Temporal and Spatial Distribution of Microplastics in a Coastal Region of the Pearl River Estuary, China
Abstract
:1. Introduction
2. Materials and Methods
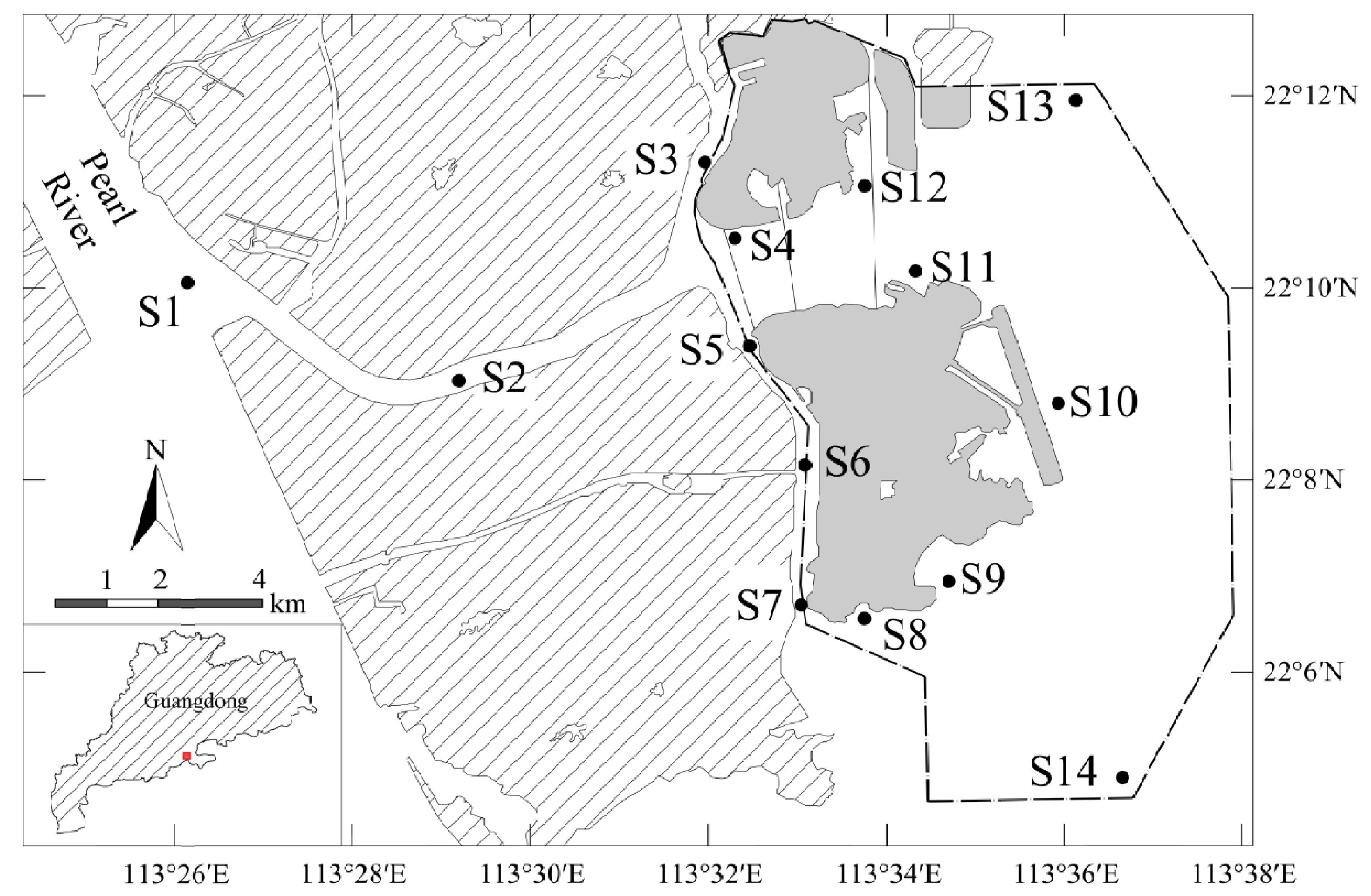
2.1. Study Area
2.2. Collection of Water Surface Samples
2.3. Sample Processing and Analysis
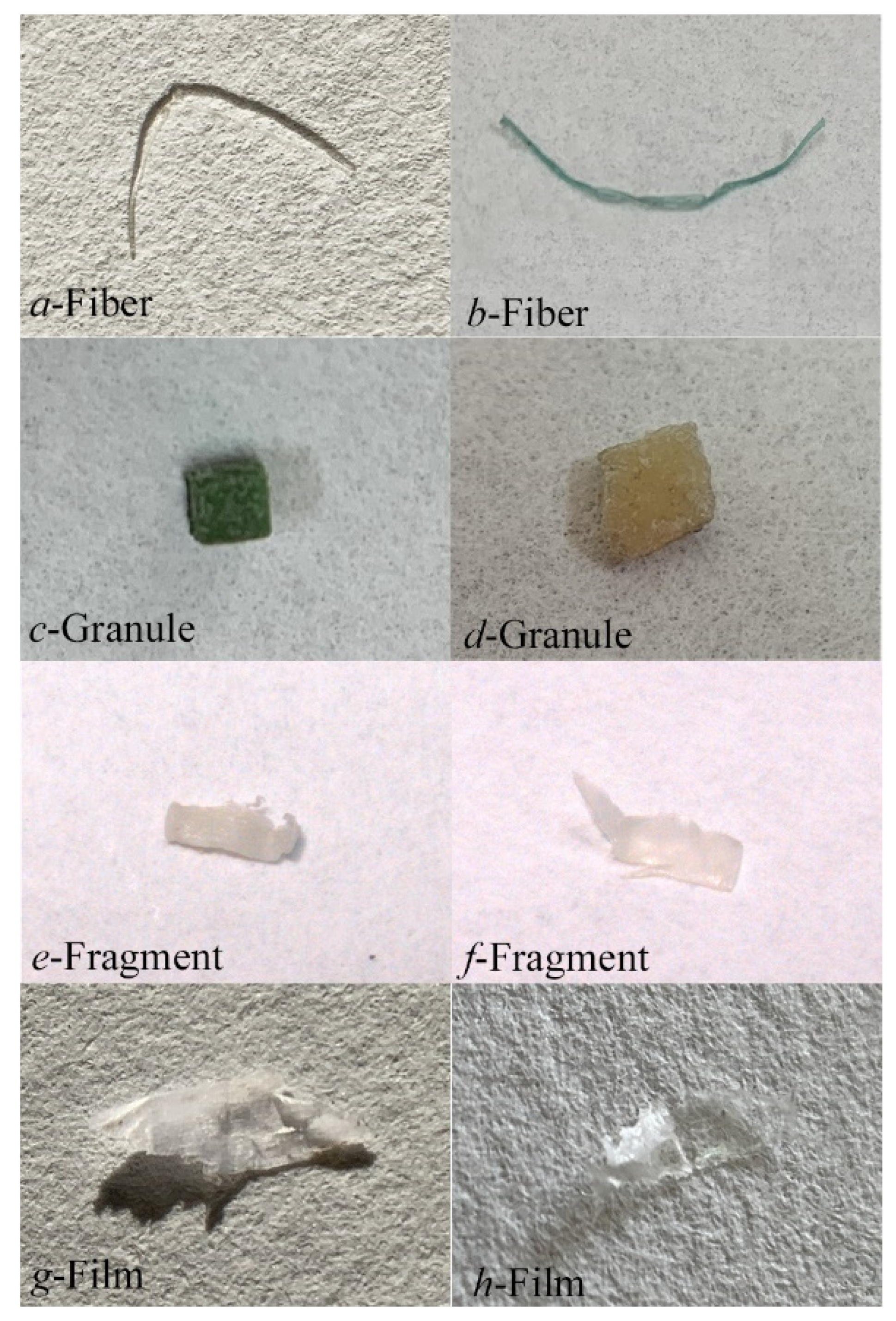
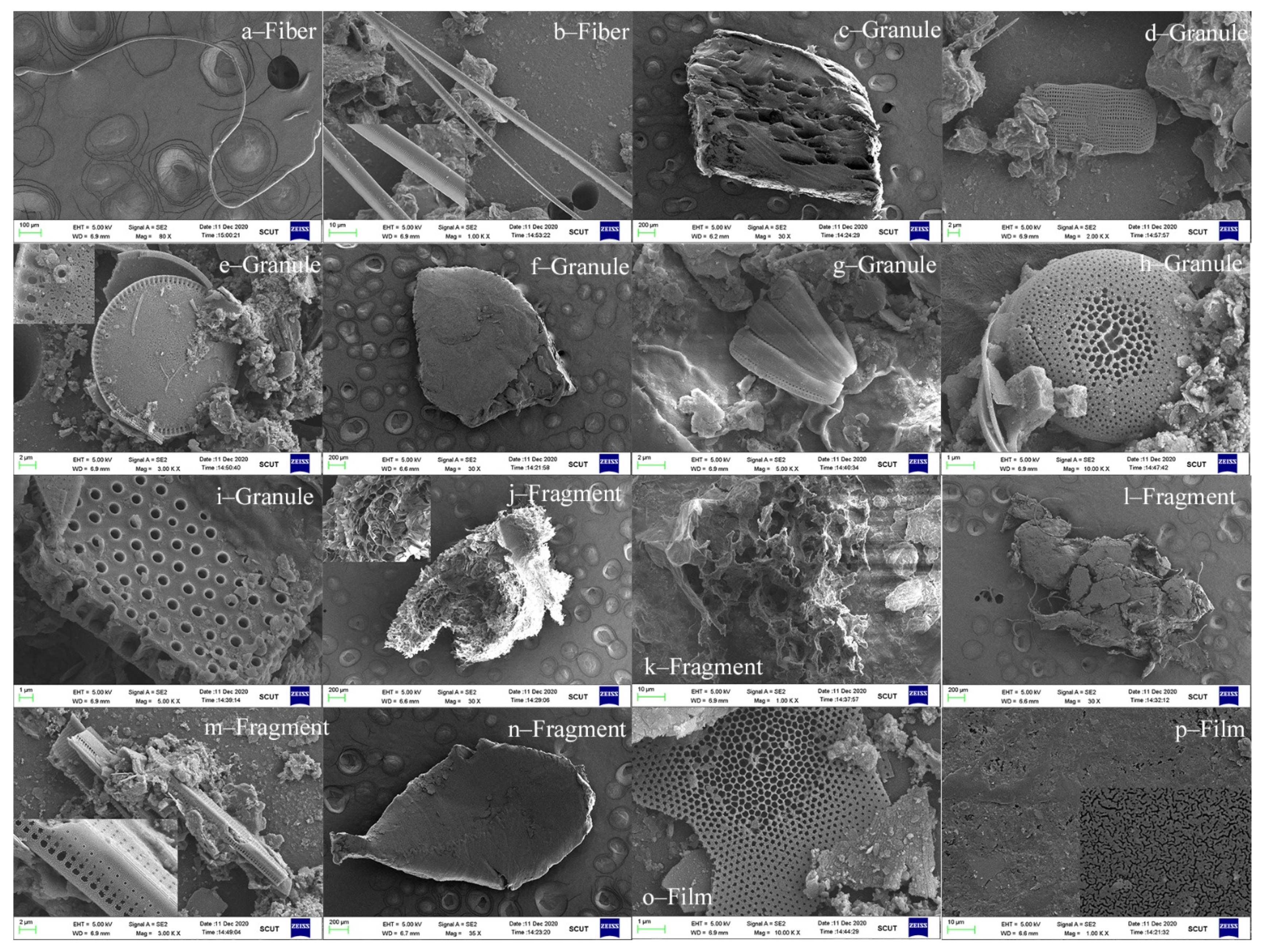
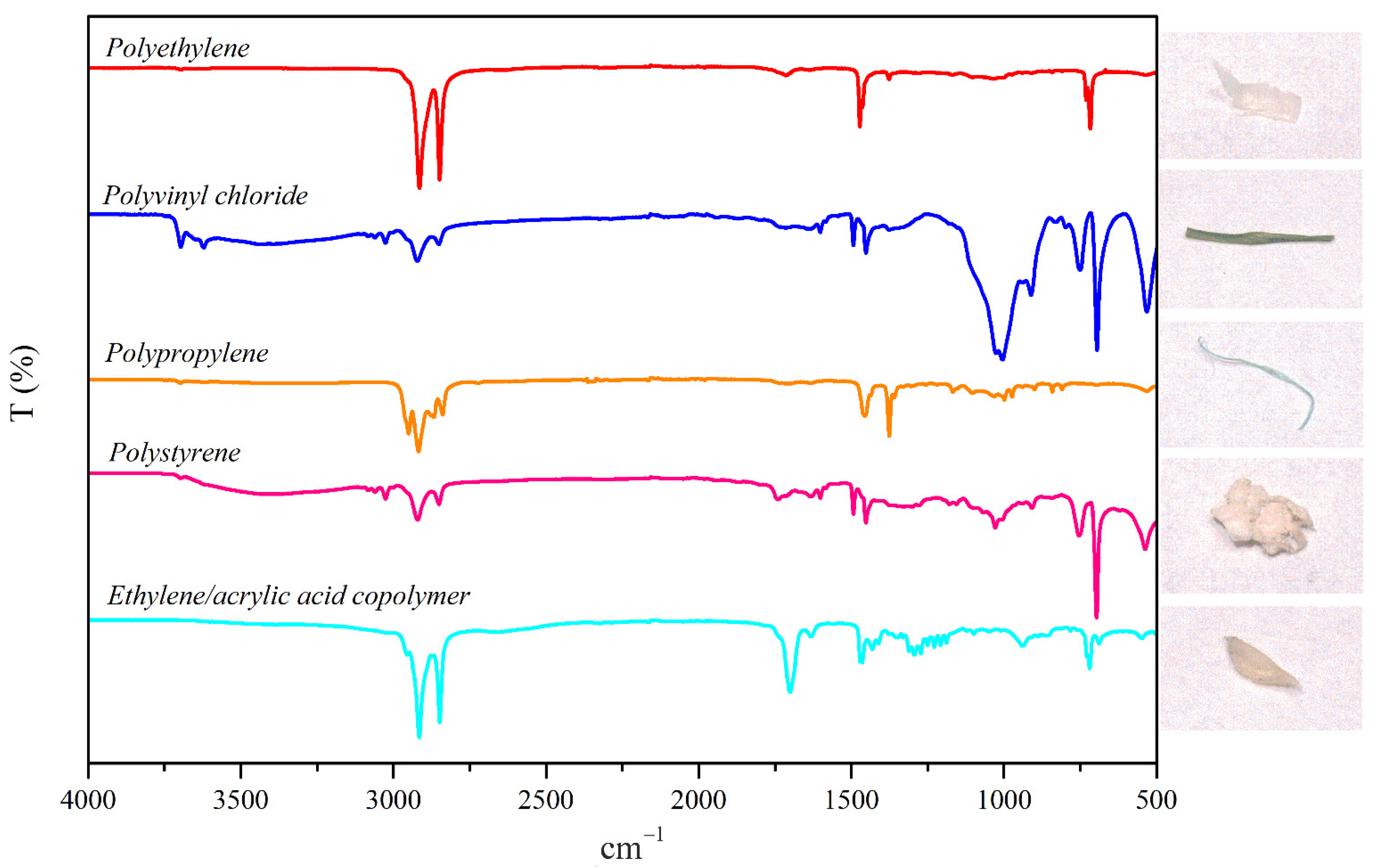
2.4. MPs Identification
2.5. Data Analysis
3. Result and Discussion
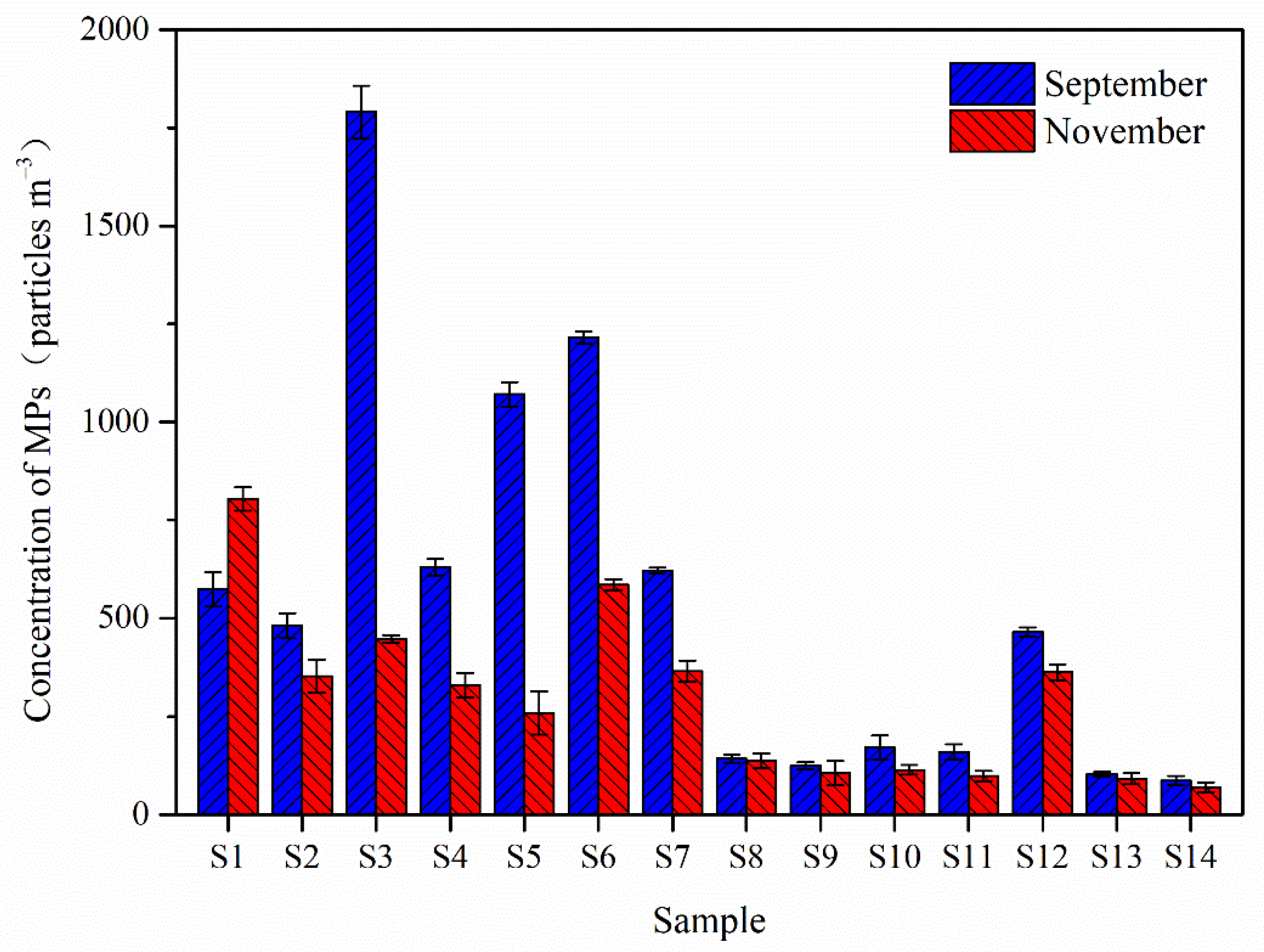
3.1. Abundance
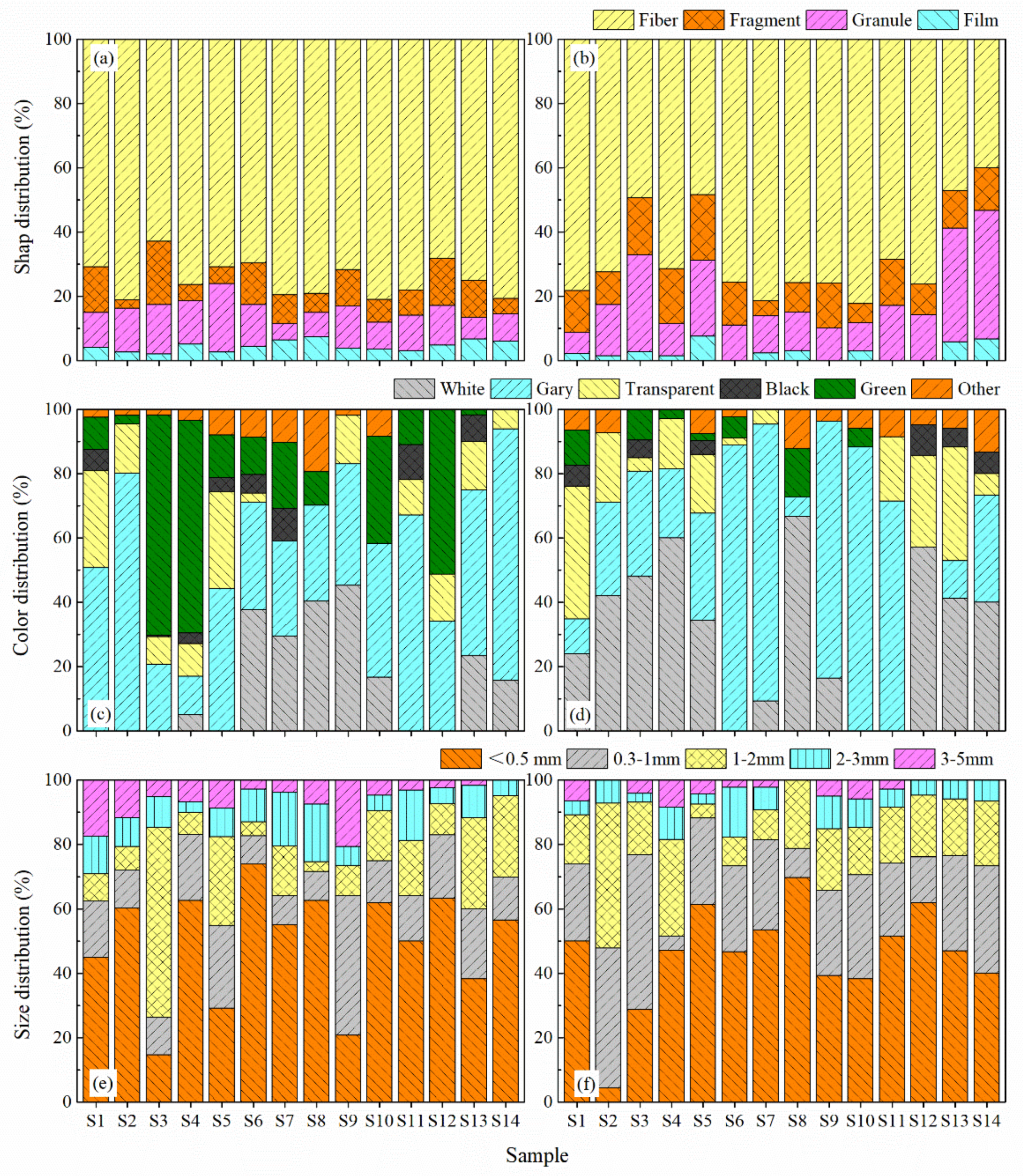
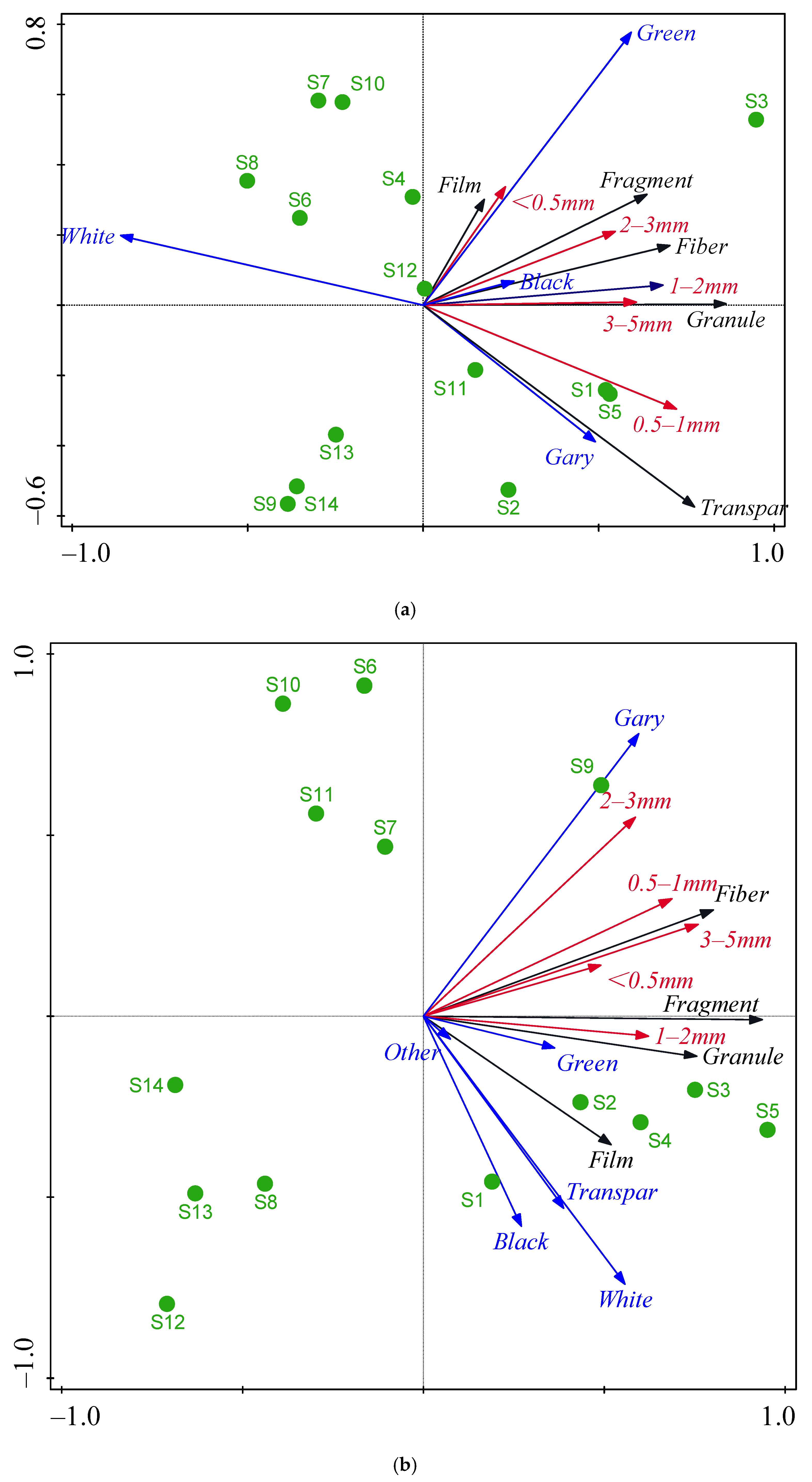
3.2. Spatial Distribution of MP Characteristics
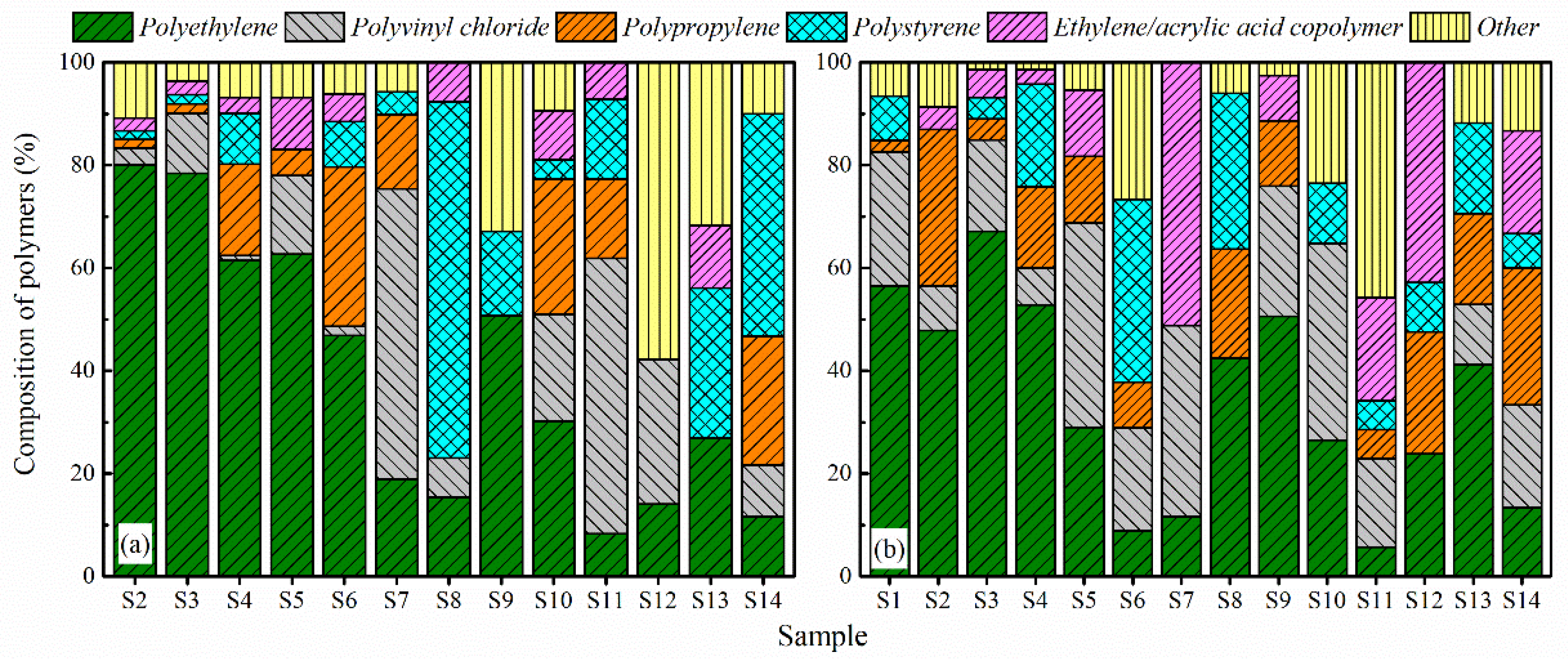
3.3. MPs Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Longitude | Latitude |
|---|---|---|
| S1 | 113°26′37.21″ | 22°10′02.17″ |
| S2 | 113°28′57.19″ | 22°09′23.42″ |
| S3 | 113°32′06.25″ | 22°11′51.77″ |
| S4 | 113°32′25.14″ | 22°10′32.51″ |
| S5 | 113°32′18.60″ | 22°09′38.75″ |
| S6 | 113°32′57.06″ | 22°08′01.08″ |
| S7 | 113°32′55.44″ | 22°06′48.36″ |
| S8 | 113°33′37.26″ | 22°06′37.87″ |
| S9 | 113°34′30.60″ | 22°07′10.50″ |
| S10 | 113°35′51.65″ | 22°08′47.48″ |
| S11 | 113°34′11.02″ | 22°10′16.24″ |
| S12 | 113°33′36.60″ | 22°11′23.58″ |
| S13 | 113°35′40.00″ | 22°12′14.80″ |
| S14 | 113°36′36.80″ | 22°04′36.00″ |
References
- Taïbi, N.-E.; Bentaallah, M.E.A.; Alomar, C.; Compa, M.; Deudero, S. Micro- and macro-plastics in beach sediment of the Algerian western coast: First data on distribution, characterization, and source. Mar. Pollut. Bull. 2021, 165, 112168. [Google Scholar] [CrossRef]
- Ma, J.; Niu, X.; Zhang, D.; Lu, L.; Ye, X.; Deng, W.; Li, Y.; Lin, Z. High levels of microplastic pollution in aquaculture water of fish ponds in the Pearl River Estuary of Guangzhou, China. Sci. Total Environ. 2020, 744, 140679. [Google Scholar] [CrossRef]
- Prata, J.C.; Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Ocaya, H.; Pabire, W.G. Microplastic pollution in surface water of Lake Victoria. Sci. Total Environ. 2020, 741, 140201. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.A.; Ferreira, G.V.B.; Barrows, A.P.W.; Christiansen, K.S.; Treinish, G.; Toshack, M.C. Global patterns for the spatial distribution of floating microfibers: Arctic Ocean as a potential accumulation zone. J. Hazard. Mater. 2021, 403, 123796. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.; Cabrera, M.; Capparelli, M.V. Assessment of Microplastic and Organophosphate Pesticides Contamination in Fiddler Crabs from a Ramsar Site in the Estuary of Guayas River, Ecuador. Bull. Environ. Contam. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Akanyange, S.N.; Lyu, X.; Zhao, X.; Li, X.; Zhang, Y.; Crittenden, J.C.; Anning, C.; Chen, T.; Jiang, T.; Zhao, H. Does microplastic really represent a threat? A review of the atmospheric contamination sources and potential impacts. Sci. Total Environ. 2021, 777, 146020. [Google Scholar] [CrossRef]
- Mbachu, O.; Jenkins, G.; Kaparaju, P.; Pratt, C. The rise of artificial soil carbon inputs: Reviewing microplastic pollution effects in the soil environment. Sci. Total Environ. 2021, 780, 146569. [Google Scholar] [CrossRef]
- Suaria, G.; Perold, V.; Lee, J.R.; Lebouard, F.; Aliani, S.; Ryan, P.G. Floating macro- and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020, 136, 105494. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, D.K.; Gardfeldt, K.; Krumpen, T.; Thompson, R.C.; O’Connor, I. Microplastics in sea ice and seawater beneath ice floes from the Arctic Ocean. Sci. Rep. 2020, 10, 5004. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, P.; Pizzul, E.; Bertoli, M.; Anselmi, S.; Kusce, M.; Menconi, V.; Prearo, M.; Renzi, M. First insights into plastic and microplastic occurrence in biotic and abiotic compartments, and snow from a high-mountain lake (Carnic Alps). Chemosphere 2021, 265, 129121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. Int. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester Textiles as a Source of Microplastics from Households: A Mechanistic Study to Understand Microfiber Release during Washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.; Ragas, A.M. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Kovač Viršek, M.; Palatinus, A.; Koren, Š.; Peterlin, M.; Horvat, P.; Kržan, A. Protocol for Microplastics Sampling on the Sea Surface and Sample Analysis. JoVE 2016, e55161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xing, R.; Sun, M.; Ling, W.; Shi, W.; Cui, S.; An, L. Microplastics profile in a typical urban river in Beijing. Sci. Total Environ. 2020, 743, 140708. [Google Scholar] [CrossRef] [PubMed]
- Koongolla, J.B.; Andrady, A.; Kumara, P.T.P.; Gangabadage, C. Evidence of microplastics pollution in coastal beaches and waters in southern Sri Lanka. Mar. Pollut. Bull. 2018, 137, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Eo, S.; Jang, M.; Han, G.M.; Isobe, A.; Shim, W.J. Horizontal and vertical distribution of microplastics in Korean coastal waters. Environ. Sci. Technol. 2018, 52, 12188–12197. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics? -Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616, 1620–1627. [Google Scholar] [CrossRef]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Tsang, Y.Y.; Mak, C.W.; Liebich, C.; Lam, S.W.; Sze, E.T.; Chan, K.M. Microplastic pollution in the marine waters and sediments of Hong Kong. Mar. Pollut. Bull. 2017, 115, 20–28. [Google Scholar] [CrossRef]
- Liu, S.; Jian, M.; Zhou, L.; Li, W. Distribution and characteristics of microplastics in the sediments of Poyang Lake, China. Water Sci. Technol. 2019, 79, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Sruthy, S.; Ramasamy, E. Microplastic pollution in Vembanad Lake, Kerala, India: The first report of microplastics in lake and estuarine sediments in India. Environ. Pollut. 2017, 222, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.-M.; Bolan, N.S.; Tsang, D.C.W.; Li, Y.; Qin, M.; Hou, D. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. J. Hazard. Mater. 2021, 401. [Google Scholar] [CrossRef]
- Su, L.; Sharp, S.M.; Pettigrove, V.J.; Craig, N.J.; Nan, B.; Du, F.; Shi, H. Superimposed microplastic pollution in a coastal metropolis. Water Res. 2020, 168, 115140. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- Pastrnak, A.; Henriquez, A.; La Saponara, V. Parametric study for tensile properties of molded high-density polyethylene for applications in additive manufacturing and sustainable designs. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Ciacci, L.; Passarini, F.; Vassura, I. The European PVC cycle: In-use stock and flows. Resour. Conserv. Recycl. 2017, 123, 108–116. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef]
| Area * | Rain Season | Dry Season | ||
|---|---|---|---|---|
| Range | Average | Range | Average | |
| River | 481.2–1790.5 | 948.5 | 352.6–803.6 | 534.5 |
| Estuary | 629.6–1215 | 884.3 | 259.2–585.0 | 385.0 |
| Sea | 87.4–264.9 | 150.5 | 69.7–162.4 | 111.5 |
| Location | Regions | Sample Type | Microplastic Particle Detection Range (mm) | MP Abundance | Source * |
|---|---|---|---|---|---|
| Sri Lanka | Southern seas | Ocean | 1.5–2.5 | 0–29 | (Koongolla et al., 2018) |
| Korea | Coastal oceans | Ocean | 0.02–5 | 448–2000 | (Song et al., 2018) |
| Britain | Coastal oceans | Ocean | 0.005–5 | 1500–6700 | (Li et al., 2018) |
| China | Wuhan | Lake | 0.05–5 | 1160–8925 | (Wang et al., 2017) |
| Yangtze River | River | 0.03–5 | 500–10,200 | (Zhao et al., 2014) | |
| Sanjiangkou | Estuary | 0.33–5 | 100–4100 | (Zhao et al., 2015) | |
| Danjiangkou | Reservoir | 0.05–5 | 467–15,017 | (Di et al., 2019) | |
| Hong Kong | Ocean | 0.03–4.96 | 0.51–279.1 | (Tsang et al., 2017) | |
| Boyang Lake | Sediment | 0.002–5 | 11–3153 * | (Liu et al., 2019) | |
| Hong Kong | Sediment | 0.135–5 | 106–15,554 ** | (Fok and Cheung, 2015) | |
| India | Vembanad Lake | Sediment | less than 5 | 252.8 ** | (Sruthy and Ramasamy, 2017) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, Y.; Liu, L.; Lai, H.; Zeng, X.; Chen, J.; Liu, C.; Luo, Q. Temporal and Spatial Distribution of Microplastics in a Coastal Region of the Pearl River Estuary, China. Water 2021, 13, 1618. https://doi.org/10.3390/w13121618
Li S, Wang Y, Liu L, Lai H, Zeng X, Chen J, Liu C, Luo Q. Temporal and Spatial Distribution of Microplastics in a Coastal Region of the Pearl River Estuary, China. Water. 2021; 13(12):1618. https://doi.org/10.3390/w13121618
Chicago/Turabian StyleLi, Siyang, Yilin Wang, Lihong Liu, Houwei Lai, Xiancan Zeng, Jianyu Chen, Chang Liu, and Qijin Luo. 2021. "Temporal and Spatial Distribution of Microplastics in a Coastal Region of the Pearl River Estuary, China" Water 13, no. 12: 1618. https://doi.org/10.3390/w13121618
APA StyleLi, S., Wang, Y., Liu, L., Lai, H., Zeng, X., Chen, J., Liu, C., & Luo, Q. (2021). Temporal and Spatial Distribution of Microplastics in a Coastal Region of the Pearl River Estuary, China. Water, 13(12), 1618. https://doi.org/10.3390/w13121618





