Synchronous Cycle of Available Phosphorus, Iron, and Sulfur in the Sediment of Lancang River Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
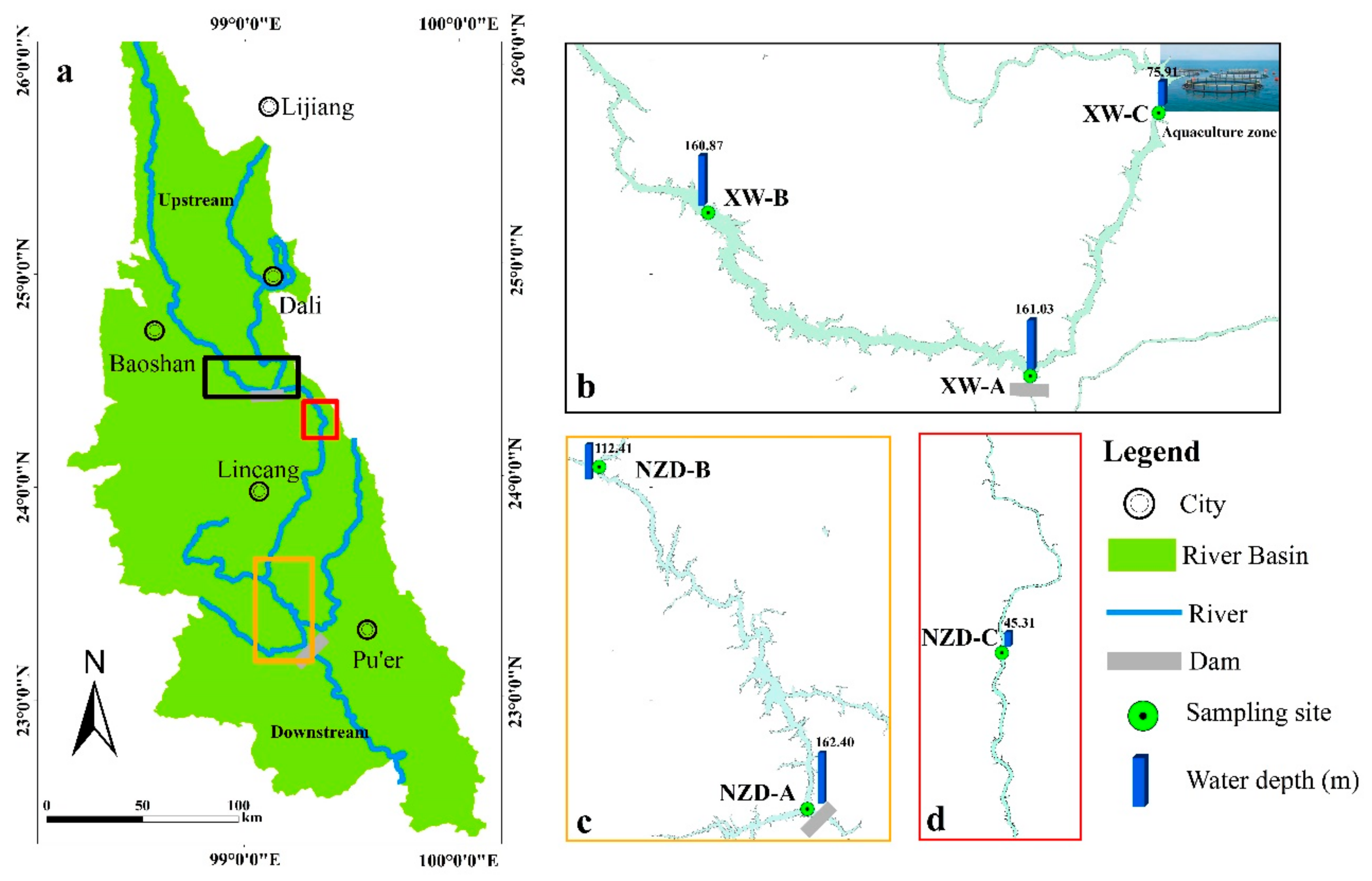
2.1. Survey Area
2.2. Sample Collection
2.3. P Fractions Analysis
2.4. Calculations
2.5. Statistical Tests
3. Results and Discussion
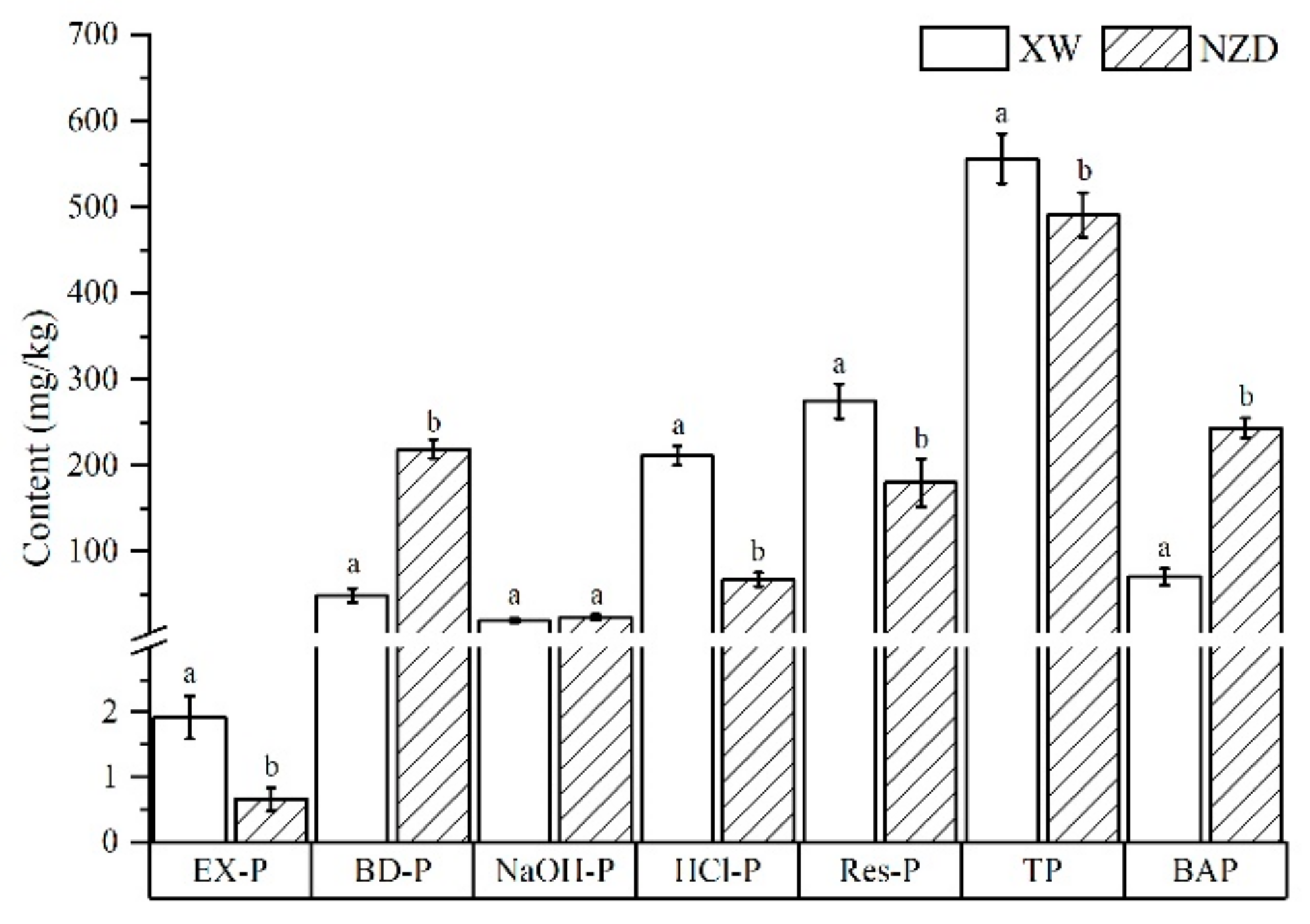
3.1. P Fractions in Sediment
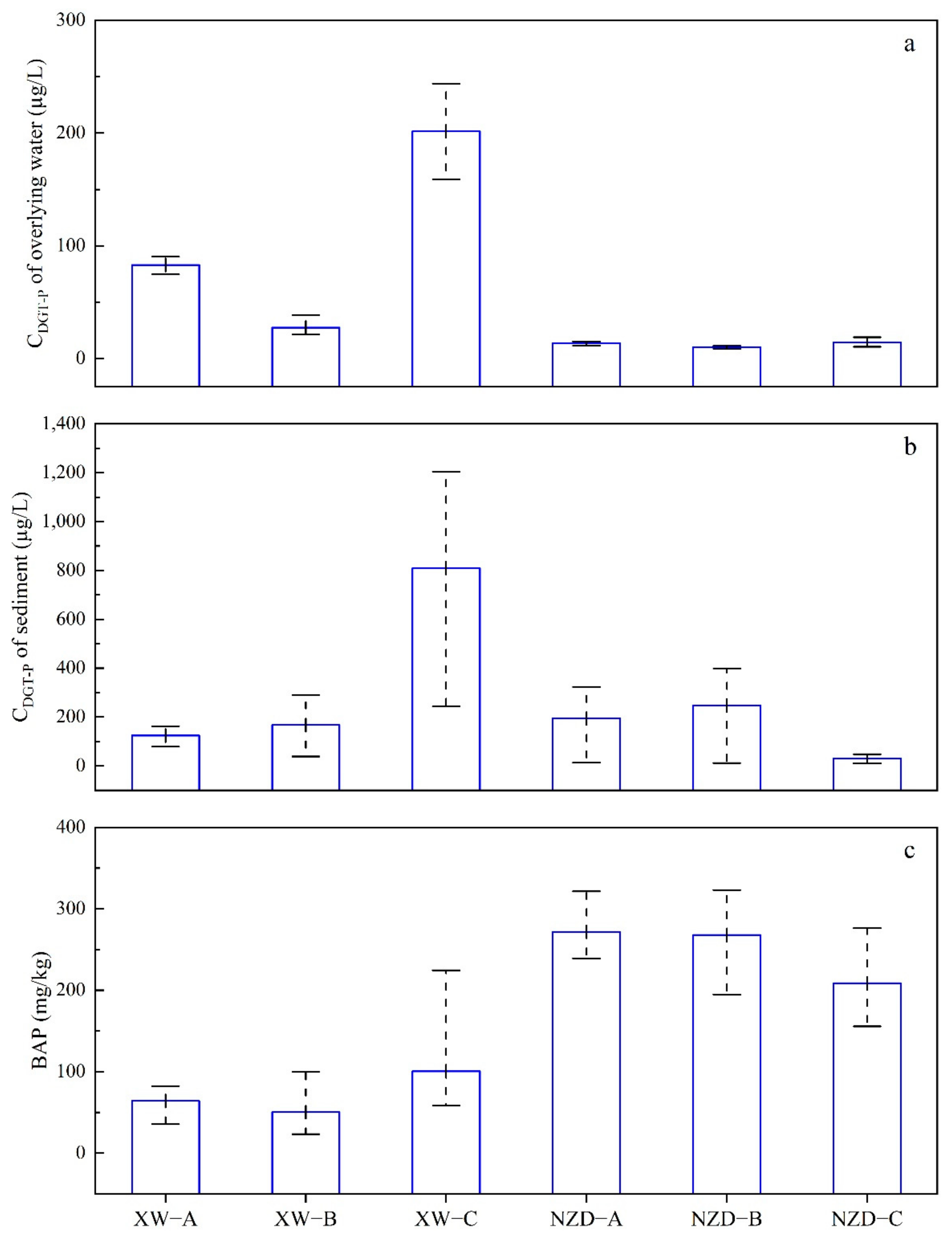
3.2. Available P in Sediment
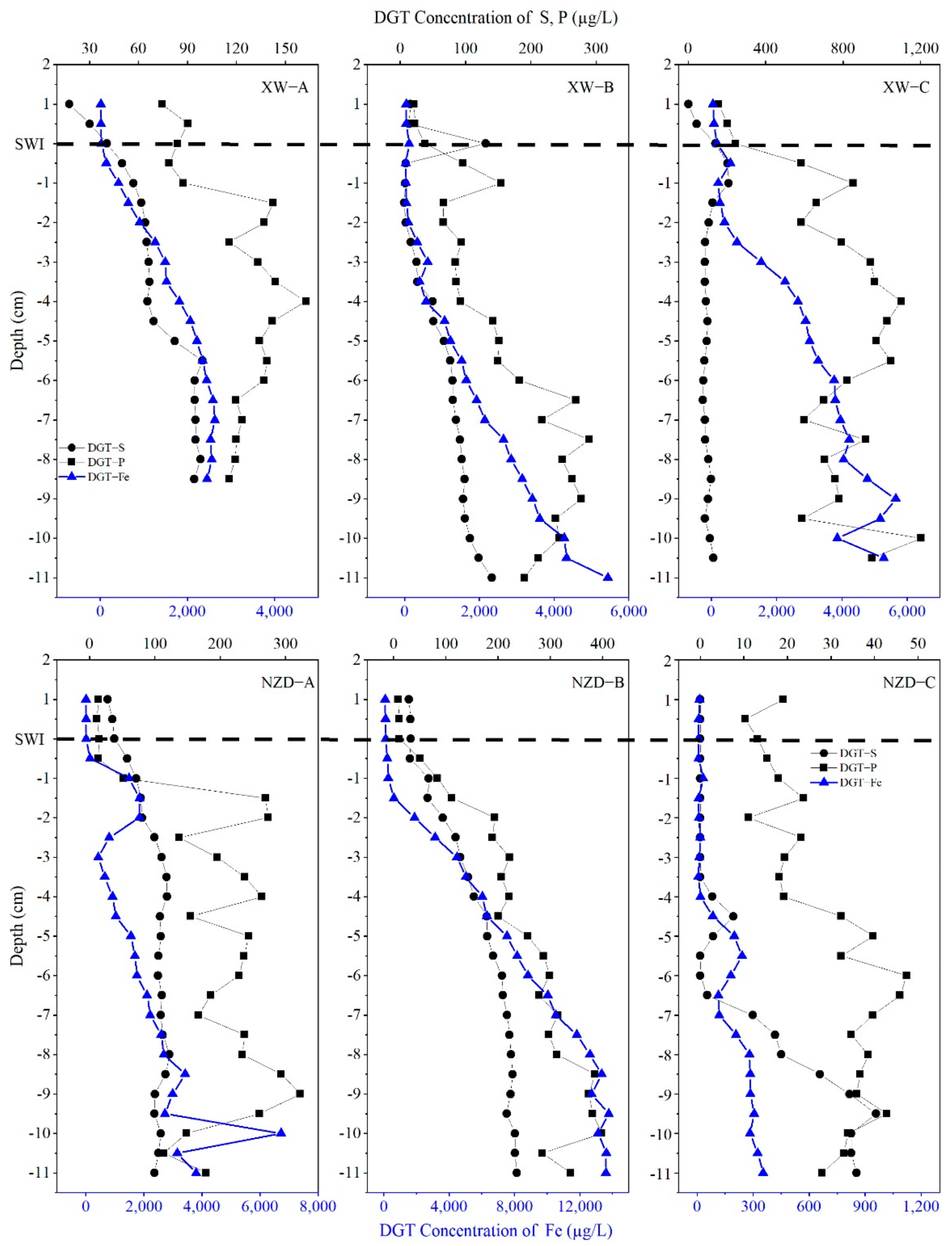
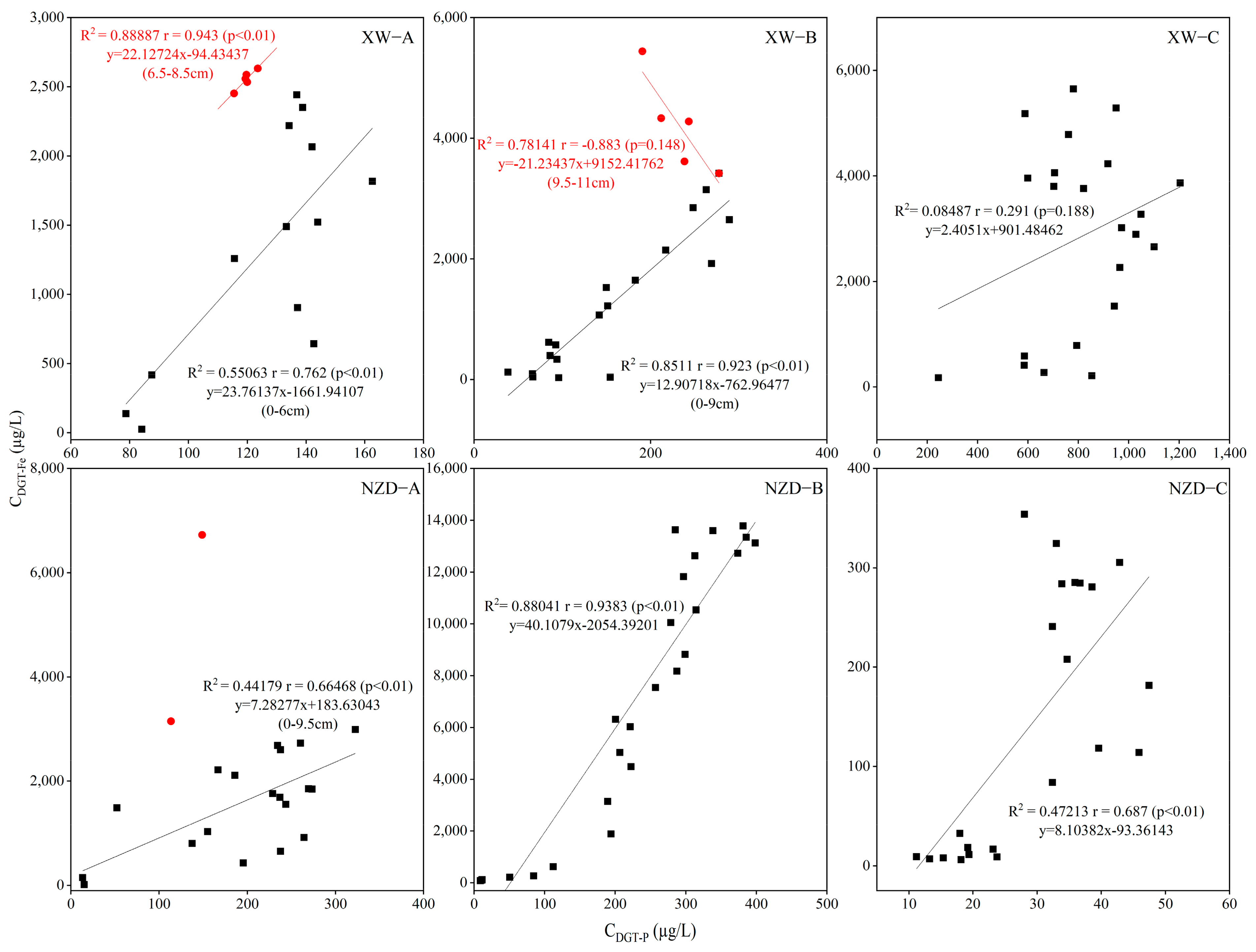
3.3. Vertical Distribution of Available P, Fe, and S in Sediment
3.4. Release Mechanism of P in Sediment
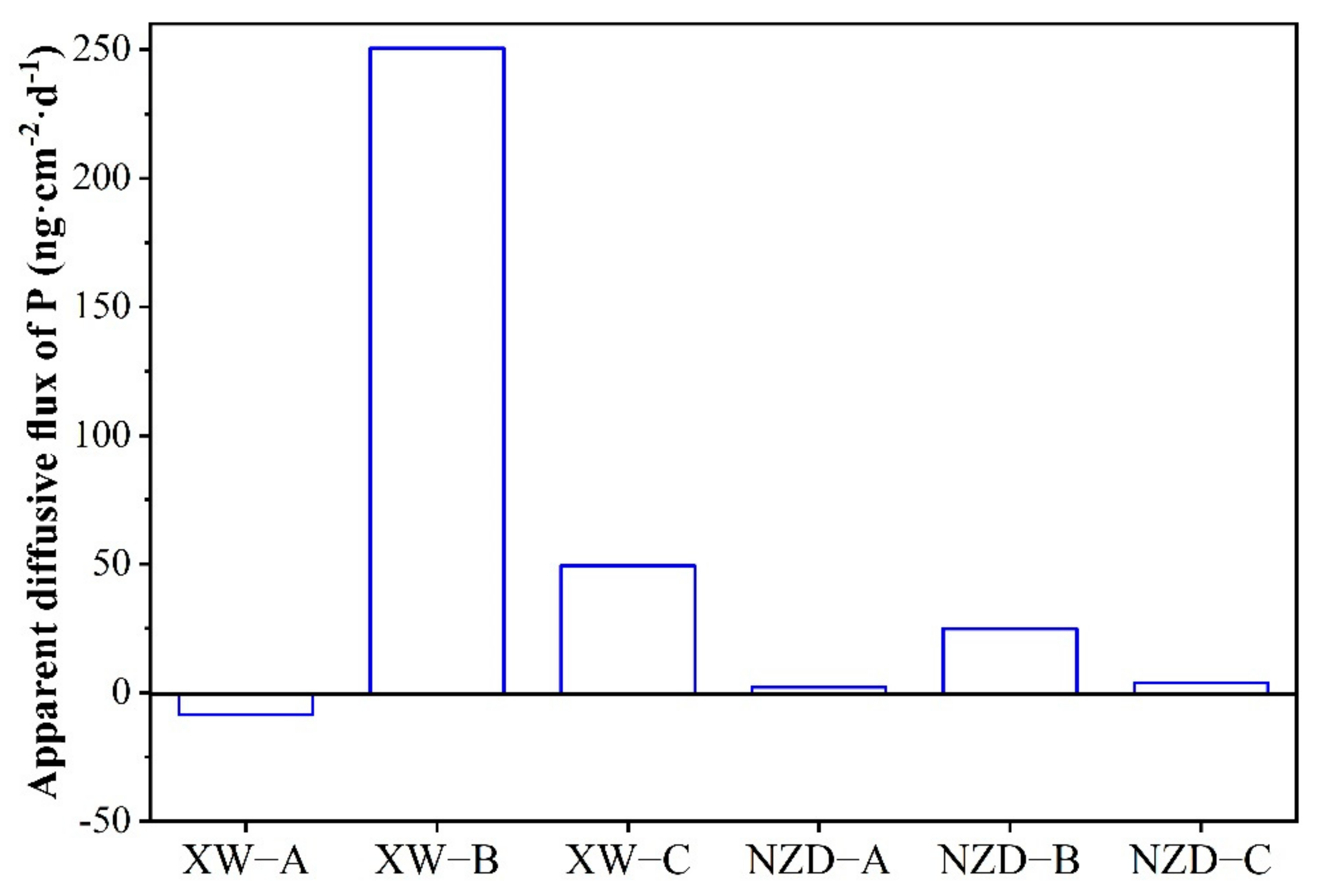
3.5. Apparent Diffusive Flux of P
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Christiane, L.; Likens, G.E. Ecology. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014. [Google Scholar] [CrossRef]
- Elser, J.; Bracken, M.; Cleland, E.; Gruner, D.; Harpole, W.; Hillebrand, H.; Ngai, J.; Seabloom, E.; Shurin, J.; Smith, J. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W. Eutrophication and recovery in experimental lakes: Implications for lake management. Science 1974, 184, 897–899. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 1999, 408, 145–152. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, S.; Wang, Y. Characteristics of bioavailable organic phosphorus in sediment and its contribution to lake eutrophication in China. Environ. Pollut. 2016, 219, S0269749116304754. [Google Scholar] [CrossRef]
- Søndergaard, M. Nutrient Dynamics in Lakes-With Emphasis on Phosphorus, Sediment and Lake Restoration; National Environmental Research Institute: Maharashtra, Denmark; University of Aarhus: Aarhus, Denmark, 2007. [Google Scholar]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, G.; Li, W.; Zhang, Y.; Zhao, L.; Gu, Z. Estimation of the algal-available phosphorus pool in sediments of a large, shallow eutrophic lake (Taihu, China) using profiled SMT fractional analysis. Environ. Pollut. 2013, 173, 216–223. [Google Scholar] [CrossRef]
- Ding, S.; Chen, M.; Gong, M.; Fan, X.; Qin, B.; Xu, H.; Gao, S.; Jin, Z.; Tsang, D.C.W.; Zhang, C. Internal phosphorus loading from sediments causes seasonal nitrogen limitation for harmful algal blooms. Sci. Total Environ. 2018, 625, 872–884. [Google Scholar] [CrossRef]
- House, W.A. Geochemical cycling of phosphorus in rivers. Appl. Geochem. 2003, 18, 739–748. [Google Scholar] [CrossRef]
- Marsden, M.W. Lake restoration by reducing external phosphorus loading: The influence of sediment phosphorus release. Freshw. Biol. 2010, 21, 139–162. [Google Scholar] [CrossRef]
- Pettersson, K. Mechanisms for internal loading of phosphorus in lakes. Hydrobiologia 1998, 373, 21–25. [Google Scholar] [CrossRef]
- Katsaounos, C.Z.; Giokas, D.L.; Leonardos, I.D.; Karayannis, M.I. Speciation of phosphorus fractionation in river sediments by explanatory data analysis. Water Res. 2007, 41, 406–418. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, F.; He, Z.; Guo, J.; Qu, X.; Xie, F.; Giesy, J.P.; Liao, H.; Gue, F. Characterization of organic phosphorus in lake sediments by sequential fractionation and enzymatic hydrolysis. Environ. Sci. Technol. 2013, 47, 7679–7687. [Google Scholar] [CrossRef]
- Condron, L.M.; Newman, S. Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J. Soils Sediments 2011, 11, 830–840. [Google Scholar] [CrossRef]
- Davlson, W.; Zhang, H. In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef] [Green Version]
- Davison, W.; Zhang, H. Progress in understanding the use of diffusive gradients in thin films (DGT)-back to basics. Environ. Chem. 2012, 9, 1–13. [Google Scholar] [CrossRef]
- Ding, S.; Han, C.; Wang, Y.; Yao, L.; Wang, Y.; Xu, D.; Sun, Q.; Williams, P.N.; Zhang, C. In situ, high-resolution imaging of labile phosphorus in sediments of a large eutrophic lake. Water Res. 2015, 74, 100–109. [Google Scholar] [CrossRef]
- Hesslein, R.H. An in situ sampler for close interval pore water studies. Limnol. Oceanogr. 1976, 21, 912–914. [Google Scholar] [CrossRef]
- Fones, G.; Davison, W.; Grime, G. Development of constrained DET for measurements of dissolved iron in surface sediments at sub-mm resolution. Sci. Total Environ. 1998, 221, 127–137. [Google Scholar] [CrossRef]
- Mei, X.; Dai, Z.; Gelder, P.H.A.J.M.; Gao, J. Linking Three Gorges Dam and downstream hydrological regimes along the Yangtze River, China. Earth Space Sci. 2015, 2, 94–106. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Zhang, L.; Zhou, Y. A bibliometric review of nitrogen research in eutrophic lakes and reservoirs. J. Environ. Sci. 2018, 66, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, J.; Wang, J.; Guo, J.; Jin, Z.; Yu, P.; Ma, Z. In situ, high-resolution evidence of phosphorus release from sediments controlled by the reductive dissolution of iron-bound phosphorus in a deep reservoir, southwestern China. Sci. Total Environ. 2019, 666, 39–45. [Google Scholar] [CrossRef]
- Hart, D.D.; Johnson, T.E.; Bushawnewton, K.L.; Horwitz, R.J.; Bednarek, A.T.; Charles, D.F.; Kreeger, D.A.; Velinsky, D.J. Dam Removal: Challenges and Opportunities for Ecological Research and River Restoration We develop a risk assessment framework for understanding how potential responses to dam removal vary with dam and watershed characteristics, which can lead to more effe. Bioscience 2002, 52, 669–682. [Google Scholar] [CrossRef]
- Stanley, E.H.; Doyle, M.W. A Geomorphic Perspective on Nutrient Retention Following Dam Removal. Bioscience 2002, 52, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Friedl, G.; Wüest, A. Disrupting biogeochemical cycles-Consequences of damming. Aquat. Sci. 2002, 64, 55–65. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Zhao, H.; Deng, L.; Wang, C.; Zhao, Q.; Dong, S. The phosphorus speciations in the sediments up- and down-stream of cascade dams along the middle Lancang River. Chemosphere 2015, 120, 653–659. [Google Scholar] [CrossRef]
- Kummu, M.; Vans, O. Sediment-related impacts due to upstream reservoir trapping, the Lower Mekong River. Geomorphology 2007, 85, 275–293. [Google Scholar] [CrossRef]
- Wei, G.L.; Yang, Z.F.; Cui, B.S.; Bing, L.; He, C.; Bai, J.H.; Dong, S.K. Impact of Dam Construction on Water Quality and Water Self-Purification Capacity of the Lancang River, China. Water Resour. Manag. 2009, 23, 1763–1780. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Zhao, Q.; Deng, L.; Dong, S. Spatial variation and contamination assessment of heavy metals in sediments in the Manwan Reservoir, Lancang River. Ecotoxicol. Environ. Saf. 2012, 82, 32–39. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, S.; Deng, L.; Dong, S.; Yang, J.; Wang, C. The effects of dam construction and precipitation variability on hydrologic alteration in the Lancang River Basin of southwest China. Stoch. Environ. Res. Risk Assess. 2012, 26, 993–1011. [Google Scholar] [CrossRef]
- Yujun, Y.I.; Tang, C.; Yang, Z.; Chen, X.I. Influence of Manwan Reservoir on fish habitat in the middle reach of the Lancang River. Ecol. Eng. 2014, 69, 106–117. [Google Scholar]
- Fan, H.; He, D.; Wang, H. Environmental consequences of damming the mainstream Lancang-Mekong River: A review. Earth-Sci. Rev. 2015, 146, 77–91. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, Y.; Wu, J.; Cheng, Y.; Lu, J.; Chen, C.; Zhao, F.; Li, Y.; Hu, M.; Bao, Y. The influence of cascade reservoir construction on sediment biogenic substance cycle in Lancang River from the perspective of phosphorus fractions. Ecol. Eng. 2020, 158, 106051. [Google Scholar] [CrossRef]
- Sun, Q.; Sheng, Y.; Yang, J.; Di Bonito, M.; Mortimer, R.J.G. Dynamic characteristics of sulfur, iron and phosphorus in coastal polluted sediments, north China. Environ. Pollut. 2016, 219, 588–595. [Google Scholar] [CrossRef]
- Ruban, V.; López-Sánchez, J.F.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—A synthesis of recent works. Fresenius J. Anal. Chem. 2001, 370, 224–228. [Google Scholar] [CrossRef]
- Hupfer, M.; Gächter, R.G.; Giovanoli, R. Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat. Sci. 1995, 57, 305–324. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard methods for the examination of water and wastewater. Am. J. Public Health Nations Health 1966, 56, 387–388. [Google Scholar]
- Zhou, A.M.; Wang, D.S.; Tang, H.X. Phosphorus fractionation and bio-availability in Taihu Lake (China) sediments. J. Environ. Sci. 2005, 17, 384–388. [Google Scholar]
- Tao, Y.; Dan, D.; Xuejiao, H.; Changda, H.; Guo, F.; Fengchang, W. Characterization of phosphorus accumulation and release using diffusive gradients in thin films (DGT)-linking the watershed to Taihu Lake, China. Sci. Total Environ. 2019, 673, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Estimation of releasing fluxes of sediment nitrogen and phosphorus in Fubao Bay in Dianchi Lake. Huan Jing Ke Xue = Huanjing Kexue 2008, 29, 114–120. [Google Scholar]
- Ding, S.; Wang, Y.; Wang, D.; Li, Y.Y.; Gong, M.; Zhang, C. In situ, high-resolution evidence for iron-coupled mobilization of phosphorus in sediments. Sci. Rep. 2016, 6, 24341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, G.; Lesven, L.; Gillan, D.; Sabbe, K.; Billon, G.; Galan, S.D.; Elskens, M.; Baeyens, W.; Leermakers, M. Geochemical behavior of trace elements in sub-tidal marine sediments of the Belgian coast. Mar. Chem. 2009, 117, 88–96. [Google Scholar]
- Li, Y.H. Diffusion of ions in seawater and deep sea sediments. Geochim. Cosmochim. Acta 1974, 38, 708. [Google Scholar]
- Ullman, W.J.; Aller, R.C. Diffusion coefficients in nearshore marine sediments. Limnol. Oceanogr. 1982, 27, 552–556. [Google Scholar] [CrossRef]
- Cavalcante, H.; Araújo, F.; Noyma, N.P.; Becker, V. Phosphorus fractionation in sediments of tropical semiarid reservoirs. Sci. Total Environ. 2018, 619–620, 1022–1029. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, S.; Xu, D.; Tang, Y.; Ming, H.W. Bioavailability assessment of phosphorus and metals in soils and sediments: A review of diffusive gradients in thin films (DGT). Environ. Monit. Assess. 2014, 186, 7367–7378. [Google Scholar] [CrossRef]
- Jin, X.; Wang, S.; Pang, Y.; Wu, F.C. Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake, China. Environ. Pollut. 2006, 139, 288–295. [Google Scholar] [CrossRef]
- Pedro, T.; Kimberley, S.; Fernando, P. Dynamics of phosphorus in sediments of a naturally acidic lake. Int. J. Sediment. Res. 2013, 28, 90–102. [Google Scholar] [CrossRef]
- Ting, D.S.; Appan, A. General characteristics and fractions of phosphorus in aquatic sediments of two tropical reservoirs. Water Sci. Technol. 1996, 34, 53–59. [Google Scholar]
- Rydin, E.; Huser, B.; Welch, E.B. Amount of phosphorus inactivated by alum treatments in Washington lakes. Limnol. Oceanogr. 2000, 45, 226–230. [Google Scholar] [CrossRef]
- Christophoridis, C.; Fytianos, K. Study of the conditions affecting the release of phosphorus from the top sediments of two lakes of Northern Greece. Proc. Int. Conf. Environ. Sci. Technol. 2005, 1, 222–231. [Google Scholar]
- Wang, J.; Chen, J.; Ding, S.; Guo, J.; Christopher, D.; Dai, Z.; Yang, H. Effects of seasonal hypoxia on the release of phosphorus from sediments in deep-water ecosystem: A case study in Hongfeng Reservoir, Southwest China. Environ. Pollut. 2016, 219, 858–865. [Google Scholar] [CrossRef]
- Xia, J.; Jin, X.; Yang, Y.; Li, L.; Wu, F. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China. Water Res. 2008, 42, 2251–2259. [Google Scholar]
- Kang, M.; Peng, S.; Tian, Y.; Zhang, H. Effects of dissolved oxygen and nutrient loading on phosphorus fluxes at the sediment–water interface in the Hai River Estuary, China. Mar. Pollut. Bull. 2018, 130, 132–139. [Google Scholar] [CrossRef]
- Zhang, Y.; He, F.; Liu, Z.; Liu, B.; Zhou, Q.; Wu, Z.; Zhang, Y.; He, F.; Liu, Z.; Liu, B. Release characteristics of sediment phosphorus in all fractions of West Lake, Hang Zhou, China. Ecol. Eng. 2016, 95, 645–651. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Zhao, H.; Zhou, X.; Wu, F. Effects of organic matter on phosphorus release kinetics in different trophic lake sediments and application of transition state theory-ScienceDirect. J. Environ. Manag. 2008, 88, 845–852. [Google Scholar] [CrossRef]
- Doan, P.; Watson, S.B.; Markovic, S.; Liang, A.; Dittrich, M. Phosphorus retention and internal loading in the Bay of Quinte, Lake Ontario, using diagenetic modelling. Sci. Total Environ. 2018, 636, 39–51. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, S.; Zhang, B.-T.; Wang, Y.; Li, H. Response of sediment organic phosphorus composition to lake trophic status in China. Sci. Total Environ. 2019, 652, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Jin, X.; Zhao, H.; Zhou, X.; Wu, F. Effect of organic matter on the sorption of dissolved organic and inorganic phosphorus in lake sediments. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 154–162. [Google Scholar] [CrossRef]
- Kaiserli, A.; Voutsa, D.; Samara, C. Phosphorus fractionation in lake sediments—Lakes Volvi and Koronia, N. Greece. Chemosphere 2002, 46, 1147–1155. [Google Scholar] [CrossRef]
- Bottrell, S.H.; Mortimer, R.; Davies, I.M.; Harvey, S.M.; Krom, M.D. Sulphur cycling in organic-rich marine sediments from a Scottish fjord. Sedimentology 2010, 56, 1159–1173. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Wang, G.; Li, X.; Ma, J.; Chen, S.; Deng, H.; Annalisa, O.-H. High sulfide production induced by algae decomposition and its potential stimulation to phosphorus mobility in sediment. Sci. Total Environ. 2018, 650, 163–172. [Google Scholar] [CrossRef]
- Rao, A.M.F.; Malkin, S.Y.; Hidalgo-Martinez, S.; Meysman, F.J.R. The impact of electrogenic sulfide oxidation on elemental cycling and solute fluxes in coastal sediment. Geochim. Cosmochim. Acta J. Geochem. Soc. Meteorit. Soc. 2016, 172, 265–286. [Google Scholar] [CrossRef] [Green Version]
- Kraal, P.; Burton, E.D.; Rose, A.L.; Kocar, B.D.; Lockhart, R.S.; Grice, K.; Bush, R.T.; Tan, E.; Webb, S.M. Sedimentary iron–phosphorus cycling under contrasting redox conditions in a eutrophic estuary. Chem. Geol. 2015, 392, 19–31. [Google Scholar] [CrossRef]

| Step | Sequential Extraction Method | P Fractions |
|---|---|---|
| 1 | 1 g of sediment added to 50 mL of 1 M NH4Cl at a pH of 7, shaken for 4 h | EX-P |
| 2 | Residual sample added to 0.11 M Na2S2O4/NaHCO3, shaken for 1 h at 40 °C | BD-P |
| 3 | Residual sample added to 0.1 M NaOH, shaken for 16 h | NaOH-P |
| 4 | Residual sample added to 0.5 M HCl, shaken for 16 h | HCl-P |
| XW-A | XW-B | XW-C | NZD-A | NZD-B | NZD-C | ||
|---|---|---|---|---|---|---|---|
| P fractions in sediments | EX-P (mg/kg) | 1.37–4.9 | 0.1–4.0 | 0.5–4.2 | 0.05–1.6 | 0–2.26 | 0–2.0 |
| BD-P (mg/kg) | 25.5–47.5 | 18.3–59.7 | 41.0–178.6 | 218.4–281.3 | 181.6–299.6 | 137.7–267.3 | |
| NaOH-P (mg/kg) | 6–34.9 | 4.2–36.1 | 10.6–41.8 | 12.8–50.4 | 11.1–64.0 | 8.9–38.8 | |
| HCl-P (mg/kg) | 179.8–319.2 | 140.9–281.2 | 166.3–318.0 | 28.7–51.8 | 25.1–109.7 | 79.1–131.7 | |
| Res-P (mg/kg) | 174.9–320.8 | 144.7–312.6 | 196.1–536.2 | 31.1–318.0 | 4.4–506.3 | 47.6–372.5 | |
| BAP (mg/kg) | 35.7–82.2 | 23.3–99.9 | 58.6–224.6 | 239.0–321.5 | 194.7–323.0 | 155.7–276.1 | |
| TP (mg/kg) | 463.6–592.2 | 363.4–553.3 | 552.7–927.0 | 359.4–622.4 | 331.7–784.1 | 364.8–683.6 | |
| Overlying water | DO (mg/L) | 4.8 | 5.1 | 2.6 | 0.9 | 3.2 | 7.8 |
| Temp (°C) | 13.7 | 13.8 | 16.5 | 17.5 | 17.7 | 18.1 | |
| Conductivity (µS/cm) | 435.2 | 444.9 | 436.1 | 382.7 | 389.6 | 407.7 | |
| pH | 7.9 | 7.9 | 7.8 | 7.6 | 7.7 | 8.3 | |
| Redox potential (mv) | −58.1 | −59.3 | −52.9 | −43.2 | −45.9 | −80.5 |
| EX-P | BD-P | NaOH-P | HCl-P | Res-P | TP | BAP | ||
|---|---|---|---|---|---|---|---|---|
| XW (n = 29) | CDGT-P | −0.17 | 0.632 ** | 0.25 | 0.32 | 0.597 ** | 0.731 ** | 0.571 ** |
| CDGT-Fe | −0.076 | 0.340 | 0.105 | 0.128 | 0.285 | 0.350 | 0.301 | |
| CDGT-s | −0.053 | 0.530 ** | 0.197 | 0.387 * | 0.440 * | 0.616 ** | 0.478 ** | |
| NZD (n = 33) | CDGT-P | 0.20 | 0.689 ** | 0.29 | −0.644 ** | −0.30 | −0.18 | 0.708 ** |
| CDGT-Fe | 0.309 | 0.446 ** | 0.244 | −0.230 | −0.133 | 0.017 | 0.477 ** | |
| CDGT-s | 0.173 | 0.758 ** | 0.356 * | −0.613 ** | −0.368 * | −0.202 | 0.790 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Z.; Cheng, Y.; Huang, Q.; Hu, M.; Dong, W.; Fan, J.; Wang, Y. Synchronous Cycle of Available Phosphorus, Iron, and Sulfur in the Sediment of Lancang River Reservoirs. Water 2021, 13, 2691. https://doi.org/10.3390/w13192691
Mu Z, Cheng Y, Huang Q, Hu M, Dong W, Fan J, Wang Y. Synchronous Cycle of Available Phosphorus, Iron, and Sulfur in the Sediment of Lancang River Reservoirs. Water. 2021; 13(19):2691. https://doi.org/10.3390/w13192691
Chicago/Turabian StyleMu, Zheng, Yao Cheng, Qiang Huang, Mingming Hu, Wei Dong, Jingjing Fan, and Yuchun Wang. 2021. "Synchronous Cycle of Available Phosphorus, Iron, and Sulfur in the Sediment of Lancang River Reservoirs" Water 13, no. 19: 2691. https://doi.org/10.3390/w13192691
APA StyleMu, Z., Cheng, Y., Huang, Q., Hu, M., Dong, W., Fan, J., & Wang, Y. (2021). Synchronous Cycle of Available Phosphorus, Iron, and Sulfur in the Sediment of Lancang River Reservoirs. Water, 13(19), 2691. https://doi.org/10.3390/w13192691






