Estimating the Reducing Power of Carbon Nanotubes and Granular Activated Carbon Using Various Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Setup and Procedure
2.3. Analytical Methods
3. Results and Discussion
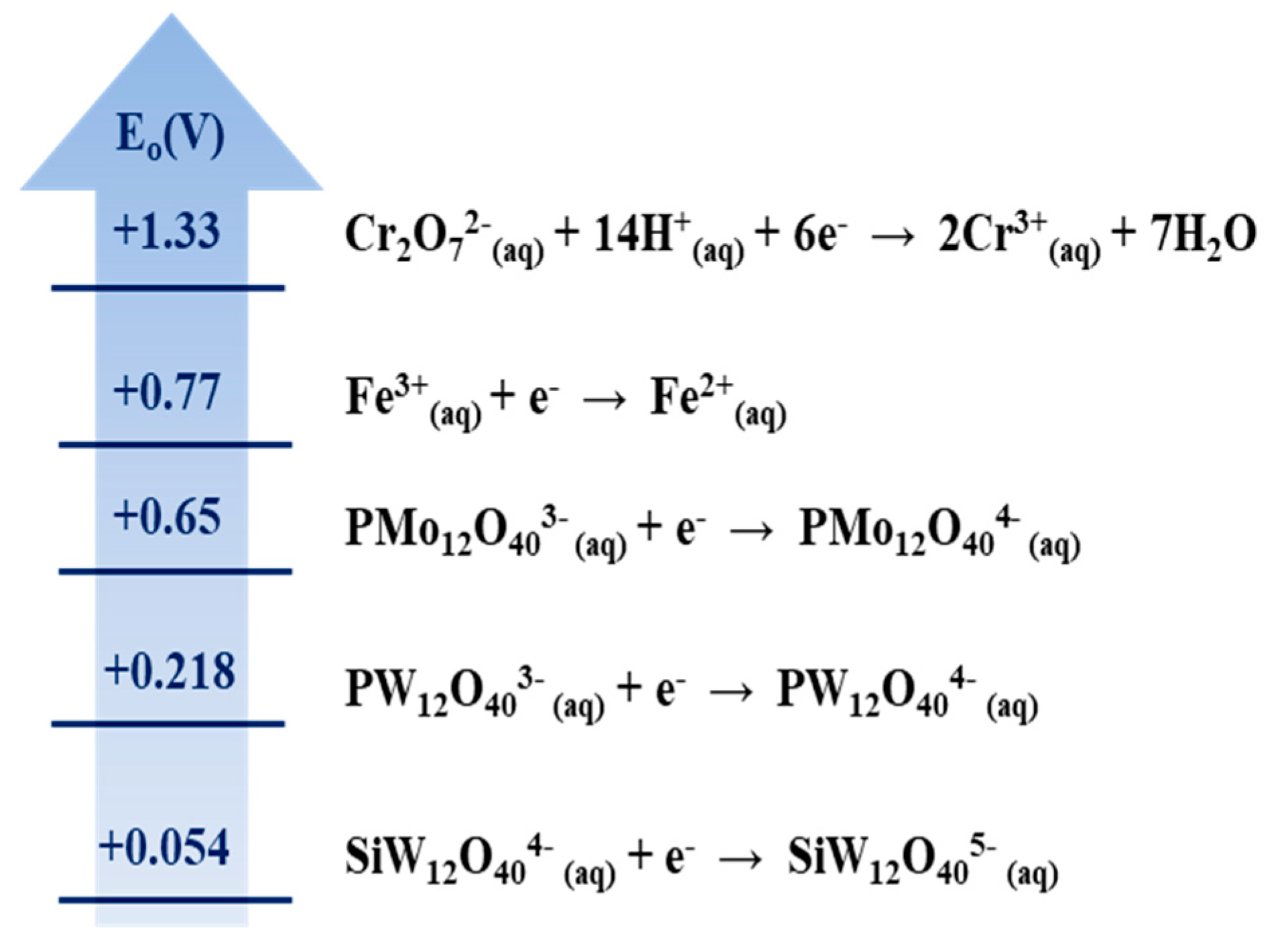
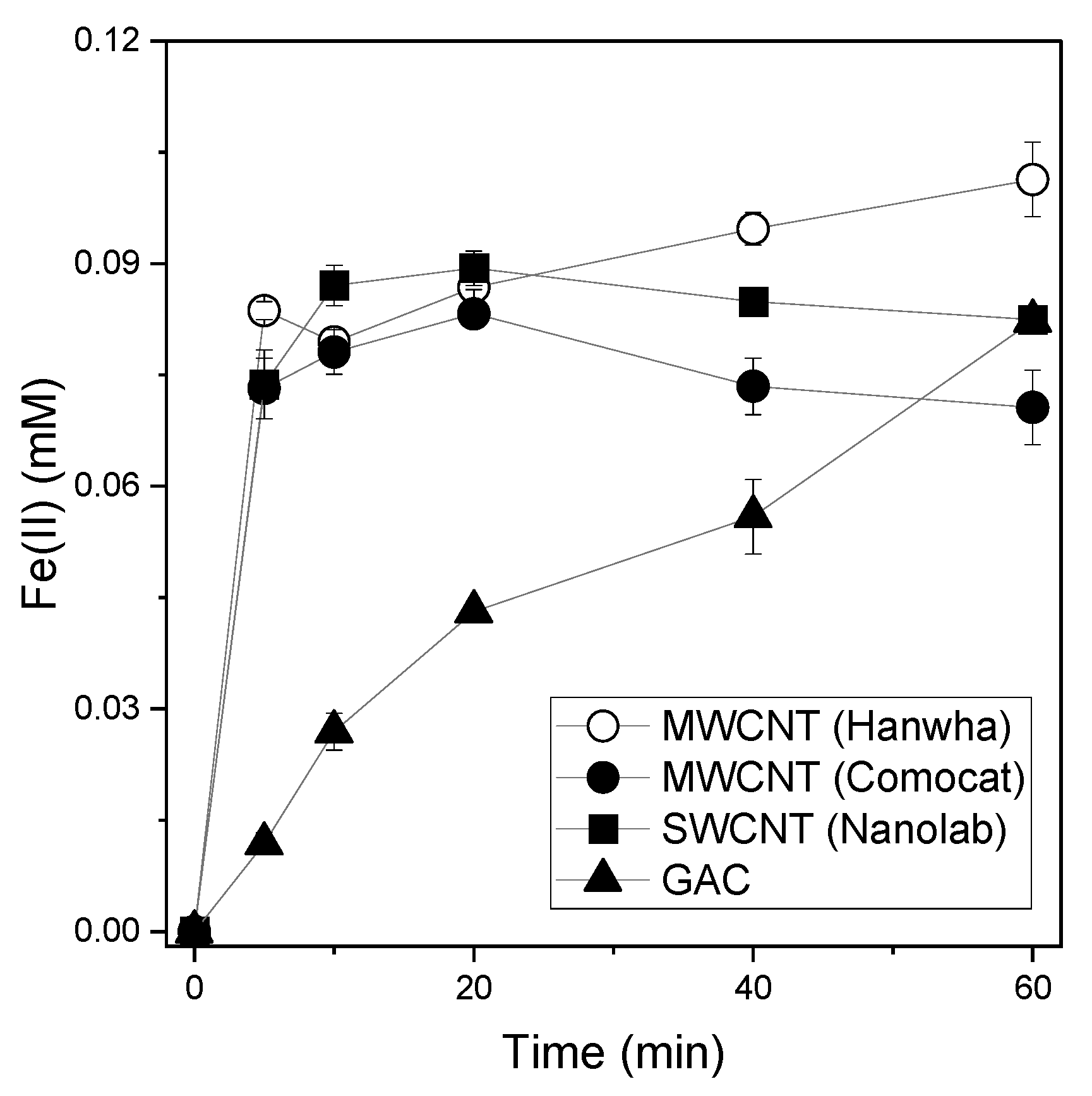
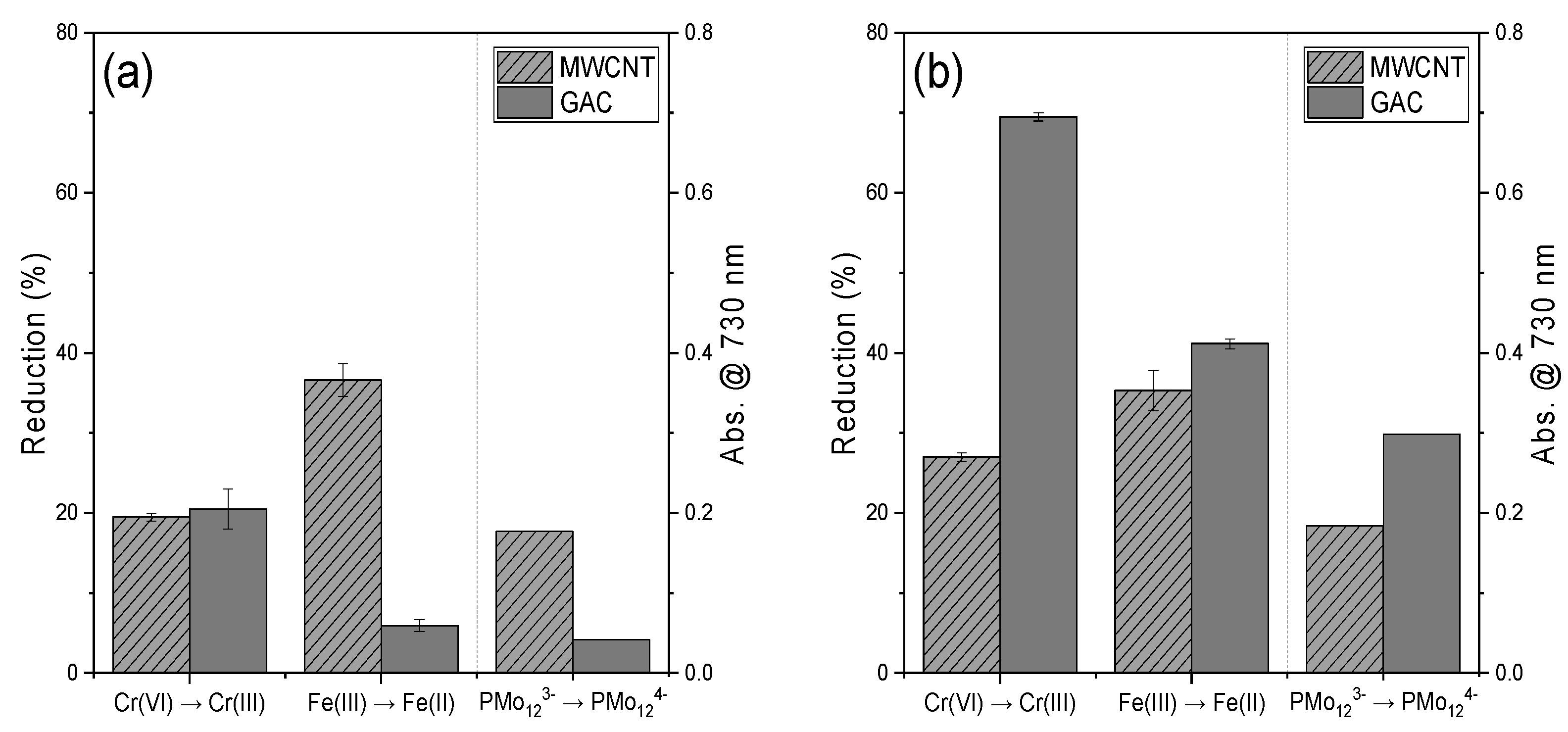
3.1. Estimating the Reducing Power of CNTs and GAC Using Various Compounds
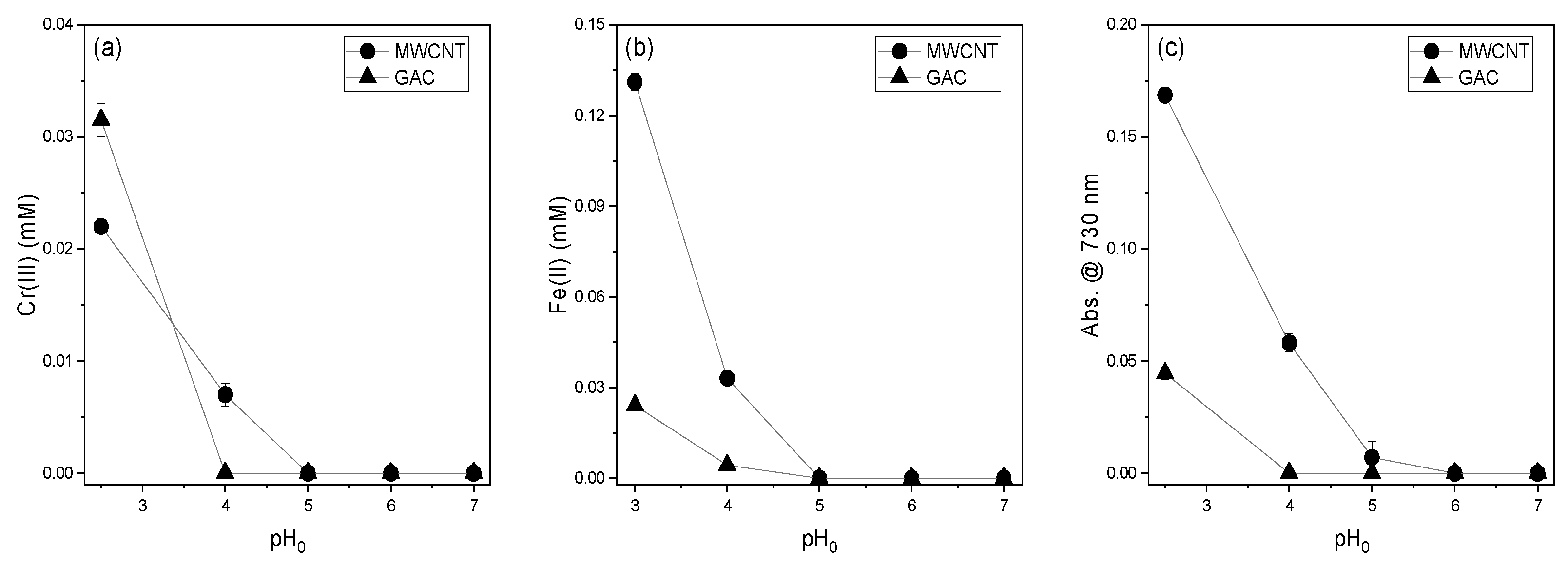
3.2. Effect of Initial pH on the Reducing Power of MWCNTs and GAC
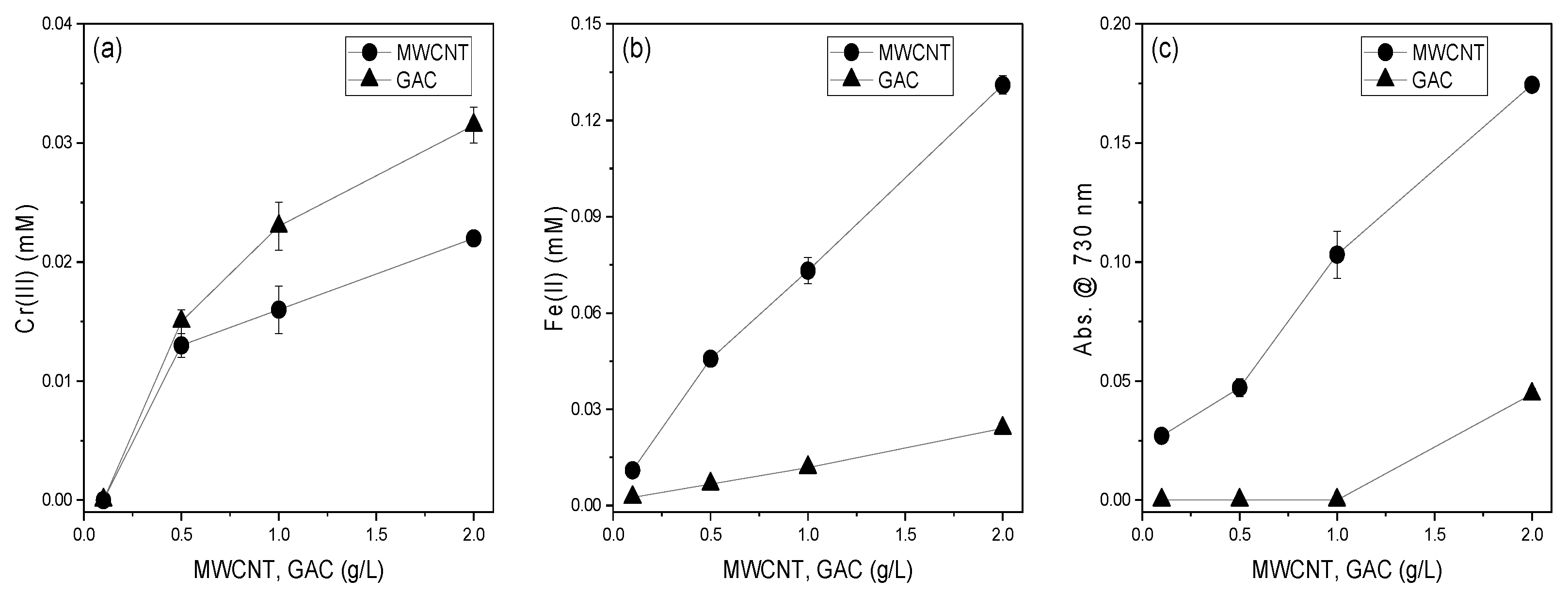
3.3. Effect of MWCNTs and GAC Dosage on the Reducing Power
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Wang, X.; Yi, P.; Zhang, P.; Zhang, L.; Wu, M.; Pan, B. Key Roles of Electron Cloud Density and Configuration in the Adsorption of Sulfonamide Antibiotics on Carbonaceous Materials: Molecular Dynamics and Quantum Chemical Investigations. Appl. Surf. Sci. 2021, 536, 147757. [Google Scholar] [CrossRef]
- Wei, C.; Akinwolemiwa, B.; Yu, L.; Hu, D.; Chen, G.Z. 7-Polymer Composites with Functionalized Carbon Nanotubes and Graphene. In Polymer Composites with Functionalized Nanoparticles Synthesis, Properties, and Applications Micro and Nano. Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–248. [Google Scholar]
- Bartoli, M.; Frediani, M.; Rosi, L. Carbon-Based Material for Environmental Protection and Remediation; IntechOpen: London, UK, 2020. [Google Scholar]
- Huang, Y.; Lee, X.; Macazo, F.C.; Grattieri, M.; Cai, R.; Minteer, S.D. Fast and Efficient Removal of Chromium (VI) Anionic Species by a Reusable Chitosan-Modified Multi-Walled Carbon Nanotube Composite. Chem. Eng. J. 2018, 339, 259–267. [Google Scholar] [CrossRef]
- Ko, Y.J.; Choi, K.; Lee, S.; Cho, J.M.; Choi, H.J.; Hong, S.W.; Choi, J.W.; Mizuseki, H.; Lee, W.S. Chromate Adsorption Mechanism on Nanodiamond-Derived Onion-Like Carbon. JHM 2016, 320, 368–375. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.; Zhou, C.; Su, S.; Lai, B. The Carbon Nanotubes-Based Materials and Their Applications for Organic Pollutant Removal: A Critical Review. Chin. Chem. Lett. 2020, 32, 1626–1636. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Hoang, T.V.; Malwade, K.; Kanel, S.R.; Harper, W.F., Jr.; Struckhoff, G. Application of Carbon Nanotubes for Removal of Emerging Contaminants of Concern in Engineered Water and Wastewater Treatment Systems. Nanotechnol. Environ. Eng. 2019, 4, 12. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Chemical regeneration of granular activated carbon: Preliminary evaluation of alternative regenerant solutions. Environ. Sci. Water Res. Technol. 2020, 6, 2043–2056. [Google Scholar] [CrossRef]
- Sontheimer, H.; Crittenden, J.C.; Summers, R.S.; Frick, B.R.; Fettig, J.; Hörner, G.; Hubele, C.; Zimmer, G. Activated Carbon for Water Treatment, 2nd ed.; DVGW-Forschungstelle: Karlsruhe, Germany, 1988. [Google Scholar]
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the Science and Technology of Carbon Nanotubes and Their Composites: A Review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.G.; Zhao, X.H.; Yu, L.Y.; Jiao, F.P.; Jiang, J.H.; Chen, X.Q. Removal, Recovery and Enrichment of Metals from Aqueous Solutions Using Carbon Nanotubes. J. Radioanal. Nucl. Chem. 2014, 299, 1155–1163. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J.B. High Efficiency Removal of Heavy Metals Using Tire-Derived Activated Carbon vs Commercial Activated Carbon: Insights into the Adsorption Mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A Review on Conventional and Novel Materials Towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Shang, Q.; Sun, H.; Wu, J.; Tan, H. Functionalized Construction of Biochar with Hierarchical Pore Structures and Surface O-/N-containing Groups for Phenol Adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Masjoudi, M.; Golgoli, M.; Ghobadi Nejad, Z.G.; Sadeghzadeh, S.; Borghei, S.M. Pharmaceuticals Removal by Immobilized Laccase on Polyvinylidene Fluoride Nanocomposite with Multi-Walled Carbon Nanotubes. Chemosphere 2021, 263, 128043. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, L.; Yang, Z.; Li, S. Physical and Mechanical Properties and Micro Characteristics of Fly Ash-Based Geopolymer Paste Incorporated with Waste Granulated Blast Furnace Slag (GBFS) and Functionalized Multi-Walled Carbon Nanotubes (MWCNTs). J. Hazard. Mater. 2021, 401, 123339. [Google Scholar] [CrossRef]
- Marques, R.R.N.; Machado, B.F.; Faria, J.L.; Silva, A.M.T. Controlled Generation of Oxygen Functionalities on the Surface of Single-Walled Carbon Nanotubes by HNO3 Hydrothermal Oxidation. Carbon 2010, 48, 1515–1523. [Google Scholar] [CrossRef]
- Romanos, G.E.; Likodimos, V.; Marques, R.R.N.; Steriotis, T.A.; Papageorgiou, S.K.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T.; Falaras, P. Controlling and Quantifying Oxygen Functionalities on Hydrothermally and Thermally Treated Single-Wall Carbon Nanotubes. J. Phys. Chem. C 2011, 115, 8534–8546. [Google Scholar] [CrossRef]
- Likodimos, V.; Steriotis, T.A.; Papageorgiou, S.K.; Romanos, G.E.; Marques, R.R.N.; Rocha, R.P.; Faria, J.L.; Pereira, M.F.R.; Figueiredo, J.L.; Silva, A.M.T.; et al. Controlled Surface Functionalization of Multiwall Carbon Nanotubes by HNO3 Hydrothermal Oxidation. Carbon 2014, 69, 311–326. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Tawabini, B.S.; Bukhari, A.A.; Khaled, M.; Alharthi, M.; Fettouhi, M.; Abuilaiwi, F.A. Removal of Chromium (III) from Water by Using Modified and Nonmodified Carbon Nanotubes. J. Nanomater. 2010, 2010, 232378. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Al-Degs, Y.S.; Sweileh, J.A.; Said, A.J. Separation and Flame Atomic Absorption Spectrometric Determination of Total Chromium and Chromium (III) in Phosphate Rock Used for Production of Fertilizer. Talanta 2013, 116, 482–487. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Yang, H.; Sun, Y.; Liu, Y. Influence of the Different Oxidation Treatment on the Performance of Multi-Walled Carbon Nanotubes in the Catalytic Wet Air Oxidation of Phenol. J. Hazard. Mater. 2012, 233–234, 18–24. [Google Scholar] [CrossRef]
- Bach, A.; Semiat, R. The Role of Activated Carbon as a Catalyst in GAC/Iron Oxide/H2O2 Oxidation Process. Desalination 2011, 273, 57–63. [Google Scholar] [CrossRef]
- Seo, J.; Lee, H.J.; Lee, H.; Kim, H.E.; Lee, J.Y.; Kim, H.S.; Lee, C. Enhanced Production of Reactive Oxidants by Fenton-Like Reactions in the Presence of Carbon Materials. Chem. Eng. J. 2015, 273, 502–508. [Google Scholar] [CrossRef]
- Peng, J.; Xue, J.; Li, J.; Du, Z.; Wang, Z.; Gao, S. Catalytic Effect of Low Concentration Carboxylated Multi-Walled Carbon Nanotubes on the Oxidation of Disinfectants with Cl-Substituted Structure by a Fenton-Like System. Chem. Eng. J. 2017, 321, 325–334. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, A.; Shan, C.; Gao, G.; Pan, B. Enhanced Fe(III)-Mediated Fenton Oxidation of Atrazine in the Presence of Functionalized Multi-Walled Carbon Nanotubes. Water Res. 2018, 137, 37–46. [Google Scholar] [CrossRef]
- Pope, M.T.; Varga, G.M., Jr. Heteropoly Blues. I. Reduction Stoichiometries and Reduction Potentials of Some 12-Tungstates. Inorg. Chem. 1966, 5, 1249–1254. [Google Scholar] [CrossRef]
- Bae, E.; Lee, J.W.; Hwang, B.H.; Yeo, J.; Yoon, J.; Cha, H.J.; Choi, W. Photocatalytic Bacterial Inactivation by Polyoxometalates. Chemosphere 2008, 72, 174–181. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 1985. [Google Scholar]
- Wielinga, B.; Mizuba, M.M.; Hansel, C.M.; Fendorf, S. Iron promoted reduction of chromate by dissimilatory iron-reducing bacteria. Environ. Sci. Technol. 2001, 35, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Voelz, J.L.; Johnson, N.W.; Chun, C.L.; Arnold, W.A.; Penn, R.L. Quantitative dissolution of environmentally accessible iron residing in iron-rich minerals: A review. Environ. Sci. Technol. 2019, 3, 1371–1392. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in solution: Speciation under spotlight. Chem. Soc. Rev. 2020, 49, 7481–7846. [Google Scholar] [CrossRef]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric Determination of Iron(II) with 1,10-phenanthroline in the Presence of Large Amounts of Iron(III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Determination of Water and Wastewater, 20th ed.; United Book Press, Inc.: Baltimore, MD, USA, 1998. [Google Scholar]
- Zardini, H.Z.; Amiri, A.; Shanbedi, M.; Maghrebi, M.; Baniadam, M. Enhanced Antibacterial Activity of Amino Acids-Functionalized Multi Walled Carbon Nanotubes by a Simple Method. Colloids Surf. B Biointerfaces 2012, 92, 196–202. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Das, R.; Abd Hamid, S.B.; Ali, M.E.; Ismail, A.F.; Annuar, M.S.M.; Ramakrishna, S. Multifunctional Carbon Nanotubes in Water Treatment: The Present, Past and Future. Desalination 2014, 354, 160–179. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for Strong Adsorption of Tetracycline to Carbon Nanotubes: A Comparative Study Using Activated Carbon and Graphite as Adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, M.J.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.E.; Hayball, J.D. Activated Carbon, Carbon Nanotubes and Graphene: Materials and Composites for Advanced Water Purification. C 2017, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Xing, B.S. Adsorption of Organic Compounds by Carbon Nanomaterials in Aqueous Phase: Polanyi Theory and Its Application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.; Chen, R.; Poblete, H.; Vergara-Jaque, A.; Riviere, J.E. Predicting Adsorption Affinities of Small Molecules on Carbon Nanotubes Using Molecular Dynamics Simulation. ACS Nano 2015, 9, 11761–11774. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Candela, M.; Martín-Martínez, J.M.; Torregrosa-Maciá, R. Chromium (VI) removal with activated carbons. Water Res. 1995, 29, 2174–2180. [Google Scholar] [CrossRef]
- Liu, P.; Ptacek, C.J.; Blowes, D.W.; Finfrock, Y.Z.; Liu, Y.Y. Characterization of Chromium Species and Distribution During Cr(VI) Removal by Biochar Using Confocal Micro-Ray Fluorescence Redox Mapping and X-Ray Adsorption Spectroscopy, Environmental. International 2020, 134, 105216. [Google Scholar] [CrossRef]
- Lakatos, J.; Brown, S.D.; Snape, C.E. Coals as Sorbents for the Removal and Reduction of Hexavalent Chromium from Aqueous Waste Streams. Fuel 2002, 81, 691–698. [Google Scholar] [CrossRef]
- Benazzi, E.; Karlsson, J.; Ben M’Barek, Y.; Chabera, P.; Blanchard, S.; Alves, S.; Proust, A.; Pullerits, T.; Izzet, G.; Gibson, E.A. Acid-Triggering of Light-Induced Charge-Separation in Hybrid Organic/Inorganic Molecular Photoactive Dyads for Harnessing Solar Energy. Inorg. Chem. Front. 2021, 8, 1610–1618. [Google Scholar] [CrossRef]
- Ueda, T. Recent Achievements in the Analysis of the Electrochemical Properties of Polyoxometalates. Rev. Polarogr. 2014, 61, 11–19. [Google Scholar] [CrossRef]
- Kolodyʼnska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Matias, M.; Andrade, K.R.; Jesus, A.N.; Cunha, G.D.C.; Andrade, R.S. Aged Biochar Changed Copper Availability and Distribution Among Soil Fractions and Influenced Corn Seed Germination in a Copper-Contaminated Soil. Chemosphere 2020, 240, 124828. [Google Scholar] [CrossRef] [PubMed]

| Carbon-Based Materials | BET Specific Surface Area (m2/g) | Pore Volume (m3/g) | Average Pore Size (nm) |
|---|---|---|---|
| MWCNTs | 236.25 | 1.92 | 32.53 (Mesoporous) |
| GAC | 648.24 | 0.26 | 1.63 (Microporous) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, H.; Kim, I.; Park, S. Estimating the Reducing Power of Carbon Nanotubes and Granular Activated Carbon Using Various Compounds. Water 2021, 13, 1959. https://doi.org/10.3390/w13141959
Woo H, Kim I, Park S. Estimating the Reducing Power of Carbon Nanotubes and Granular Activated Carbon Using Various Compounds. Water. 2021; 13(14):1959. https://doi.org/10.3390/w13141959
Chicago/Turabian StyleWoo, Heesoo, Ilho Kim, and Saerom Park. 2021. "Estimating the Reducing Power of Carbon Nanotubes and Granular Activated Carbon Using Various Compounds" Water 13, no. 14: 1959. https://doi.org/10.3390/w13141959






