacterial biofilms, which can negatively affect filtration performance [16,17].
Sand filtration is a cost-effective and commonly used method to improve the microbial quality of drinking water [18,19], and has been used for agricultural water in order to prevent sediment from clogging drip irrigation lines [20]. Modifying sand filters by adding zero-valent iron (ZVI or Fe0) has been shown to reduce viral pathogens and bacteria in filtered water. In iron–sand filtration, ZVI is oxidized to Fe2+ and/or Fe3+ by water and dissolved oxygen, generating various iron oxides and hydroxides [21]. A redox transformation of ZVI can also result in degradation or adsorption of chemical pollutants. Several previous studies have demonstrated that ZVI can reduce the level of a multitude of chemicals in water, including bromate [22], chloropicrin [23], haloacetic acids [24,25], N-nitrosodimethylamine [26], and several classes of antibiotics [27].
Adding ZVI to sand may be a practical strategy to improve the microbiological quality of irrigation water for growers and producers who use sand filtration on a regular basis during irrigation of their crops. Marik et al. (2019) reported reductions in 2 log CFU Escherichia coli in surface water in over twenty filtration events using ZVI–sand filters [28]. Kim et al. (2020) found that ZVI–sand filtration reduced Escherichia coli levels by a significantly greater extent than sand filtration in inoculated surface water [29]. Sand filters containing iron-oxide-coated sand reduced E. coli levels by 1 log CFU compared to sand filters without iron oxide [30]. Other ZVI/sand filtration research has shown a reduction of human viral surrogates and virus-like particles and in agricultural or treated wastewater [31,32].
The reduction in bacteria or viruses in agricultural water by ZVI–sand filtration may be affected by the contact time with iron particles, which is influenced by iron particle size and loading in the filter [33], as well as influent water quality parameters such as pH [34] and dissolved oxygen [35]. However, those studies did not evaluate if different levels of ZVI can affect E. coli reduction levels by filtration, or evaluate longitudinal aspects of the effectiveness of ZVI–sand filtration. Evaluating specific factors in surface irrigation water that affect bacterial inactivation can help identify conditions where utilization of ZVI–sand filtration by small-scale farmers can optimally improve irrigation water quality.
The objectives of this study were to: (1) determine the effect of different water types (pond vs. deionized water) on E. coli removal by ZVI–sand filtration, (2) evaluate water quality parameters that affect E. coli reduction by ZVI-sand filtration, and (3) assess the effect of ZVI loading (35% vs. 50%) on E. coli removal.
2. Materials and Methods
2.1. Construction of ZVI and Sand Filter
Each filter system was made using 10 cm (diameter) by 30 cm (length) pieces of Charlotte polyvinyl chloride (PVC) with a 2.36 L interior volume. Columns were packed following the same procedures that were used previously [29]. To construct 50% ZVI–sand filters, each filter was filled with 1.18 L of ZVI particles (0.43–0.60 mm size, Peerless Metals, Detroit MI) and 1.18 L of sand (0.45–55 mm, Northern Filter Media, Muscatine, IA) using graduated cylinders. Filters consisting of 35% ZVI–sand filters were constructed by adding 0.83 L of ZVI particles to 1.53 L of sand particles. ZVI and sand particles were mixed in a sterile plastic bag. PVC columns were fitted with landscape cloth, which was used at the top and bottom of the cylinder to avoid leakage of particles.
2.2. Influent Water Collection and Characteristics
Water was collected from the Wye pond located at the University of Maryland Wye Research and Education Center (Wye REC, Queenstown, MD, USA). Pond water was collected in sterile 20 L carboys and stored at 4 °C for up to 7 days before use. DI water was collected at the USDA-ARS Environmental Microbial and Food Safety Laboratory. Pond water and DI water quality characteristics (turbidity, pH, DO, ORP, and conductivity) were measured using a ProDSS multiparameter water quality sonde/meter (YSI, Yellow Springs, OH, USA) at each trial before filtration events. Each parameter was measured in triplicate and mean values were calculated (Table 2).

Table 2.
Summary of microbial reductions and associated water quality parameters of influent water for each filtration trial.
2.3. Inoculum Preparation
A non-pathogenic, rifampicin-resistant E. coli strain, TVS 353, previously isolated from agricultural water [36], was cultured from frozen stock onto MacConkey agar (Neogen, Lansing, MI, USA) supplemented with 80 µg/mL rifampicin (Sigma-Aldrich, St. Louis, MO, USA) (MACR). For each filtration trial, a single colony of E. coli TVS 353 was inoculated into 10 mL tryptic soy broth (Neogen, Lansing, MI, USA) supplemented with 80 µg/mL rifampicin (TSBR) and grown for 18–24 h. Overnight populations were determined by serial dilution in phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO, USA) (PBS), spiral-plated onto MACR using an easySpiral Dilute (Interscience, Woburn, MA, USA), and incubated at 37 °C for 18–24 h. Colony counts were determined and recorded. E. coli TVS 353, suspended in PBS, was diluted into 2 L of either DI or pond water to achieve a level of 4 log CFU/mL. Specific populations in the inoculated waters were determined using the method described above. In order to measure E. coli TVS 353 populations in sample fractions, bottles were homogenized by shaking for one minute, either spiral- or spread-plated onto MACR, and counts were determined as described above.
2.4. Filtration and Recovery of E. coli TVS 353
Filters were flushed with 2 L of DI water or pond water before testing. Inoculated water samples were pumped (Flex-Pro A4 ProSeries, standard peristaltic metering pump, Cole-Parmer, Vernon Hills, IL, USA) vertically up through filters to avoid preferential flow, at a rate of 0.5 L/min. Approximately 1.2 L of water was pumped through the filter. For each trial, pond water (2 L) or DI water (2 L) was inoculated with 4 log CFU/mL E. coli TVS 353 and pumped through filters, followed by 8 L of uninoculated pond water or DI water. Each 2 L of filtered effluent was collected to determine E. coli TVS 353 populations via serial dilution and spiral plating, as described previously, on to MACR media. Six filtration trials were performed with both inoculated DI and pond water through 50% ZVI–sand filters, while four filtration trials were performed for pond water through 35% ZVI–sand filters. After each filtration event, valves receiving influent water and controlled effluent flow were closed so that filters did not lose water and remained wet.
2.5. Statistical Analysis
For statistical analysis, E. coli TVS 353 levels from each 2 L of filtered effluent were transformed to log CFU/mL and plotted against the volume of effluent (X) in the equation below. Data from each trial for each water type/filter were used to fit a linear regression model, leading to Equation (1):
where (y-intercept) and (slope) are fitted parameters, and is the error term.
In Equation (2), dummy variable and were used, which were both set to 0 if the data originated from deionized water passed through 50% ZVI–sand filters; was 1 and was 0 if the data originated from pond water passed through 50% ZVI–sand filters; and was 0 and was 1 if the data originated from pond water passed through 35% ZVI–sand filters. That is:
By combining Equations (1) and (2), the following equation is obtained:
where , and are the fitting coefficients and and are dummy variables. Three different equations were acquired based on the different water type and ZVI ratio:
The null hypothesis was that coefficient is equal to zero and the alternative hypothesis was that is not zero for the linear regression model. Coefficients , , and are y-intercepts, and , , and are slopes for fitting the linear regression model. E. coli reductions (log- and percentage-based) in inoculated pond and DI water effluents were calculated by comparing the area under the curve (AUC) generated from E. coli recovered in effluents to the initial inoculum level for each trial.
For this study, principal component analysis (PCA) was used to characterize variations in water quality parameters and reduce dimensionality. R version 3.53 was used to compute PCA [37]. PCA, which can characterize explanatory variables, was applied to this study. For the PCA analysis, water quality parameters (turbidity, pH, DO, ORP, and conductivity) measured from deionized and pond water before filtration and inoculation with E. coli were used from Table 3. Water quality parameters, along with the time variable, were applied to PCA analysis as explanatory variables. Principal components 1 (PC1) and 2 (PC2) accounted for 34.9% and 23.8% of the variance, respectively.

Table 3.
The reduction in E. coli TVS 353 in (a) deionized water filtered through 50% ZVI/50% sand filters (ZVI 50% DI), (b) in pond water filtered through 50% ZVI–sand filters (ZVI 50% pond) or (c) in pond water filtered through 35% ZVI–sand filters (ZVI 35% pond) in each individual trial as shown by percentage and log reduction. E. coli reductions were calculated using the area under the curve (AUC) method.
3. Results
E. coli levels were reduced more significantly (p < 0.001) when inoculated deionized (DI) water was passed through a 50% ZVI filter compared to when pond water was passed through either 50% ZVI or 35% ZVI filters (Figure 1).
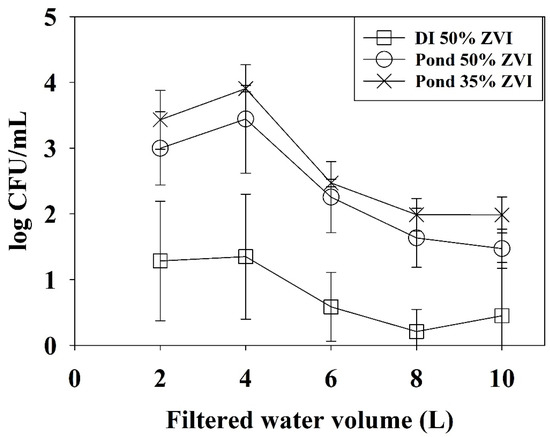
Figure 1.
Mean E. coli reductions in effluents after filtration of pond water through 50% ZVI–sand filters (x); pond water through though 35% ZVI–sand filters (squares); and deionized water through 50% ZVI–sand filters (open circles). E. coli levels were determined from every 2 L aliquot of effluent water after filtration.
There was no statistical difference (p = 0.48) in reduction in E. coli levels between pond water filtered through 50% ZVI–sand and pond water filtered through 35% ZVI–sand filters. Results obtained from the linear regression analysis are summarized below. The regression model had a R2 value of 0.74. Coefficient α1 (1.61 log CFU/mL) is the y-intercept for DI water filtered through 50% ZVI–sand filters (Equation (4)). Coefficient α3 (2.2 log CFU/mL) represents the difference between y-intercept value α1 (DI water passed through 50% ZVI–sand filters) and the y-intercept value for pond water filtered through 50% ZVI–sand filters (α1 + α3, Equation (5)).
In practical terms these findings show E. coli levels in the first 2 L of effluent of DI passed through a 50% ZVI–sand filter were 2.2 log CFU/mL lower than E. coli levels in the first 2 L of effluent of pond water passed through a 50% ZVI–sand filter. Similarly, coefficient α5 (2.58 log CFU/mL) is the difference between α1 and the y-intercept value for pond water filtered through 35% ZVI–sand filters (α1 + α5, Equation (6)). Practically, E. coli levels in the first 2 L of effluent of DI passed through a 50% ZVI–sand filter were 2.58 log CFU/mL lower than pond water passed through 35% ZVI–sand filters. Coefficient α1, α3, and α5 were all statistically significant (p < 0.001). Of the three calculated slope values, α2 (0.14), α4 (0.10), and α6 (0.10), only α2—the slope coefficient for DI water filtered through 50% ZVI–sand filters—was statistically significant (p < 0.001), indicating that the rate of E. coli decline in deionized water passed through 50% ZVI–sand filters was significantly different than E. coli in pond water passed through 35% or 50% ZVI–sand filters.
Table 3 shows the reduction in E. coli in each combination of water type and ZVI concentration by individual experiment trials. E. coli reductions were determined by the AUC. As Table 3 shows, deionized water and pond water have different water quality parameters, which influenced the reduction efficacy of E. coli by ZVI–sand filtration.
At least a 2-log reduction (99%) of E. coli levels in deionized water filtered through 50% ZVI–sand filters was achieved in the first five trials, resulting in a mean reduction of 98.8% ± 1.7% compared to levels of E. coli in influent water. In comparison, E. coli in pond water filtered through 50% ZVI–sand filters was reduced by 63.0% ± 24.0%. The level of E. coli in pond water filtered through 35% ZVI–sand filters was reduced by 20.3% ± 45.7%. The comparatively high levels of reductions in E. coli in deionized water filtered through 50% ZVI–sand filters are in contrast to the pond water being passed through 50% ZVI–sand filters. In pond water passed through a 50% ZVI–sand filter, only trial one showed a 1.85-log reduction in E. coli levels, with trials 2–6 (0.19–0.78-log reduction) showing decreasing effectiveness in reducing E. coli in pond water.
The most dramatic difference in water quality parameters between pond water and deionized water were observed with turbidity and conductivity values. Figure 2 presents the principal component analysis (PCA) of the percentage reduction in E. coli with regard to water quality factors and time, based on which day each trial occurred compared to the first trial.
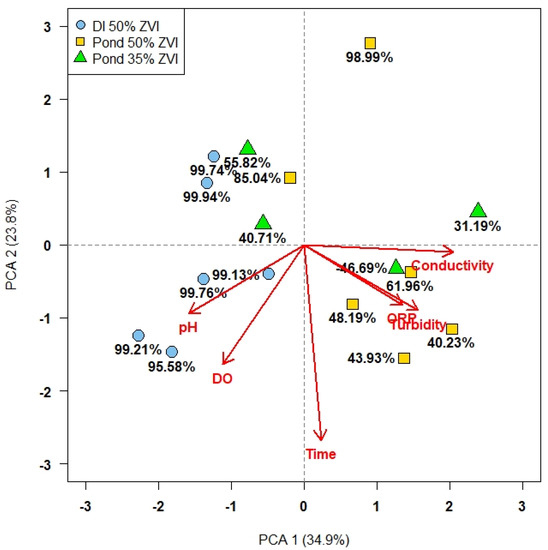
Figure 2.
Principal component analysis (PCA) of reduction in E. coli by all ZVI-sand filtration combinations with water quality factors. Prior to PCA modeling, all variables were centered to the mean of each variable and scaled by their respective standard deviations. Percentage reductions in E. coli are plotted on the bottom (PC1) and leftmost (PC2) axes. The smaller the size of the angle between specific water quality variables, the more closely correlated they are; the length and direction of each vector line indicates how much each water quality variable contributes to either PC1 or PC2.
PC1 and PC2 accounted for 34.9% and 23.8% of the variance, respectively. Trials with lower E. coli reductions were correlated with higher conductivity, ORP, and turbidity values. Higher pH and DO values were measured in deionized water compared to pond water (Table 3) and were associated with larger reductions in E. coli.
Values for pH and dissolved oxygen values were positively correlated; however, these two variables were negatively correlated with conductivity. Conductivity was positively correlated with ORP (oxidation–reduction potential) and turbidity. ORP and turbidity were extremely well-correlated, but ORP contributed less to PC1 than turbidity. The time variable contributed more than any other variable to PC2 but was not correlated with any water quality factor. Overall, trials with E. coli reductions greater than 90%, as determined by the area under the curve (AUC) method, were associated with higher pH and DO values.
4. Discussion
Zero-valent iron filtration more effectively reduced levels of E. coli in deionized water than in pond water. E. coli levels in pond water were not reduced by ZVI/sand filtration in later trials. These results indicate that different water types, with different levels of water quality parameters (specifically turbidity and conductivity), influence the effectiveness of ZVI–sand filters in reducing E. coli levels. E. coli levels in pond water filtered through 35% ZVI–sand filters in trials 1–3 showed a mean reduction of 42%, with a negative reduction in E. coli occurring in trial 4. The lack of reductions in effluent may be due to viable E. coli cells being physically trapped but not inactivated in 35% ZVI–sand filters. These E. coli cells may emerge in later trials with the same filters as additional influent water dislodges these viable E. coli into the effluent, causing the level of E. coli in the effluent to exceed levels in the influent for that specific trial. Higher levels of turbidity and conductivity in pond water compared to deionized water may account for the lower levels of inactivation of E. coli in pond water during our study. In addition, the presence of other microbes in pond water may have affected the reduction in E. coli TVS 353 in pond water.
Findings from several previous studies demonstrated that bacterial cells are inactivated from contact with ZVI particles [28,35,38]. Exposure to ZVI particles may incur oxidative stress lethal to bacterial cells during the transition of iron from a zero-valent state (Fe0) to ferrous (Fe2+) [35,39]. Other research evaluating the longevity (ability of ZVI–sand to reduce E. coli levels in water over time) of 35% ZVI–sand filters reported that E. coli levels in inoculated pond water (ca. 7 log CFU/mL) were consistently reduced by an average of 2.3 log CFU/mL over 13 trials (390 L) [28]. Different physicochemical factors in the influent water and the original inoculum levels may result in varying levels of longevity between the results of Marik et al. (2019) and this study [28]. Previous studies reported that influent alkalinity, reduction potential, and pH were likely factors influencing the performance of ZVI permeable reactive barriers (PRB), with higher pH values in influent water diminishing the performance of ZVI filters [40]. The decreased ability of 50% ZVI–sand filters to reduce E. coli in pond water after the fourth trial may be due to higher turbidity levels in pond water compared to deionized water (Table 3), due to the organic load accumulating within the filter over time effectively reducing the removal and inactivation efficiencies. The results of the linear regression model are in agreement with Kim et al. (2020), where E. coli reduction by ZVI–sand filtration was reported to occur immediately after filtration [29].
The results of the principal component analysis showed E. coli reductions, regardless of influent water type and filter composition, were strongly related with water quality parameters. PC1 and PC2 explained 34.9% and 23.8% of the variability, respectively. Higher conductivity values may have led to the development of a passivation layer on the iron particles, decreasing the interaction between the surface area of iron particles in ZVI filters and E. coli, reducing the inactivation of E. coli [41]. Previous studies reported that iron hydroxides can form a passivation layer on the surface of iron particles. In addition, we hypothesize that organic matter (measured as turbidity) blocked iron surfaces from contacting E. coli cells, resulting in decreased E. coli reductions in trials conducted with pond water. Previous studies have also shown that reduction in E. coli in pond water decreased with repeated ZVI–sand filtration through the same system over time [29]. Many studies have shown that the initial pH values played a critical role in the removal of metals or inactivated microbes [42,43,44]. Kim et al. (2011) found that inactivated E. coli through nano-ZVI (nZVI) was greater under de-aerated conditions (low DO values) than under air-saturated conditions (higher DO values) [35,44]. These results indicate that reductions in E. coli through ZVI–sand filtration can be improved if turbidity and conductivity levels are actively lowered and managed during the maintenance of ZVI filtration, and if water quality is characterized in advance of ZVI–sand filtration.
ZVI–sand filtration inactivated or removed E. coli most efficiently from water containing lower turbidity values (deionized water) compared to pond water. Higher levels of conductivity and turbidity, and oxidation–reduction potential potentially negatively influenced E. coli reduction by ZVI–sand filtration. Reducing turbidity in water, as well as backflushing filters to remove organic matter before ZVI–sand filtration, may improve the removal of E. coli in surface water.
5. Conclusions
Zero-valent iron (ZVI)–sand filtration reduced Escherichia coli levels in different types of influent water. E. coli populations were reduced by larger amounts in DI when filtered through 50% ZVI/sand columns compared to pond water. The quantitative reduction in E. coli was dependent upon chemical parameters of the influent water. Higher turbidity, conductivity, and ORP levels in pond water compared to DI water may have contributed to the lower E. coli reductions observed in pond water. While ZVI filtration was effective in reducing E. coli in early trials with pond water, its effectiveness over time decreased, indicating additional modifications are needed to increase the longevity of effective filtration. These findings indicate that ZVI filtration can be used to reduce E. coli in surface waters to help small farmers and growers gain compliance with government and commercial irrigation water standards.
Author Contributions
S.K.—responsible for experimental design, experimental procedures data management, statistical analysis, and manuscript preparation; K.E.—experimental design, experimental procedures, and manuscript preparation; S.S.—experimental design, manuscript preparation, and data management; P.C.C.—experimental design, technical advice, and manuscript preparation; A.R.S.—experimental design, technical advice, and manuscript preparation; E.T.H. and C.L.E.—experimental design, experimental procedures, data management, and manuscript preparation; K.E.K.—experimental design, experimental procedures, technical advice, and manuscript preparation; M.S.—experimental design, experimental procedures, data management, data analysis, and manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the USDA NIFA Grant 20166800725064 entitled CONSERVE (Coordinating Nontraditional Sustainable watER Use in Variable climatEs): A Center of Excellence at the Nexus of Sustainable Water Reuse, Food and Health (www.conservewaterforfood.org, accessed on 24 September 2021), and by the USDA ARS project plan 8042-32420-006-00-D: Characterization and Mitigation of Bacterial Pathogens in the Fresh Produce Production and Processing Continuum.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Annalise Lower for her laboratory support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United States Environmental Protection Agency (EPA). Guidelines for Water Reuse, Development; U.S. Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Suri, M.R.; Dery, J.L.; Perodin, J.; Brassill, N.; He, X.; Ammons, S.; Gredes, M.; Rock, C.R.; Goldstein, R.E.R. U.S. farmers’ opinion on the use of nontraditional water sources for agricultural activities. Environ. Res. 2019, 172, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.R.; Brassil, N.; Dery, J.L.; Carr, D.; McLain, J.E.; Bright, K.R.; Gerba, C.P. Review of water quality criteria for water reuse and risk-based implications under the FDA Food Safety Modernization Act, Produce Safety Rule. Environ. Res. 2020, 172, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.B.; Quiñones, B.; Oryang, D.; Mandrell, R.E.; Gorski, L. Prevalence of shiga toxin producing Escherichia coli, Salmonella enterica, and Listeria monocytogenes at public access watershed sites in a California Central Coast agricultural region. Front. Cell. Infect. Microbiol. 2014, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, M.T.; Van Kessel, J.A.; Micallef, S.A. Salmonella enterica recovery from river waters of the Maryland Eastern Shore reveals high serotype diversity and some multidrug resistance. Environ. Res. 2019, 168, 7–13. [Google Scholar] [CrossRef]
- Sharma, M.; Handy, E.T.; East, C.L.; Kim, S.; Jiang, C.; Callahan, M.T.; Allard, S.M.; Micallef, S.; Craighead, S.; Anderson-Coughlin, B.; et al. Prevalence of Salmonella and Listeria monocytogenes in non-traditional irrigation waters in the Mid-Atlantic United States is affected by water type, season, and recovery method. PLoS ONE 2020, 15, e0229365. [Google Scholar] [CrossRef]
- Truitt, L.N.; Vazquez, K.M.; Pfuntner, R.C.; Rideout, S.L.; Havelaar, A.H.; Strawn, L.K. Microbial quality of agricultural water used in produce preharvest production on the eastern shore of Virginia. J. Food Prot. 2018, 81, 1661–1672. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FSMA Final Rule on Produce Safety. 2016. Available online: https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-produce-safety (accessed on 1 August 2020).
- California LGMA (LGMA). Commodity Specific Food Safety Guidelines for the Production and Harvest of Lettuce and Leafy Greens. 2019. Available online: https://lgma-assets.sfo2.digitaloceanspaces.com/downloads/CA_LGMA_METRICS_FINAL_VERSION_Accessible_Jan2020.pdf (accessed on 1 August 2020).
- World Health Organization (WHO). Guidelines for the Safe Use of Wastewater Excreta and Greywater. 2006. Available online: https://apps.who.int/iris/bitstream/handle/10665/39401/WHO_TRS_778.pdf?sequence=1&isAllowed=y (accessed on 1 August 2020).
- World Health Organization (WHO). Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture. 1989. Available online: https://apps.who.int/iris/bitstream/handle/10665/78265/9241546824_eng.pdf?sequence=1 (accessed on 24 July 2020).
- Tomato Good Agricultural Practices and Tomato Best Management Practices (T-GAP). 2006. Available online: https://www.floridatomatoes.org/wp-content/uploads/2013/01/TOMATO_QA_on_T-GAP_and_T-BMP11-8-06.pdf (accessed on 25 July 2020).
- EFSA. Commission Notice on Guidance Document on Addressing Microbiological Risks in Fresh Fruits and Vegetables at Primary Production Through Good Hygiene. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52017XC0523%2803%29 (accessed on 1 August 2020).
- Garrido, Y.; Marín, A.; Tudela, J.A.; Truchado, P.; Allende, A.; Gil, M.I. Chlorate accumulation in commercial lettuce cultivated in open field and irrigated with reclaimed water. Food Control 2020, 114, 107283. [Google Scholar] [CrossRef]
- Lonigro, A.; Montemurro, N.; Laera, G. Effects of residual disinfectant on soil and lettuce crop irrigated with chlorinated water. Sci. Total Environ. 2017, 584, 595–602. [Google Scholar] [CrossRef]
- Chang, I.S.; Clech, P.L.; Jefferson, B.; Judd, S. Membrane fouling in membrane bioreactors for wastewater treatment. J. Environ. Eng. 2002, 128, 1018–1029. [Google Scholar] [CrossRef]
- Herzberg, M.; Elimelech, M. Biofouling of reverse osmosis membranes: Role of biofilm-enhanced osmotic pressure. J. Membr. Sci. 2007, 295, 11–20. [Google Scholar] [CrossRef]
- Hu, R.; Yang, H.; Tao, R.; Cui, X.; Xiao, M.; Amoah, B.K.; Cao, V.; Lufingo, M.; Soppa-Sangue, N.P.; Ndé-Tchoupé, A.I.; et al. Metallic Iron for Environmental Remediation: Starting an Overdue Progress in Knowledge. Water 2020, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.M.M.; Begum, Z.A.; Sawai, H.; Maki, T.; Hasegawa, H. Decontamination of spent iron-oxide coated sand from filters used in arsenic removal. Chemosphere 2013, 92, 196–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duran-Ros, M.; Puig-Bargués, J.; Arbat, G.; Barragán, J.; De Cartagena, F.R. Effect of filter, emitter and location on clogging when using effluents. Agri. Water Mgmt. 2009, 96, 67–79. [Google Scholar] [CrossRef]
- Powell, R.M.; Blowes, D.W.; Gillham, R.W.; Schultz, D.; Sivavec, T.; Puls, R.W.; Vogan, J.L.; Powel, P.D.; Landis, R. Permeable Reactive Barrier Technologies for Contaminant Remediation. US EPA. 1998. Available online: https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=NRMRL&dirEntryId=90404 (accessed on 1 September 2021).
- Xie, L.I.; Shang, C. Role of humic acid and quinone model compounds in bromate reduction by zerovalent iron. Environ. Sci. Technol. 2005, 39, 1092–1100. [Google Scholar] [CrossRef]
- Pearson, C.R.; Hozalski, R.M.; Arnold, W.A. Degradation of chloropicrin in the presence of zero-valent iron. Environ. Toxicol. Chem. 2005, 24, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Hozalski, R.M.; Zhang, L.; Arnold, W.A. Reduction of haloacetic acids by Fe0: Implications for treatment and fate. Environ. Sci. Technol. 2001, 35, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Arnold, W.A.; Hozalski, R.M. Kinetics of haloacetic acid reactions with Fe (0). Environ. Sci. Technol. 2004, 38, 6881–6889. [Google Scholar] [CrossRef]
- Odziemkowski, M.S.; Gui, L.; Gillham, R.W. Reduction of N-nitrosodimethylamine with granular iron and nickel-enhanced iron. 2. Mechanistic studies. Environ. Sci. Technol. 2000, 34, 3495–3500. [Google Scholar] [CrossRef]
- Kulkarni, P.; Raspanti, G.A.; Bui, A.Q.; Bradshaw, R.N.; Kniel, K.E.; Chiu, P.C.; Sharma, M.; Sapkota, A.; Sapkota, A.R. Zerovalent iron-sand filtration can reduce the concentration of multiple antimicrobials in conventionally treated reclaimed water. Environ. Res. 2019, 172, 301–309. [Google Scholar] [CrossRef]
- Marik, C.; Anderson, B.; Gartley, S.; Craighead, S.; Bradshaw, R.; Kulkarni, P.; Sharma, M.; Kniel, K. ZVI filtration reduced Listeria innocua in pond water compared to sand filtration. Environ. Res. 2019, 173, 33–39. [Google Scholar] [CrossRef]
- Kim, S.; Bradshaw, R.; Kulkarni, P.; Allard, S.; Chiu, P.C.; Sapkota, A.R.; Newell, C.; Hand, E.; East, C.; Kniel, K.; et al. Zero-valent iron-sand filtration reduces Escherichia coli in surface water and leafy green growing environments. Front. Sustain. Food Syst. 2020, 4, 112. [Google Scholar] [CrossRef]
- George, D.; Ahammed, M. Effect of zero-valent iron amendment on the performance of biosand filters. Water Supply 2019, 19, 1612–1618. [Google Scholar] [CrossRef]
- Shearer, A.E.; Kniel, K.E. Enhanced removal of norovirus surrogates, Murine norovirus and Tulane virus, from aqueous systems by zero-valent iron. J. Food Prot. 2018, 81, 1432–1438. [Google Scholar] [CrossRef]
- Chopyk, J.; Kulkarni, P.; Nasko, D.J.; Bradshaw, R.; Kniel, K.E.; Chiu, P.; Sharma, M.; Sapkota, A.R. Zero-valent iron sand filtration reduces concentrations of virus-like particles and modifies virome community composition in reclaimed water used for agricultural irrigation. BMC Res. Notes 2019, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Yao, M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009, 43, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Callejas, A.; López-Velasco, G.; Camacho, A.B.; Artés, F.; Artés-Hernández, F.; Suslow, T.V. Survival and distribution of Escherichia coli on diverse fresh-cut baby leafy greens under preharvest through postharvest conditions. Int. J. Food Microbiol. 2011, 151, 216–222. [Google Scholar] [CrossRef]
- R Core Team. R: The R Project for Statistical Computing. 2020. Available online: http://www.R-project.org/ (accessed on 28 January 2021).
- Ingram, D.T.; Callahan, M.T.; Ferguson, S.; Hoover, D.G.; Shelton, D.R.; Millner, P.D.; Camp, M.J.; Patel, J.R.; Kniel, K.E.; Sharma, M. Use of zero-valent iron biosand filters to reduce Escherichia coli O157: H12 in irrigation water applied to spinach plants in a field setting. J. Appl. Microbiol. 2012, 112, 551–560. [Google Scholar] [CrossRef]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.A.; Chanéac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.Y. Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Phenrat, T.; Lowry, G.V. Effect of TCE concentration and dissolved groundwater solutes on NZVI-promoted TCE dechlorination and H2 evolution. Environ. Sci. Technol. 2007, 41, 7881. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.L.; Yan, X.; Zhang, B. Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: Pumice-supported nanoscale zero-valent iron. Chem. Eng. J. 2014, 245, 34–40. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.; Zhao, R.; Zhou, R. Effects of pH and particle size on kinetics of nitrobenzene reduction by zero-valent iron. J. Environ. Sci. 2010, 22, 1741–1747. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Love, D.C.; Sedlak, D.L.; Yoon, J.; Nelson, K.L. Inactivation of MS2 coliphage by ferrous ion and zero-valent iron nanoparticles. Environ. Sci. Technol. 2011, 45, 6978–6984. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).