A Functionalised Carbon Fiber for Flexible Extraction and Determination of Hg(II) Using Au(NP)-Thiol-CF Inductively Coupled Plasma Mass Spectrometry
Abstract
:1. Introduction
2. Experimental Work
2.1. Chemical and Solutions
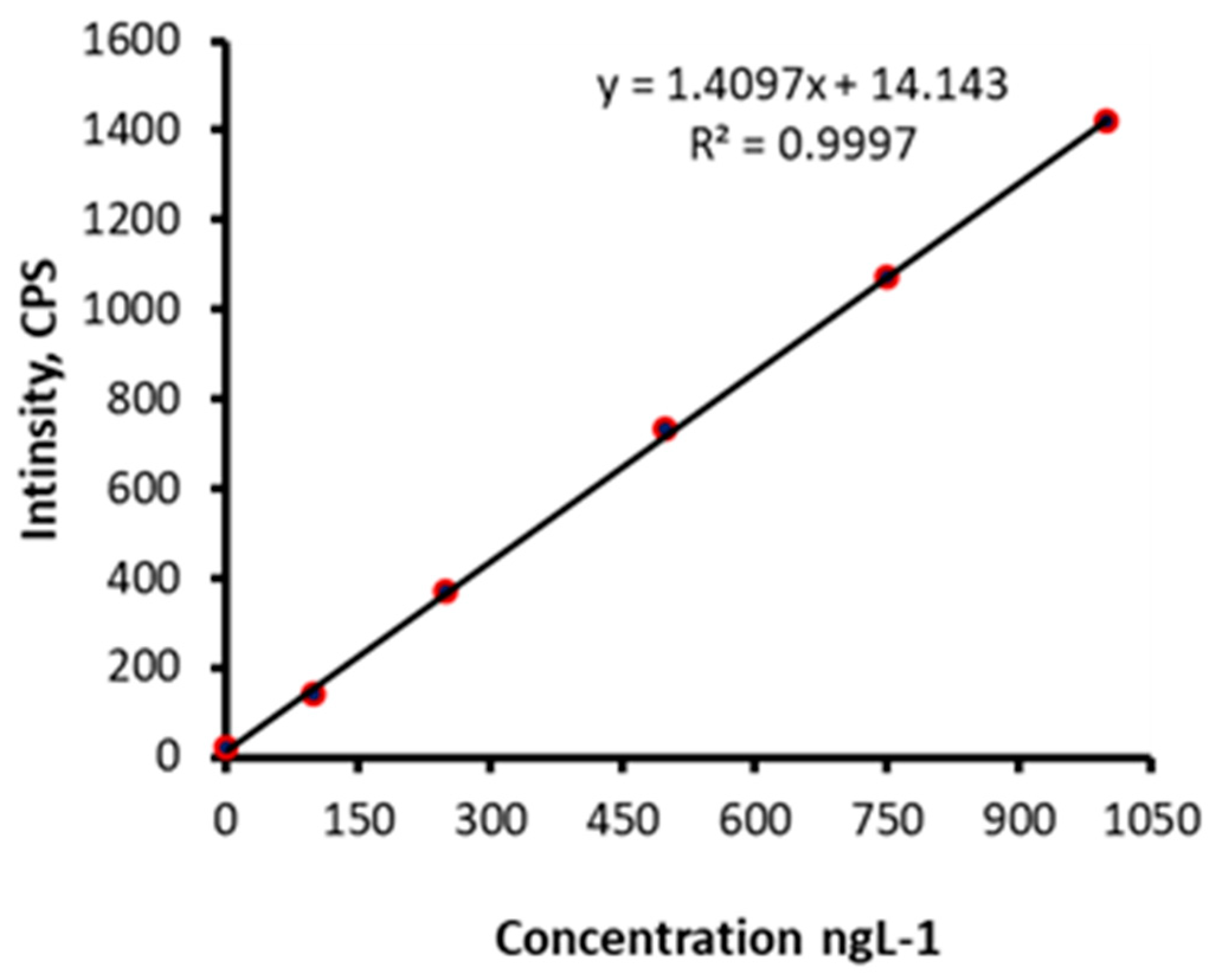
2.2. Characterisation
2.3. Experimental Procedures
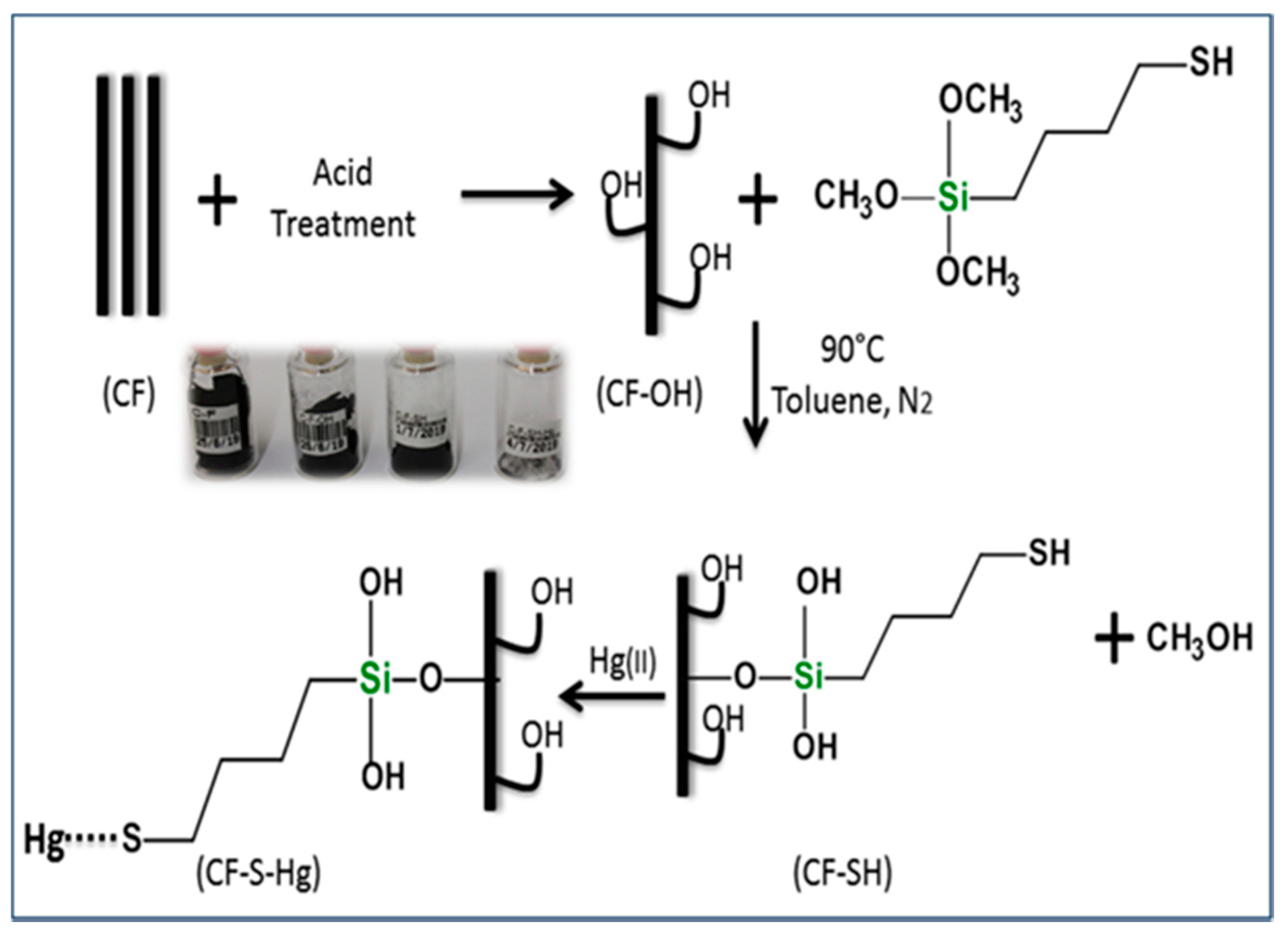
2.3.1. CF Modification
2.3.2. Adsorption Capacity of Hg(II) and Preconcentration
2.3.3. The pH Effect Experiment
2.3.4. Eluent Effect Experiment
3. Results and Discussion
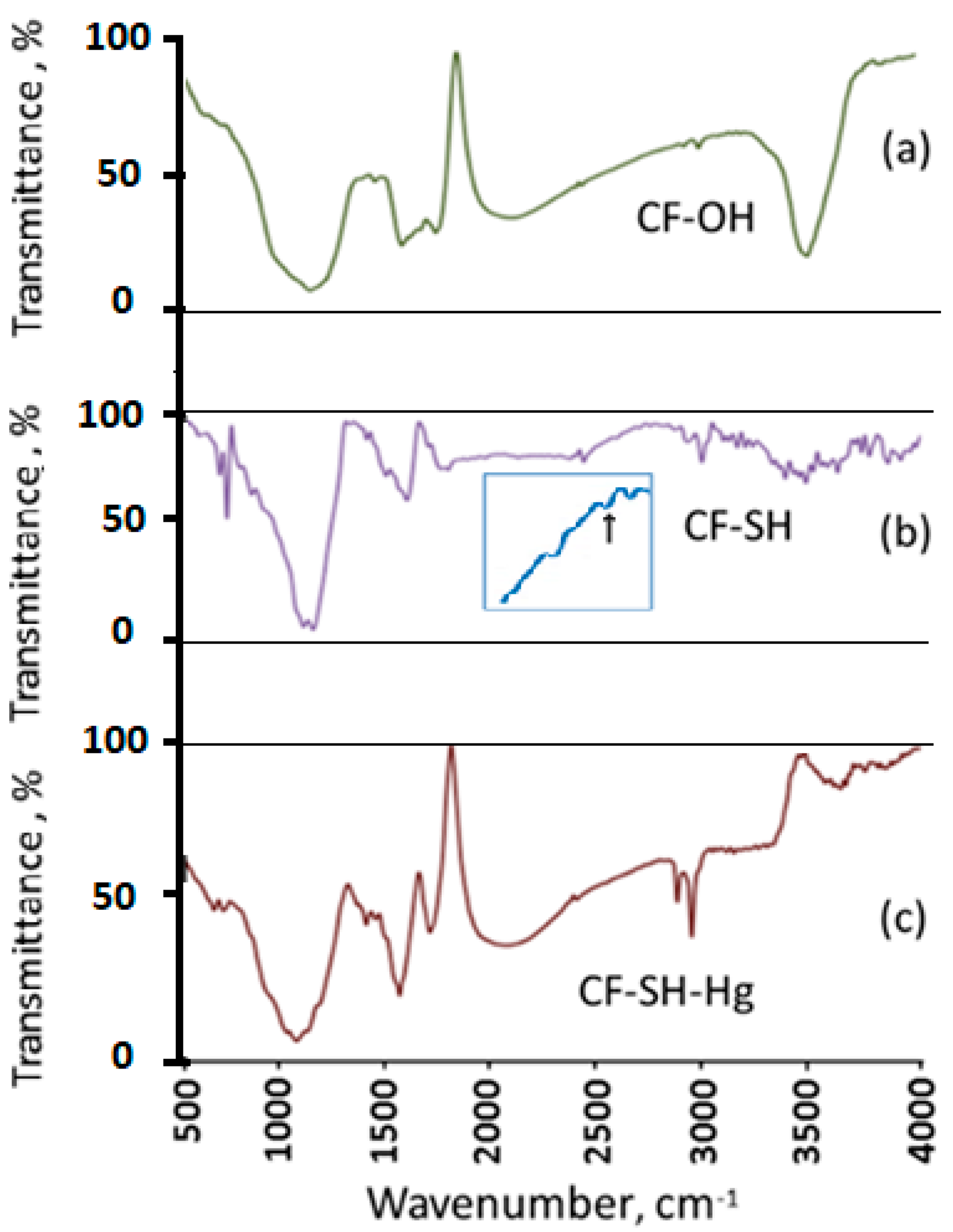
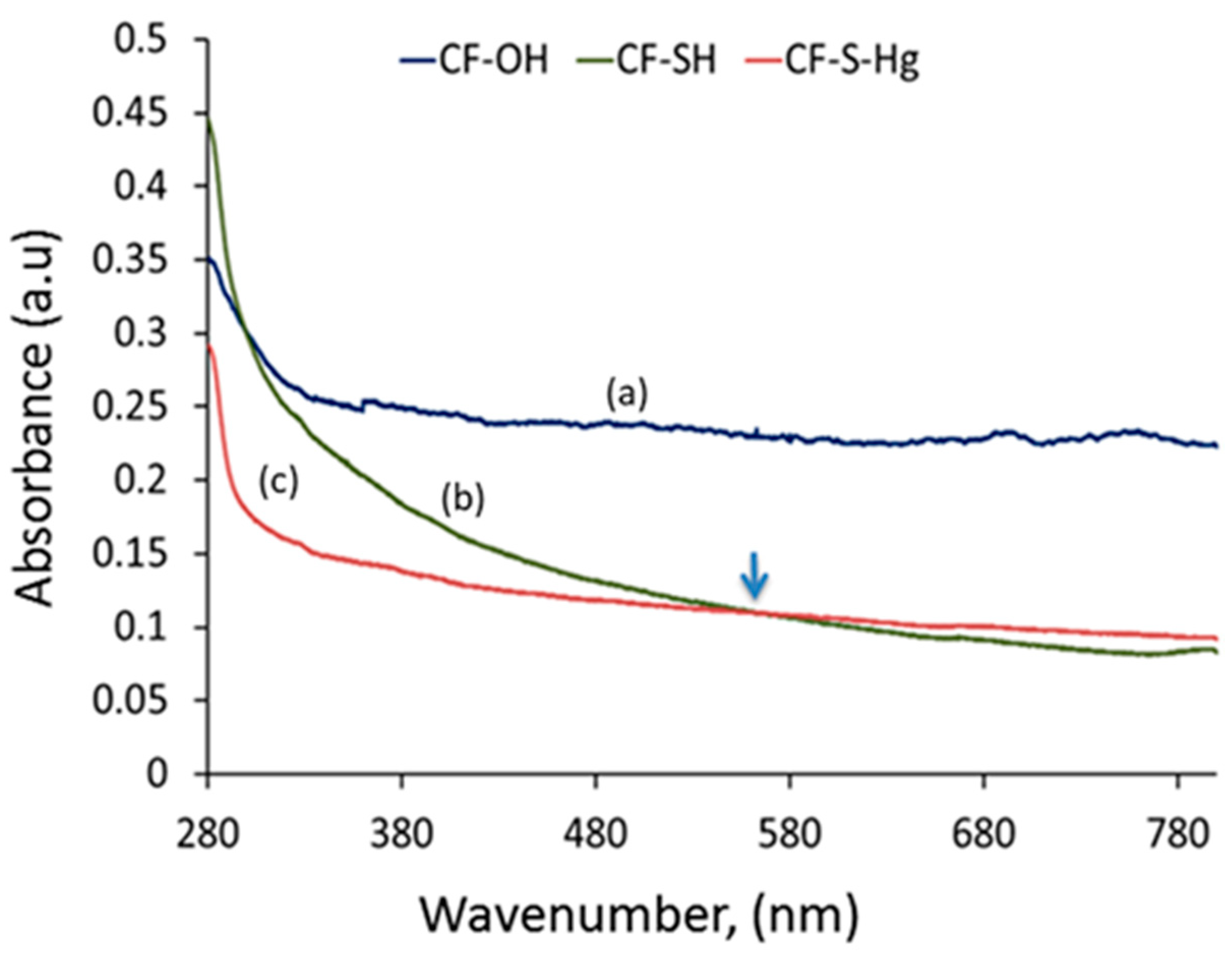
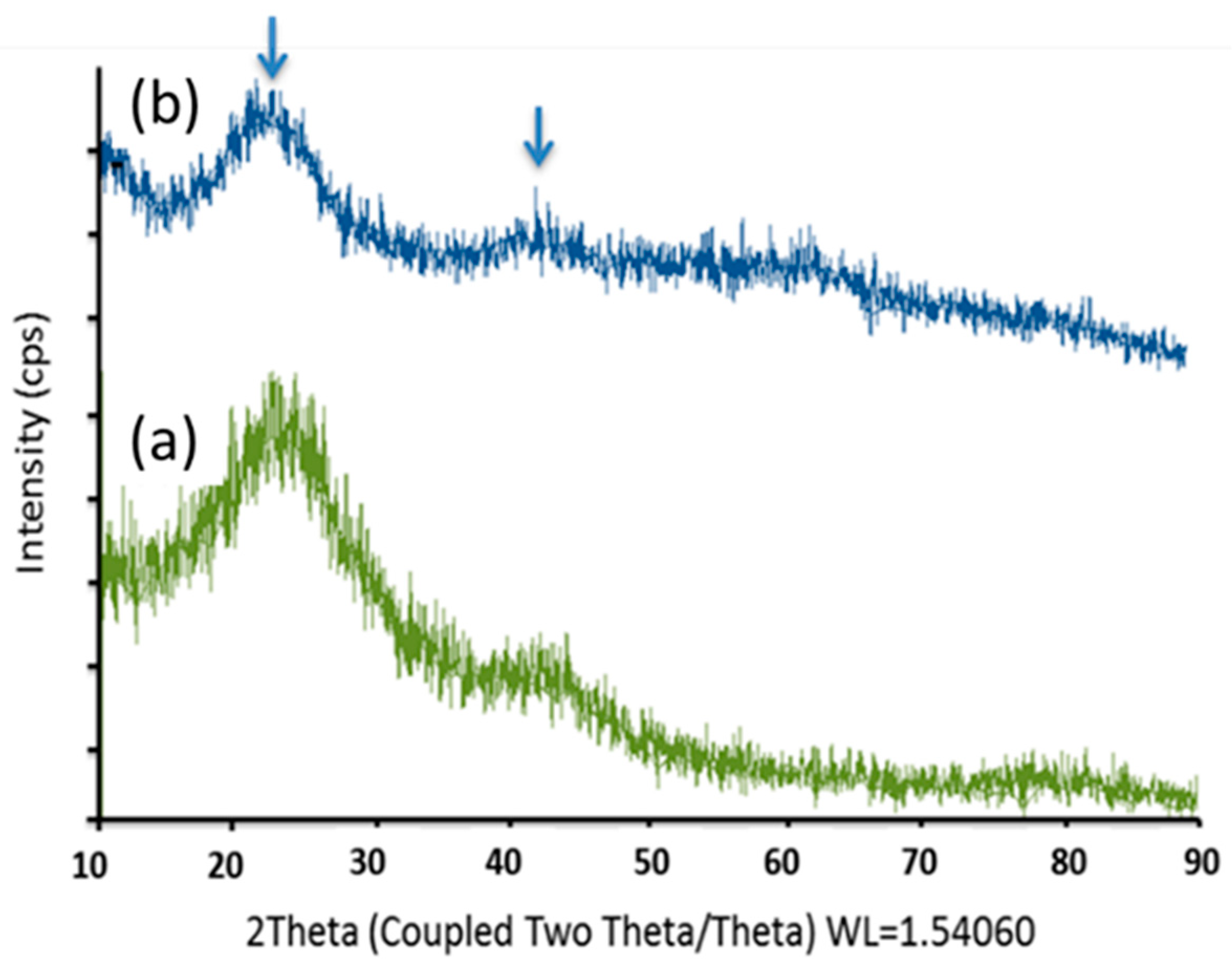
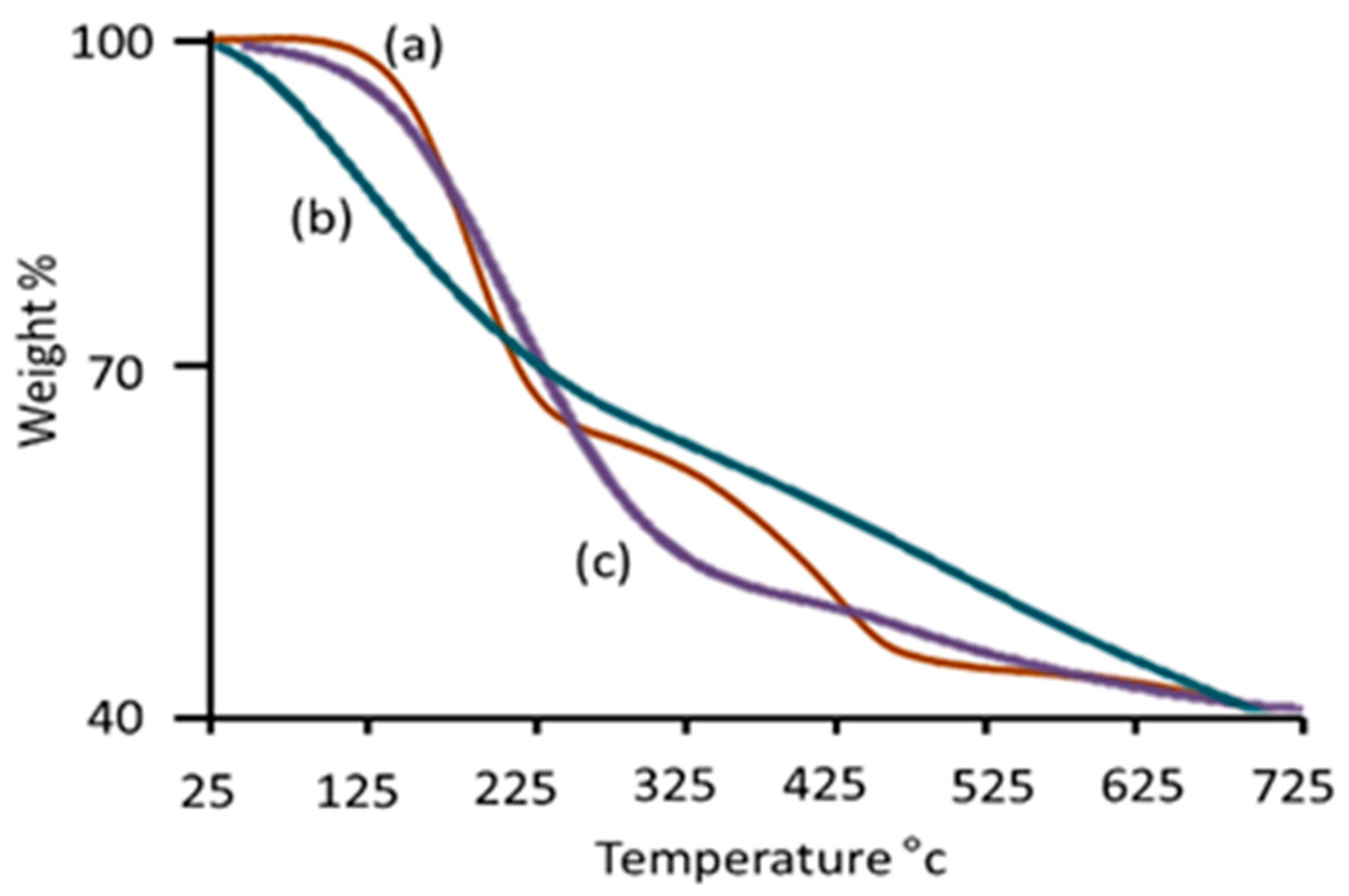
3.1. Surface Modification
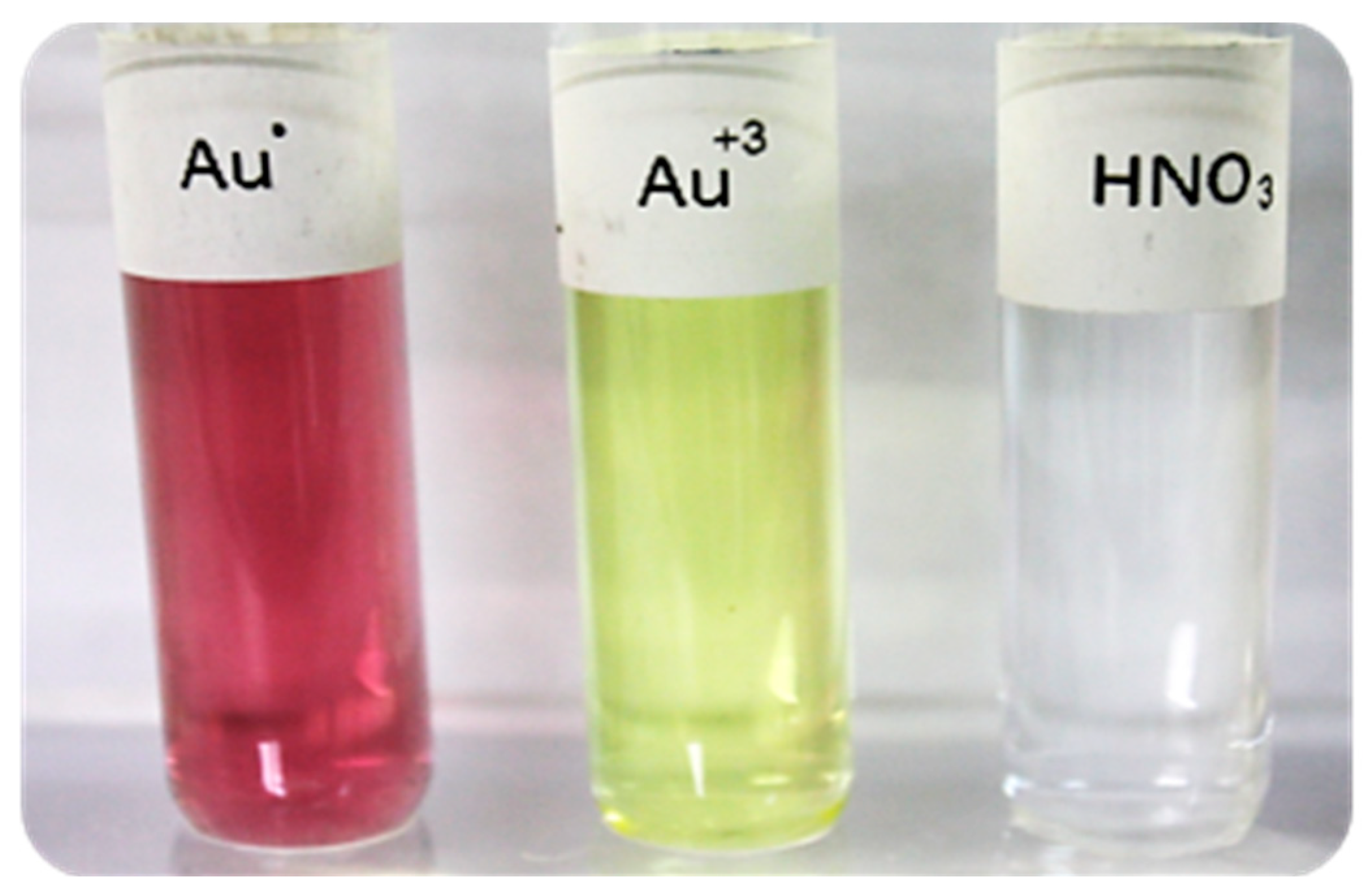
3.2. Hg CF-SH Extraction and Parameters Effect
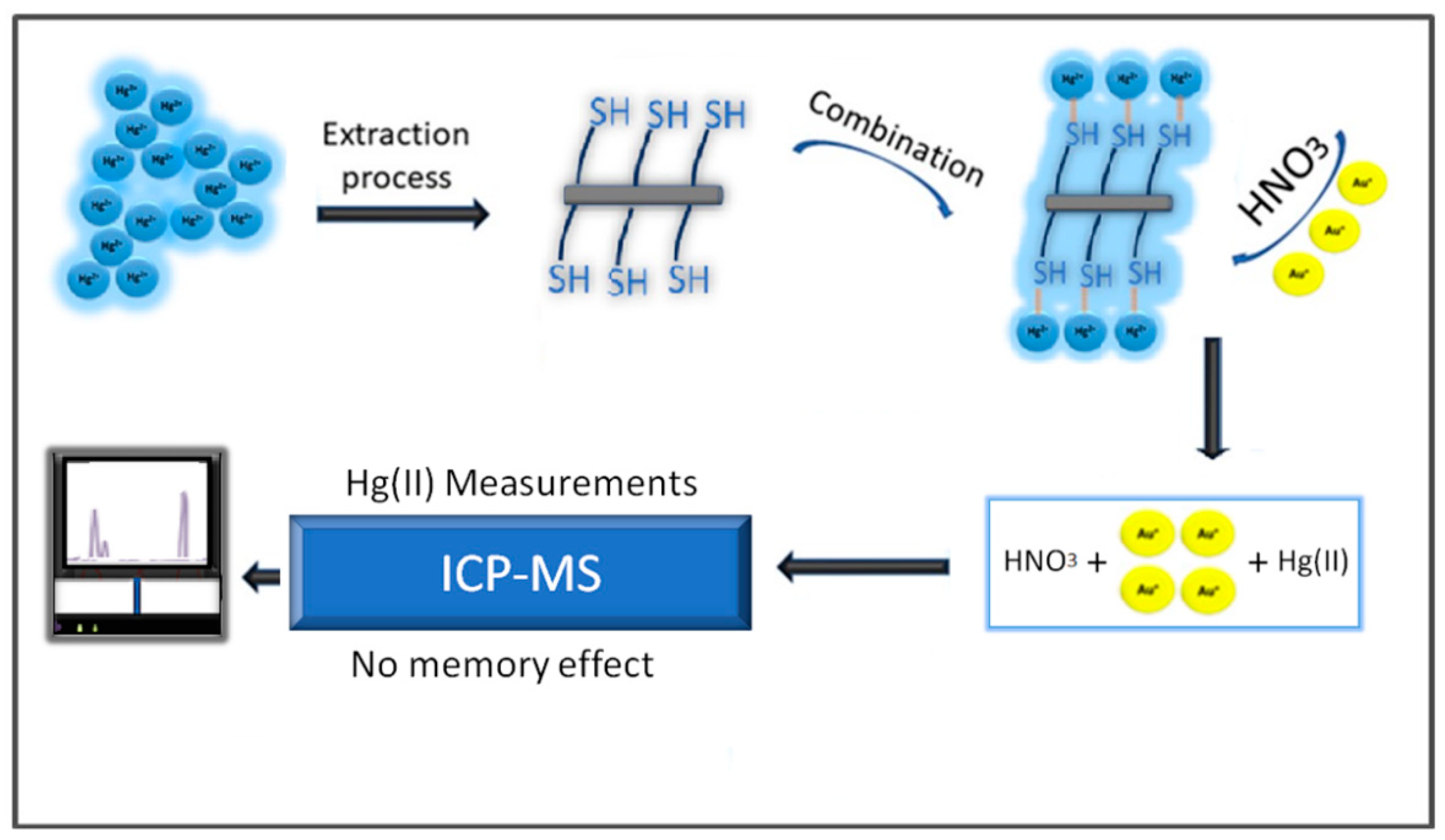
3.2.1. Memory Effect
3.2.2. The Efficiency of CF for Mercury Uptake
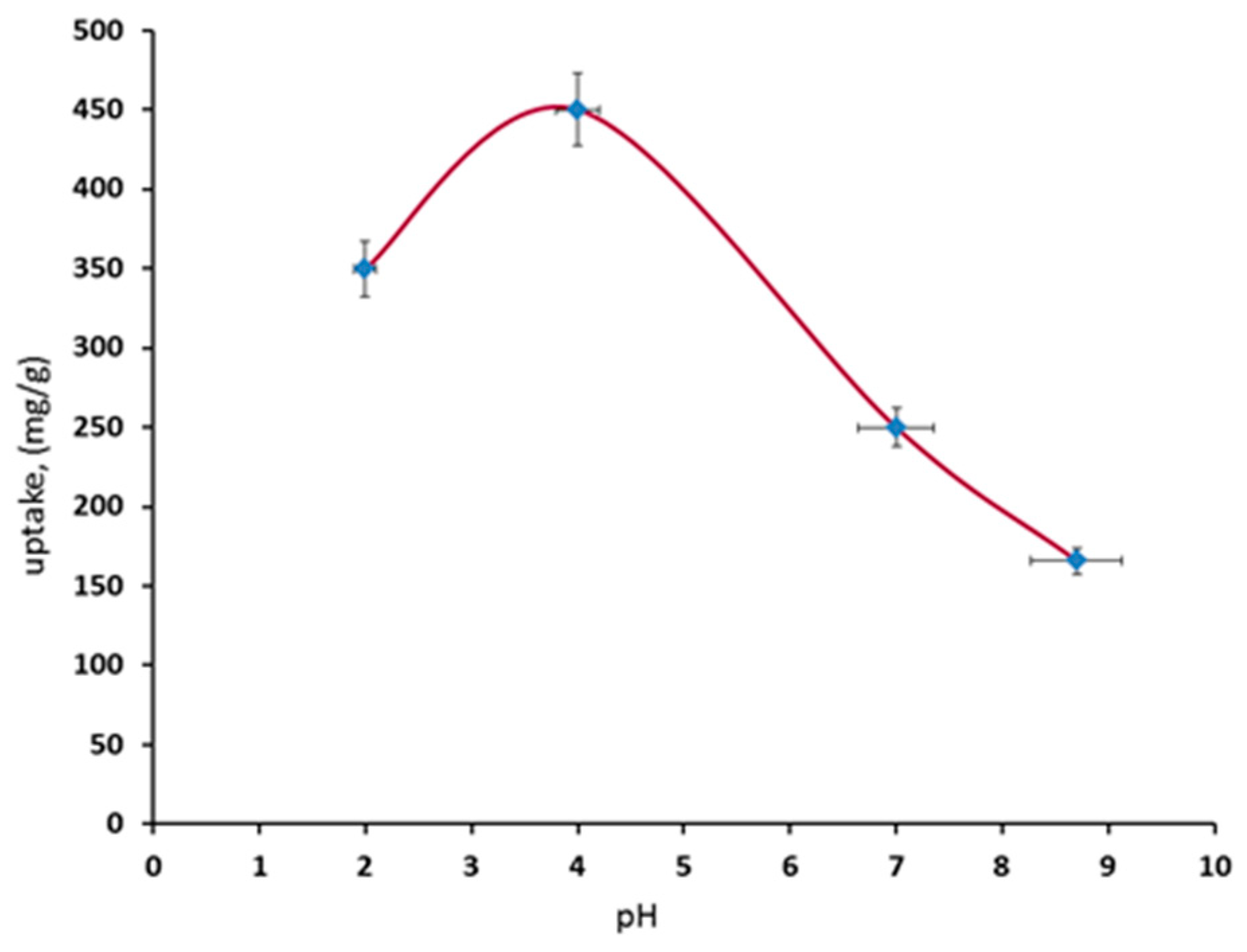
3.2.3. pH Effect
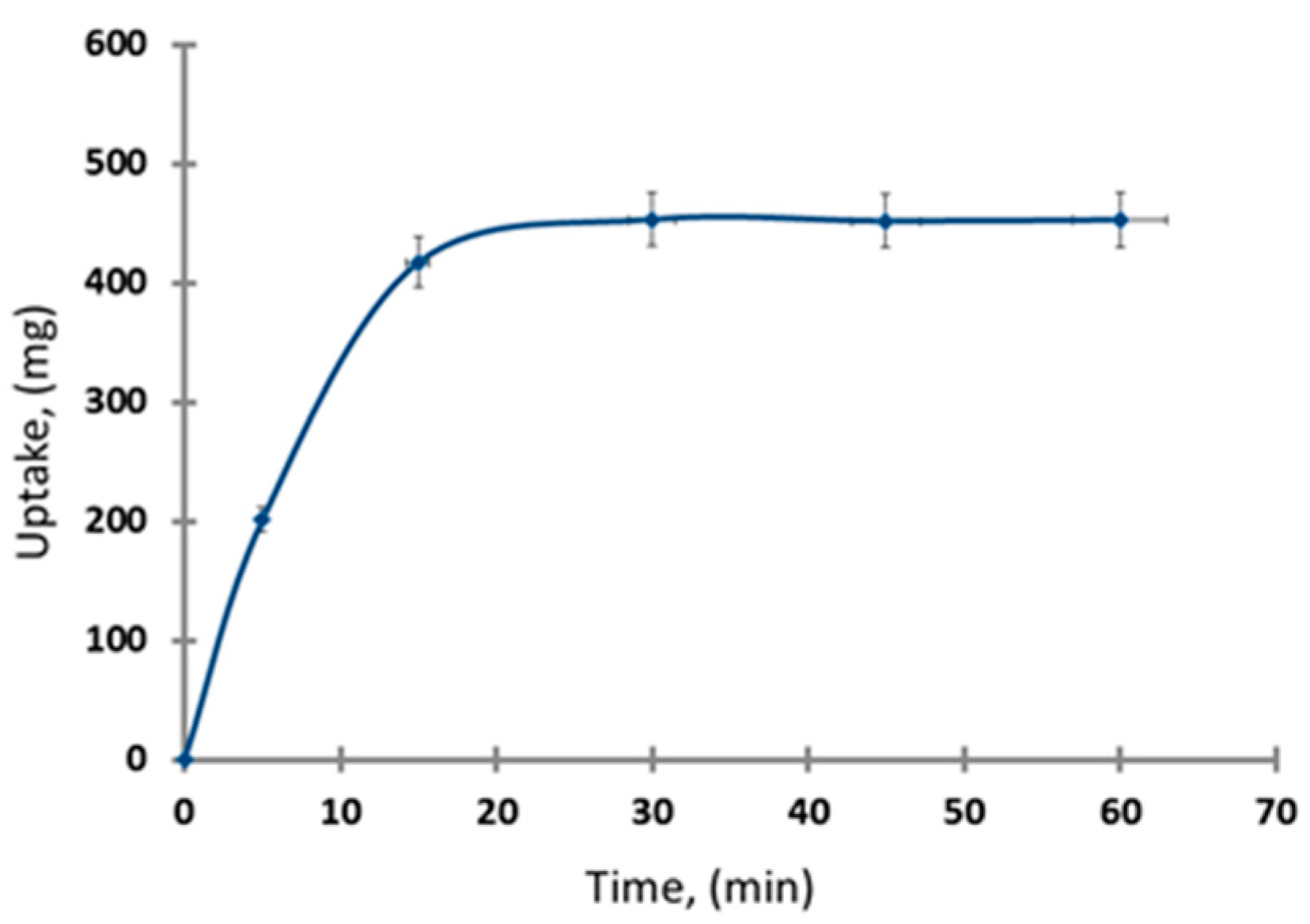
3.2.4. Equilibrium Time Effect
3.2.5. Capacity of CF
3.2.6. Preconcentration Factor (PCF)
3.2.7. Interferences with Other Cations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN Environment. Global Mercury Supply, Trade and Demand; United Nations Environment Programme, Chemicals and Health Branch: Geneva, Switzerland, 2017. [Google Scholar]
- Pirrone, N.; Mason, R. Mercury Fate and Transport in the Global Atmosphere: Emissions, Measurements and Models; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, toxicity, and remediation of mercury: An essence review. Environ. Monit. Assess. 2019, 191, 566. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef]
- Li, P.; Feng, X.B.; Qiu, G.L.; Shang, L.H.; Li, Z.G. Mercury pollution in Asia: A review of the contaminated sites. J. Hazard. Mater. 2009, 168, 591–601. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V. Mercury detoxification by absorption, mercuric ion reductase, and exopolysaccharides: A comprehensive study. Environ. Sci. Pollut. Res. 2020, 27, 27181–27201. [Google Scholar] [CrossRef]
- Dickson, J.O.; Mayes, M.A.; Brooks, S.C.; Mehlhorn, T.L.; Lowe, K.A.; Earles, J.K.; Goñez-Rodriguez, L.; Watson, D.B.; Peterson, M.J. Source relationships between streambank soils and streambed sediments in a mercury-contaminated stream. J. Soils Sediments 2019, 19, 2007–2019. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Khalid, S.; Bibi, I.; Bundschuh, J.; Niazi, N.K.; Dumat, C. A critical review of mercury speciation, bioavailability, toxicity and detoxification in soil-plant environment: Ecotoxicology and health risk assessment. Sci. Total Environ. 2020, 711, 134749. [Google Scholar]
- Deng, Y. Improvements to the Performance of Trickling Filters by Inclusion of Alternative Surface Active Media. Ph.D. Thesis, Loughborough University, Leicestershire, UK, 2018. [Google Scholar]
- Xing, G.; Sardar, M.R.; Lin, B.; Lin, J.M. Analysis of trace metals in water samples using NOBIAS chelate resins by HPLC and ICP-MS. Talanta 2019, 204, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Saint’Pierre, T.D.; Rocha, R.C.C.; Duyck, C.B. Determination of Hg in water associate to crude oil production by electrothermal vaporization inductively coupled plasma mass spectrometry. Microchem. J. 2013, 109, 41–45. [Google Scholar] [CrossRef]
- Khdary, N.H.M.; Gassim, A.E.H. The Distribution and Accretion of Some Heavy Metals in Makkah Wells. J. Water Resour. Prot. 2014, 06, 998–1010. [Google Scholar] [CrossRef] [Green Version]
- Blanco, R.M.; Villanueva, M.T.; Enrique, J.; Ur, S.; Sanz-medel, A. Field sampling, preconcentration and determination of mercury species in river waters. Anal. Chim. Acta 2000, 419, 137–144. [Google Scholar] [CrossRef]
- Zhu, X.; Alexandratos, S.D. Determination of trace levels of mercury in aqueous solutions by inductively coupled plasma atomic emission spectrometry: Elimination of the ‘memory effect’. Microchem. J. 2007, 86, 37–41. [Google Scholar] [CrossRef]
- Braaten, H.F.V.; de Wit, H.A.; Harman, C.; Hageström, U.; Larssen, T. Effects of sample preservation and storage on mercury speciation in natural stream water. Int. J. Environ. Anal. Chem. 2014, 94, 381–384. [Google Scholar] [CrossRef] [Green Version]
- Pappas, R.S. Sample preparation problem solving for inductively coupled plasma-mass spectrometry with liquid introduction systems: Solubility, chelation, and memory effects. Spectrosc. (Santa Monica) 2012, 27, 20–31. [Google Scholar]
- Allibone, J.; Fatemian, E.; Walker, P.J. Determination of mercury in potable water by ICP-MS using gold as a stabilising agent. J. Anal. At. Spectrom. 1999, 14, 235–239. [Google Scholar] [CrossRef]
- Khdary, N.H.; Howard, A.G.A.G. New solid-phase-nanoscavenger for the analytical enrichment of mercury from water. Analyst 2011, 136, 3004–3009. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, X.; Yang, D.; Guo, Y.; Meng, S. Highly selective determination of inorganic mercury (II) after preconcentration with Hg (II)-imprinted diazoaminobenzene—Vinylpyridine copolymers. Anal. Chim. Acta 2005, 538, 85–91. [Google Scholar] [CrossRef]
- Duval, B.; Gredilla, A.; de Vallejuelo, S.F.; Tessier, E.; Amouroux, D.; de Diego, A. A simple determination of trace mercury concentrations in natural waters using dispersive Micro-Solid phase extraction preconcentration based on functionalized graphene nanosheets. Microchem. J. 2020, 154, 104549. [Google Scholar] [CrossRef]
- Daye, M.; Ouddane, B.; Halwani, J.; Hamzeh, M. Solid Phase Extraction of Inorganic Mercury Using 5-Phenylazo-8-hydroxyquinoline and Determination by Cold Vapor Atomic Fluorescence Spectroscopy in Natural Water Samples. Sci. World J. 2013, 2013, 134565. [Google Scholar] [CrossRef] [Green Version]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Spedding, F.H.; Dye, J.L. The vapor pressure of mercury at 250–360°. J. Phys. Chem. 1955, 59, 581–583. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Wang, Y.; Liu, L.; Huang, Y. Interfacial properties and thermo-oxidative stability of carbon fiber reinforced methylphenylsilicone resin composites modified with polyhedral oligomeric silsesquioxanes in the interphase. RSC Adv. 2016, 6, 5032–5039. [Google Scholar] [CrossRef]
- Li, N.; Wu, Z.; Huo, L.; Zong, L.; Guo, Y.; Wang, J.; Jian, X. One-step functionalization of carbon fiber using in situ generated aromatic diazonium salts to enhance adhesion with PPBES resins. RSC Adv. 2016, 6, 70704–70714. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Sonoda, A.; Maruo, Y.; Makita, Y.; Okihara, T.; Irie, M.; Yoshida, Y.; Van Meerbeek, B. Effectiveness and stability of silane coupling agent incorporated in ‘universal’ adhesives. Dent. Mater. 2016, 32, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Huang, W.; Tang, L.; Chen, Y.; Zhang, Y.; Wu, M.; Song, Y.; Wen, S. Electrospun nanofibrous mercury filter: Efficient concentration and determination of trace mercury in water with high sensitivity and tunable dynamic range. Anal. Chim. Acta 2017, 982, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Sharma, S.; Mirajkar, S.P.; Singh, A.P. Silica functionalised sulphonic acid groups: Synthesis, characterization and catalytic activity in acetalization and acetylation reactions. J. Mol. Catal. A Chem. 2004, 212, 219–228. [Google Scholar] [CrossRef]
- Franquelo, M.L.; Duran, A.; Herrera, L.K.; de Jimenez, H.M.C.; Perez-Rodriguez, J.L. Comparison between micro-Raman and micro-FTIR spectroscopy techniques for the characterization of pigments from Southern Spain Cultural Heritage. J. Mol. Struct. 2009, 924–926, 404–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, A.; Xiong, M.; Macharia, D.K.; Liu, J.; Chen, Z.; Li, M.; Zhang, L. TiO2/BiOI p-n junction-decorated carbon fibers as weavable photocatalyst with UV–vis photoresponsive for efficiently degrading various pollutants. Chem. Eng. J. 2021, 415, 129019. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, T.; Xu, P.; Zhang, L.; Liu, J.; Chen, Z. Growth of C3N4 nanosheets on carbon-fiber cloth as flexible and macroscale filter-membrane-shaped photocatalyst for degrading the flowing wastewater. Appl. Catal. B Environ. 2017, 219, 425–431. [Google Scholar] [CrossRef]
- Luhrs, C.C.; Moberg, M.; Maxson, A.; Brewer, L.; Menon, S. IF-WS2/nanostructured carbon hybrids generation and their characterization. Inorganics 2014, 2, 211–232. [Google Scholar] [CrossRef] [Green Version]
- Tunç, I.D.; Erol, M.; Güneş, F.; Sütçü, M. Growth of ZnO nanowires on carbon fibers for photocatalytic degradation of methylene blue aqueous solutions: An investigation on the optimization of processing parameters through response surface methodology/central composite design. Ceram. Int. 2020, 46, 7459–7474. [Google Scholar] [CrossRef]
- Kaewprasit, C.; Abidi, N.; Gourlot, J.P. Effect of adsorbed water on the specific surface area of some standards cotton. In Proceedings of the Beltwide Cotton Conference, Orlandao, FL, USA, 3–7 January 1999; Volume 1, pp. 710–711. [Google Scholar]
- Dias Filho, N.L.; Caetano, L.; Do Carmo, D.R.; Rosa, A.H. Preparation of a silica gel modified with 2-amino-1,3,4-thiadiazole for adsorption of metal ions and electroanalytical application. J. Braz. Chem. Soc. 2006, 17, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F.; Zaghrioui, M.; Mabkhot, Y.N. Organically modified Silica with Pyrazole-3-carbaldehyde as a new sorbent for Solid-Liquid Extraction of heavy metals. Molecules 2014, 19, 247–262. [Google Scholar] [CrossRef]
- Bi, W.; Sun, J.; Yu, G.; Goegelein, C.; Hoch, M.; Klaassen, J.; Kirchhoff, J.; Zhao, S. Study on Interaction between Aluminum Hydroxide and Vinyltriethoxy Silane by Gas Chromatography-Mass Spectrometry; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 300. [Google Scholar]
- Congiusta, C.; Granleese, J.Y.; Graiver, D.; Hoffman, L.; Mathew, S.; Clarke, D.; Johnston, M.; Clarke, S.R. Novel grafting onto silica via aldehyde functionality. Silicon 2009, 1, 29–36. [Google Scholar] [CrossRef]
- Chen, J. Determination of Mercury in Wastewater by Inductively Coupled Plasma-Mass Spectrometry; PerkinElmer: Walthan, MA, USA, 2011. [Google Scholar]
- Dressler, V.L.; Pozebon, D.; Curtius, A.J. Determination of heavy metals by inductively coupled plasma mass spectrometry after on-line separation and preconcentration. Spectrochim. Acta Part B At. Spectrosc. 1998, 53, 1527–1539. [Google Scholar] [CrossRef]
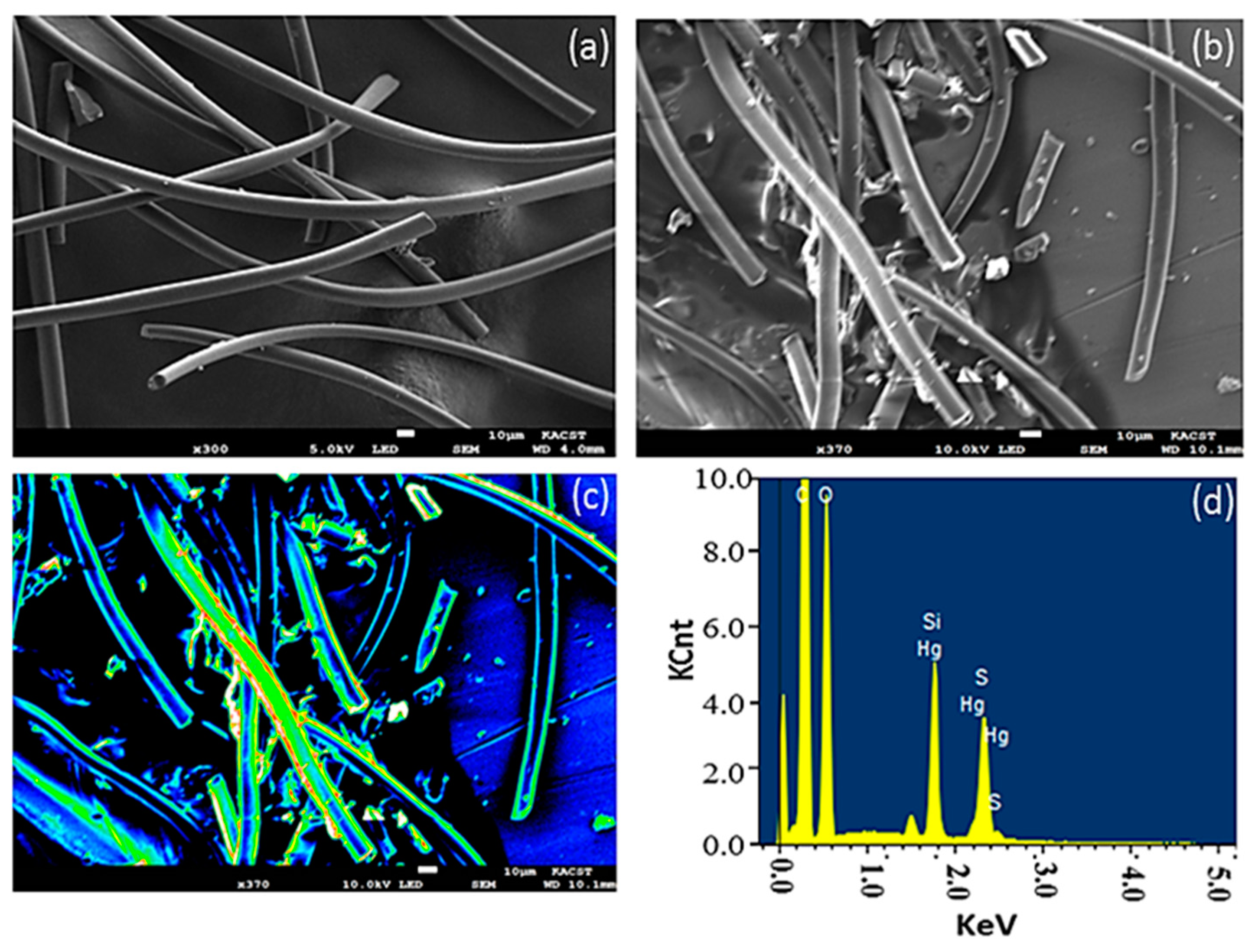
| Element | Weight % | Atomic % |
|---|---|---|
| C K | 61.63 | 72.05 |
| O K | 25.93 | 22.76 |
| Si k | 5.31 | 2.66 |
| S K | 5.52 | 2.42 |
| Hg M | 1.61 | 0.11 |
| SDT (n = 3) | Average | Recovery (%) | Eluent |
|---|---|---|---|
| 1.54 | 12.47 | 11 | 2% HNO3 |
| 14.07 | |||
| 12.35 | |||
| 1.42 | 76.12 | 74.87 | 2% HNO3-Au(III) |
| 77.66 | |||
| 75.83 | |||
| 0.75 | 99.27 | 99.84 | 2% HNO3-Au(NP) |
| 98.42 | |||
| 99.55 |
| SDT | Average (n = 3) | mg/g | Capacity |
|---|---|---|---|
| 77.17 | 76.8 | CF | |
| ±2.50 | 80.4 | ||
| 74.3 | |||
| 130.8 | 127.2 | CF-OH | |
| ±3.42 | 135.41 | ||
| 129.8 | |||
| 422.55 | 414.61 | CF-SH | |
| ±5.63 | 425.98 | ||
| 427.05 |
| Average (n = 3) | PCF | Substance |
|---|---|---|
| 98.96 ± 0.41 | 10 | CF-SH |
| 95.63 ± 1.09 | 50 | |
| 94.62 ± 1.87 | 100 | |
| 38.49 ± 3.47 | 50 | CF-OH |
| 17.85 ± 0.61 | 50 | CF |
| Recovery Percent of Hg | Concentration (mg L−1) | Cations |
|---|---|---|
| 96.1 ± 1.2 | 76.4 ± 1.2 | Na |
| 0.7 ± 0.10 | K | |
| 91.7 ± 1.4 | Mg | |
| 3.7 ± 1.3 | Ca | |
| 47.7 ± 1.3 | Ba | |
| 447.5 ± 2.2 | Cr | |
| 19.9 ± 3.8 | Fe | |
| 257.2 ± 2.4 | Co | |
| 3650.2 ± 3.4 | Ni | |
| 69.8 ± 2.2 | Cu | |
| 1.76 ± 2.0 | Zn | |
| 17.7 ± 1.3 | As | |
| 1198.8 ± 2.6 | Mo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Ateeq, M.K.; Bin Durayhim, N.M.; Sulayem, M.M.; Al-Qahtani, W.A.; Khdary, N.H.; Alhassan, A.M.; Alzahrani, F.M.A.; Katubi, K.M.M.; Alsaiari, N.S. A Functionalised Carbon Fiber for Flexible Extraction and Determination of Hg(II) Using Au(NP)-Thiol-CF Inductively Coupled Plasma Mass Spectrometry. Water 2021, 13, 1829. https://doi.org/10.3390/w13131829
Bin Ateeq MK, Bin Durayhim NM, Sulayem MM, Al-Qahtani WA, Khdary NH, Alhassan AM, Alzahrani FMA, Katubi KMM, Alsaiari NS. A Functionalised Carbon Fiber for Flexible Extraction and Determination of Hg(II) Using Au(NP)-Thiol-CF Inductively Coupled Plasma Mass Spectrometry. Water. 2021; 13(13):1829. https://doi.org/10.3390/w13131829
Chicago/Turabian StyleBin Ateeq, Mashael K., Nouf M. Bin Durayhim, Meral M. Sulayem, Waad A. Al-Qahtani, Nezar H. Khdary, Ahmed M. Alhassan, Fatimah Mohammed A. Alzahrani, Khadijah Mohammedsaleh M. Katubi, and Norah Salem Alsaiari. 2021. "A Functionalised Carbon Fiber for Flexible Extraction and Determination of Hg(II) Using Au(NP)-Thiol-CF Inductively Coupled Plasma Mass Spectrometry" Water 13, no. 13: 1829. https://doi.org/10.3390/w13131829
APA StyleBin Ateeq, M. K., Bin Durayhim, N. M., Sulayem, M. M., Al-Qahtani, W. A., Khdary, N. H., Alhassan, A. M., Alzahrani, F. M. A., Katubi, K. M. M., & Alsaiari, N. S. (2021). A Functionalised Carbon Fiber for Flexible Extraction and Determination of Hg(II) Using Au(NP)-Thiol-CF Inductively Coupled Plasma Mass Spectrometry. Water, 13(13), 1829. https://doi.org/10.3390/w13131829







