Temperature Dependence of Freshwater Phytoplankton Growth Rates and Zooplankton Grazing Rates
Abstract
1. Introduction
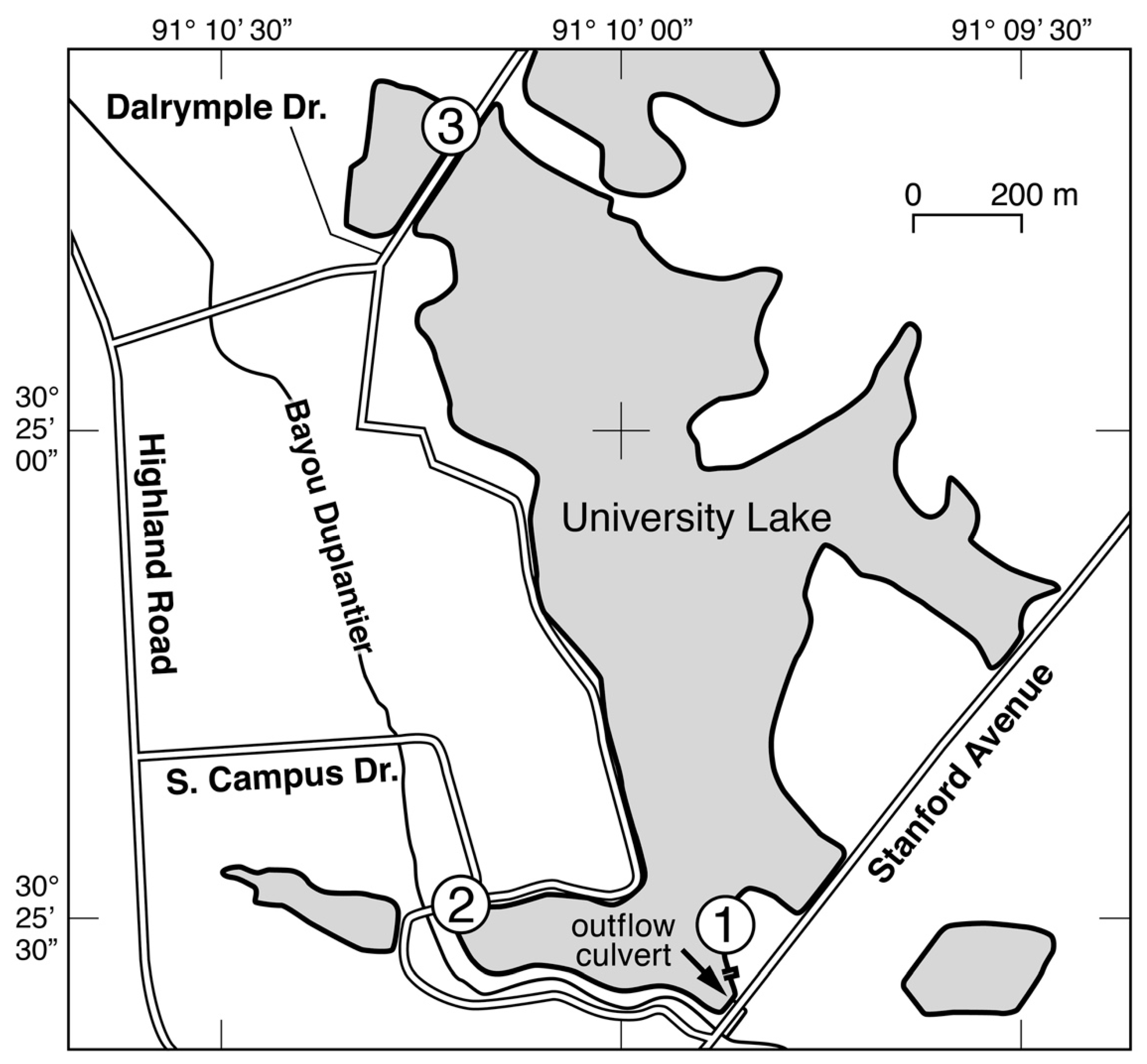
2. Materials and Methods
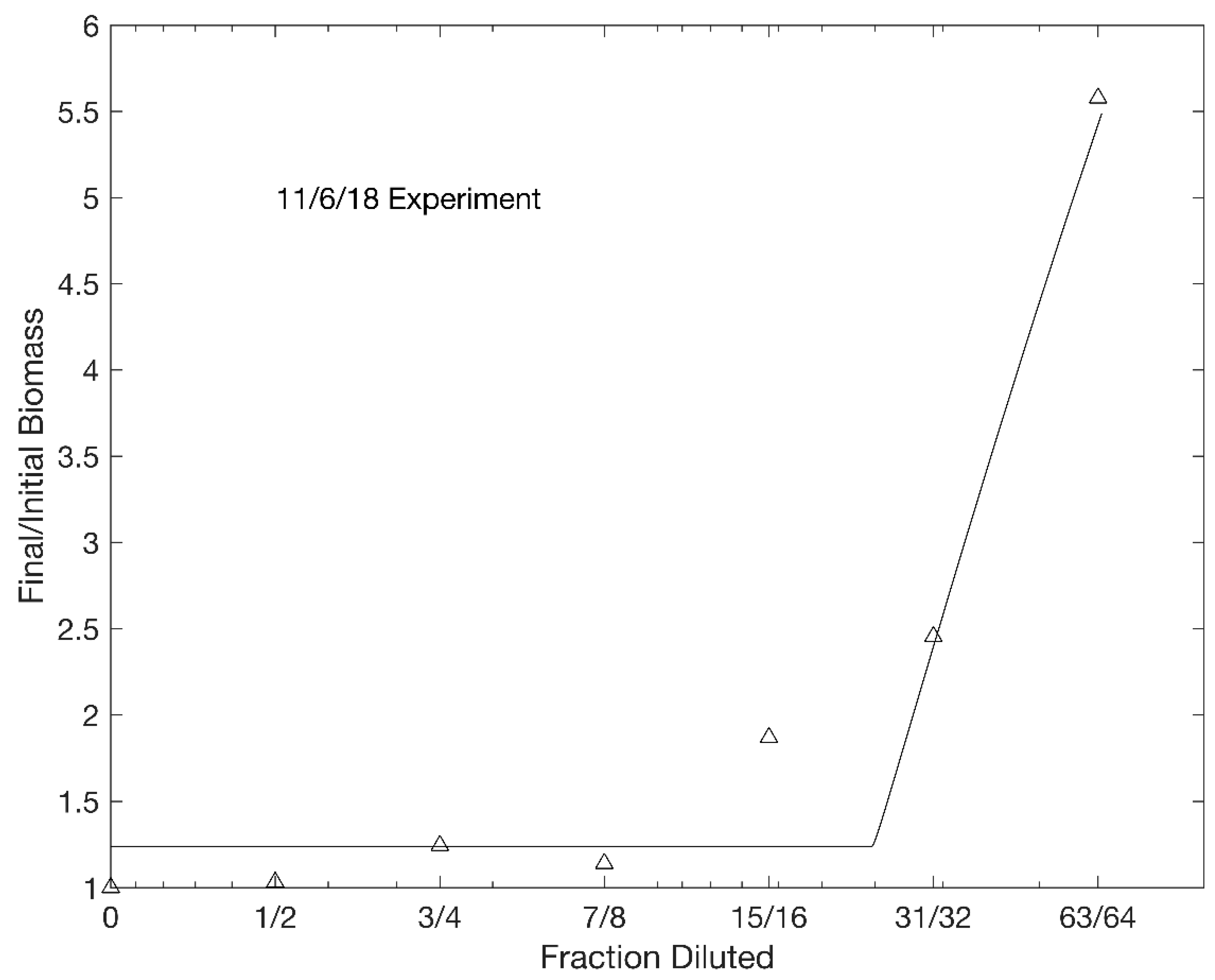
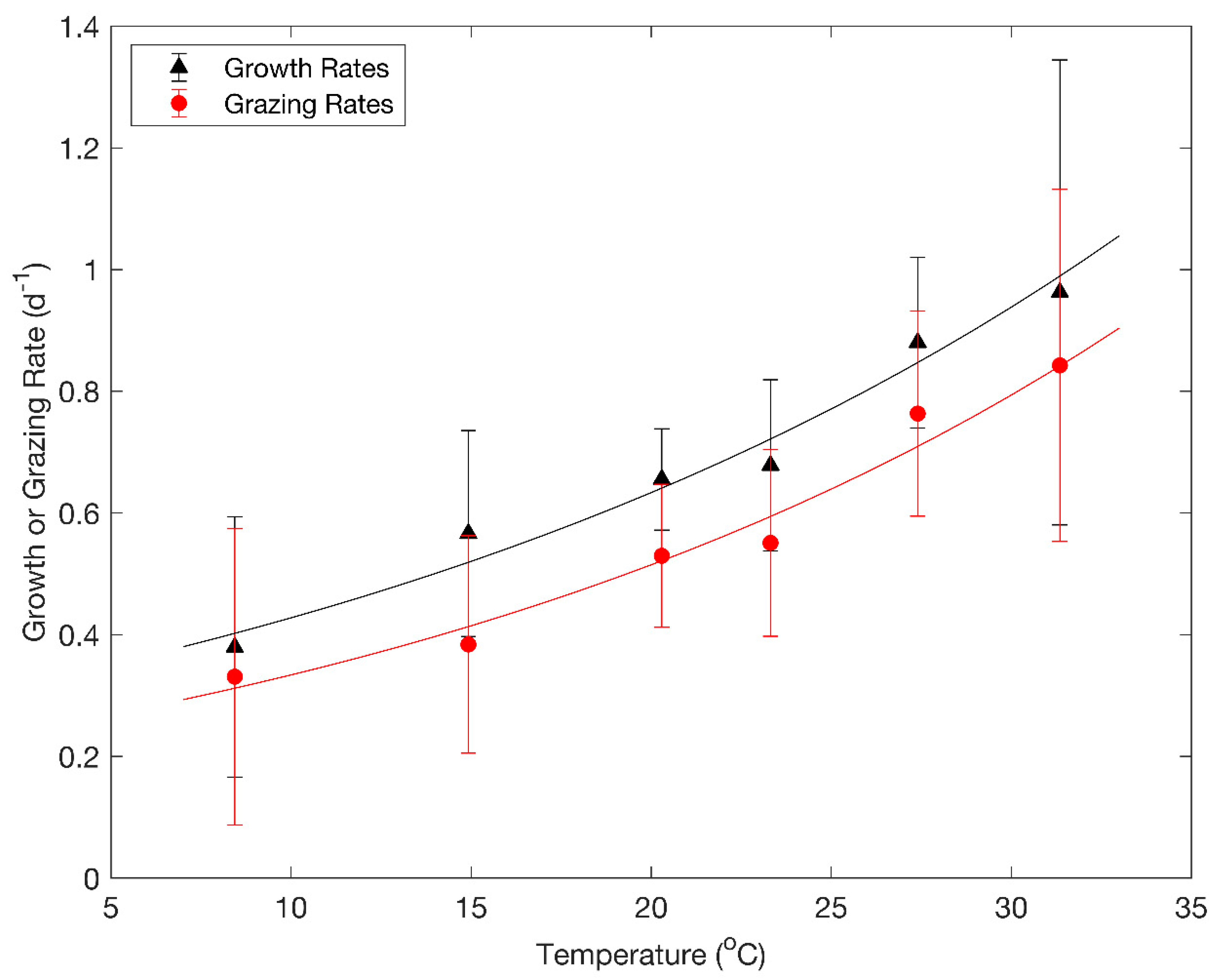
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Date | Initial Chlorophyll (mg L−1) | Temp. (°C) | μ (d−1) 1 | g (d−1) 2 | Gmax (mg chl a L−1 d−1) 3 | K (mg chl a L−1) 4 | R2 5 |
|---|---|---|---|---|---|---|---|
| 10/15/18 | 372 | 26.2 | 0.95 | 1.10 | 408 | 21 | 0.895 |
| 10/26/18 | 156 | 20.3 | 2.1 | 2.00 | 312 | 8 | 0.876 |
| 11/6/18 | 148 | 22.2 | 2.65 | 2.59 | 384 | 3 | 0.967 |
| 11/12/18 | 137 | 16.8 | 0.75 | 0.70 | 96 | 1.5 | 0.839 |
| 11/18/18 | 96.7 | 11 | 1.5 | 1.49 | 144 | 1 | 0.915 |
| 12/3/18 | 87 | 14 | 0.2 | 0.28 | 24 | 5 | 0.722 |
| 1/17/19 | 65.5 | 12.8 | 2.6 | 2.56 | 168 | 4.5 | 0.958 |
| 2/22/19 | 40.1 | 20.3 | 0.6 | 0.60 | 24 | 1 | 0.946 |
| 4/26/19 | 2012 | 23.6 | 0.35 | 0.62 | 1248 | 400 | 0.834 |
| 6/27/19 | 232 | 30.5 | 0.35 | 0.31 | 72 | 102 | 0.858 |
| 9/10/19 | 376 | 33 | 0.3 | 0.38 | 144 | 67 | 0.966 |
| 9/19/19 | 337 | 30.3 | 1.35 | 1.42 | 480 | 69.5 | 0.999 |
| 9/22/19 | 114 | 31.6 | 1.85 | 1.26 | 144 | 9.5 | 0.989 |
| 11/12/19 | 177 | 11 | 0.4 | 0.54 | 96 | 30.5 | 1.0 |
| 2/26/20 | 56.1 | 15.7 | 0.4 | 0.43 | 24 | 8.5 | 0.63 |
| 3/16/20 | 60.7 | 24.2 | 0.2 | 0.40 | 24 | 4.5 | 0.86 |
| Temperature (°C) | Phytoplankton Growth Rate (d−1) | Zooplankton Grazing Rate (d−1) | Reference |
|---|---|---|---|
| 22 | 1.23 | 1.79 | [18] |
| 9 | 1.28 | 1.62 | [18] |
| 11 | 0.7 | 0.1 | [15] |
| 11 | 0.4 | −0.3 | [15] |
| 10 | 0.9 | 0.2 | [15] |
| 19 | 1.2 | 0.3 | [15] |
| 16 | 0 | −0.1 | [15] |
| 15 | −1.3 | −1.5 | [15] |
| 16 | −0.7 | −1.3 | [15] |
| 21 | −0.2 | −1.1 | [15] |
| 20 | 0.8 | 0.4 | [15] |
| 22 | 0.9 | 0 | [15] |
| 25 | 0.4 | 0.3 | [15] |
| 22 | 1 | 0.5 | [15] |
| 20 | 1.1 | 0.8 | [15] |
| 16 | 0.9 | 0.6 | [15] |
| 13 | 0.9 | 0.7 | [15] |
| 6 | −0.8 | −0.9 | [15] |
| 18.6 | 1.33 | −1.83 | [19] |
| 18.6 | 0.19 | −0.35 | [19] |
| 21 | −0.1 | 0.03 | [16] |
| 21 | 0 | 0 | [16] |
| 17 | −0.05 | 0.3 | [16] |
| 21 | 0.34 | 0.07 | [16] |
| 21 | 0.96 | 1.34 | [16] |
| 17 | 0.87 | 0.51 | [16] |
| 21 | 0.26 | 0.21 | [16] |
| 21 | 0.1 | 0.17 | [16] |
| 17 | 1.42 | 0.27 | [16] |
| 21 | 0.43 | 0.33 | [16] |
| 21 | 0.16 | 0.4 | [16] |
| 17 | 0.6 | 0 | [16] |
| 21 | 0.32 | 0 | [16] |
| 21 | 0.16 | 0.11 | [16] |
| 17 | 0.91 | 0.27 | [16] |
| 21 | 0.5 | 0.25 | [16] |
| 21 | 0.18 | 0.1 | [16] |
| 17 | 1.34 | 0.69 | [16] |
| 21 | 0.51 | 0.53 | [16] |
| 21 | 0.32 | 0.45 | [16] |
| 17 | 0.29 | 1.13 | [16] |
| 19.2 | 0.19 | 0.32 | [20] |
| 19.2 | 1.87 | 2.79 | [20] |
| 19.2 | 1.51 | 1.65 | [20] |
| 19.2 | 1.7 | 1.01 | [20] |
| 15 | 0.09 | 0.07 | [21] |
| 20 | 0.37 | 0.26 | [21] |
| 20.2 | 0.78 | 0.87 | [21] |
| 15 | 0.24 | 1.01 | [21] |
| 20 | 1.22 | 1.34 | [21] |
| 20.2 | 1.66 | 1.92 | [21] |
| 20.7 | 0.38 | 0.27 | [17] |
| 18.6 | 0.73 | 0.62 | [17] |
| 22.1 | 1.15 | 0.66 | [17] |
| 21.4 | 0.69 | 0.42 | [17] |
| 23.2 | 0.43 | 0.21 | [17] |
| 24 | 0.37 | 0.28 | [17] |
| 21.3 | 0.52 | 0.42 | [17] |
| 19.6 | 0.23 | 0.16 | [17] |
| 20.7 | 0.5 | 0.04 | [17] |
| 18.6 | 0.34 | 0.06 | [17] |
| 22.1 | 0.19 | 0.02 | [17] |
| 21.4 | 0.3 | 0.04 | [17] |
| 23.2 | 0.25 | 0.059 | [17] |
| 24 | 0.18 | 0.07 | [17] |
| 21.3 | 0.44 | 0.04 | [17] |
| 19.6 | 0.23 | 0.03 | [17] |
| 18 | 0.58 | 0.52 | [22] |
| 18 | 2.32 | 2.29 | [22] |
| 21 | 1.07 | 1.93 | [23] |
| 21 | 0.74 | 0.88 | [23] |
| 21 | 0.33 | 0.28 | [23] |
| 21 | 0.5 | 1.6 | [23] |
| 21 | 0.31 | 0.67 | [23] |
| 21 | 0.68 | 0.18 | [23] |
| 8.5 | 0.312 | 0.264 | [24] |
| 8.5 | 0.264 | 0.144 | [24] |
| 8.5 | 0.144 | 0.264 | [24] |
| 8.5 | 0.48 | 0.528 | [24] |
| 8.5 | 0.456 | 0.528 | [24] |
| 28 | 0.61 | 0.58 | [26] |
| 28 | 1.08 | 0.61 | [26] |
| 24 | 0.25 | 0.37 | [25] |
| 24 | 0.47 | 0.68 | [25] |
| 24 | 0.8 | 0.3 | [25] |
| 24 | 0.74 | 0.69 | [25] |
| 24 | 0.65 | 0.38 | [25] |
References
- Eppley, R.W. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972, 70, 1063–1085. [Google Scholar]
- Goldman, J.C.; Carpenter, E.J. Kinetic approach to effect of temperature on algal growth. Limnol. Oceanogr. 1974, 19, 756–766. [Google Scholar] [CrossRef]
- Bissinger, J.E.; Montagnes, D.J.S.; Harples, J.; Atkinson, D. Predicting marine phytoplankton maximum growth rates from temperature: Improving on the Eppley curve using quantile regression. Limnol. Oceanogr. 2008, 53, 487–493. [Google Scholar] [CrossRef]
- Kremer, C.T.; Thomas, M.K.; Litchman, E. Temperature- and size-scaling of phytoplankton population growth rates: Reconciling the Eppley curve and the metabolic theory of ecology. Limnol. Oceanogr. 2017, 62, 1658–1670. [Google Scholar] [CrossRef]
- Norris, B.; Laws, E.A. Nutrients and Phytoplankton in a Shallow, Hypereutrophic Urban Lake: Prospects for Restoration. Water 2017, 9, 11. [Google Scholar]
- Landry, M.R.; Hassett, R.P. Estimating the grazing impact of marine micro-zooplankton. Mar. Biol. 1982, 67, 283–288. [Google Scholar] [CrossRef]
- Evans, G.T.; Paranjape, M.A. Precision of estimates of phytoplankton growth and microzooplankton grazing when the functional-response of grazers may be nonlinear. Mar. Ecol. Prog. Ser. 1992, 80, 285–290. [Google Scholar] [CrossRef]
- Gallegos, C.L. Microzooplankton grazing on phytoplankton in the Rhode River, Maryland - nonlinear feeding kinetics. Mar. Ecol. Prog. Ser. 1989, 57, 23–33. [Google Scholar] [CrossRef]
- Moigis, A.G. The clearance rate of microzooplankton as the key element for describing estimated non-linear dilution plots demonstrated by a model. Mar. Biol. 2006, 149, 743–762. [Google Scholar] [CrossRef]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Chen, B.; Laws, E.A.; Liu, H.; Huang, B. Estimating microzooplankton grazing half-saturatiion constants from dilution experiments with nonlinear feeding kinetics. Limnol. Oceanogr. 2014, 59, 639–644. [Google Scholar] [CrossRef]
- Atlas, D.; Bannister, T.T. Dependence of mean spectral extinction coefficient of phytoplankton on depth, water color, and species. Limnol. Oceanogr. 1980, 25, 157–159. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a determination: Improvements in methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Boyer, J.; Rollwagen-Bollens, G.; Bollens, S.M. Microzooplankton grazing before, during and after a cyanobacterial bloom in Vancouver Lake, Washington, USA. Aquat. Microb. Ecol. 2011, 64, 163–174. [Google Scholar] [CrossRef]
- Lavrentyev, P.J.; Vanderploeg, H.A.; Franzé, G.; Chacin, D.H.; Liebig, J.R.; Johengen, T.H. Microzooplankton distribution, dynamics, and trophic interactions relative to phytoplankton and quagga mussels in Saginaw Bay, Lake Huron. J.Great Lakes Res. 2014, 40, 95–105. [Google Scholar] [CrossRef]
- Tadonleke, R.D.; Sime-Ngando, T. Rates of growth and microbial grazing mortality of phytoplankton in a recent artificial lake. Aquat. Microb. Ecol. 2000, 22, 301–313. [Google Scholar] [CrossRef]
- Adrian, R.; Wickham, S.A.; Butler, N.M. Trophic interactions between zooplankton and the microbial community in contrasting food webs: The epilimnion and deep chlorophyll maximum of a mesotrophic lake. Aquat. Microb. Ecol. 2001, 24, 83–97. [Google Scholar] [CrossRef]
- Griniene, E.; Sulcius, S.; Kuosa, H. Size-selective microzooplankton grazing on the phytoplankton in the Curonian Lagoon (SE Baltic Sea). Oceanologia 2016, 58, 292–301. [Google Scholar] [CrossRef]
- Staniewski, M.A.; Short, C.M.; Short, S.M. Contrasting Community versus Population-Based Estimates of Grazing and Virus-Induced Mortality of Phytoplankton. Microb. Ecol. 2012, 64, 25–38. [Google Scholar] [CrossRef]
- Sterner, R.W.; Chrzanowski, T.H.; Elser, J.J.; George, N.B. Sources of nitrogen and phosphorus supporting the growth of bacterioplankton and phytoplankton in an oligotrophic Canadian shield lake. Limnol. Oceanogr. 1995, 40, 242–249. [Google Scholar] [CrossRef]
- Tijdens, M.; Van de Waal, D.B.; Slovackova, H.; Hoogveld, H.L.; Gons, H.J. Estimates of bacterial and phytoplankton mortality caused by viral lysis and microzooplankton grazing in a shallow eutrophic lake. Freshw. Biol. 2008, 53, 1126–1141. [Google Scholar] [CrossRef]
- Twiss, M.R.; Campbell, P.G.C.; Auclair, J.C. Regeneration, recycling, and trophic transfer of trace metals by microbial food-web organisms in the pelagic surface waters of Lake Erie. Limnol. Oceanogr. 1996, 41, 1425–1437. [Google Scholar] [CrossRef]
- Weisse, T.; Müller, H.; Pinto-Coelho, R.M.; Schweizer, A.; Springmann, D.; Baldringer, G. Response of the microbial loop to the phytoplankton spring bloom in a large prealpine lake. Limnol. Oceanogr. 1990, 35, 781–794. [Google Scholar] [CrossRef]
- Davis, T.W.; Koch, F.; Marcoval, M.A.; Wilhelm, S.W.; Gobler, C.J. Mesozooplankton and microzooplankton grazing during cyanobacterial blooms in the western basin of Lake Erie. Harmful Algae 2012, 15, 26–35. [Google Scholar] [CrossRef]
- Collos, Y.; Vaquer, A.; Johnston, A.M.; Pons, V.; Bibent, B.; Richard, S. Carbon fixation, ammonium uptake and regeneration in an equatorial lake: Biological versus physical control. J. Plankton Res. 2001, 23, 263–270. [Google Scholar] [CrossRef]
- Chisholm, S.W. Phytoplankton size. In Primary Productivity and Biogeochemical Cycles in the Sea; Falkowski, P.G., Woodhead, A.D., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 213–237. [Google Scholar]
- Laws, E.A. The importance of respiration losses in controlling the size distribution of marine phytoplankton. Ecology 1975, 56, 419–426. [Google Scholar] [CrossRef]
- Maranon, E. Inter-specific scaling of phytoplankton production and cell size in the field. J. Plankton Res. 2008, 30, 157–163. [Google Scholar] [CrossRef]
- Maranon, E. Cell Size as a Key Determinant of Phytoplankton Metabolism and Community Structure. Annu. Rev. Mar. Sci. 2015, 7, 241–264. [Google Scholar] [CrossRef]
- Allen, A.P.; Gillooly, J.F.; Brown, J.H. Linking the global carbon cycle to individual metabolism. Funct. Ecol. 2005, 19, 202–213. [Google Scholar] [CrossRef]
- Lopez-Urrutia, A.; San Martin, E.; Harris, R.P.; Irigoien, X. Scaling the metabolic balance of the oceans. PNAS 2006, 103, 8739–8744. [Google Scholar] [CrossRef]
- Rose, J.M.; Caron, D.A. Does low temperature constrain the growth rates of heterotrophic protists? Evidence and implications for algal blooms in cold waters. Limnol. Oceanogr. 2007, 52, 886–895. [Google Scholar] [CrossRef]
- Doney, S.C.; Glover, D.M.; Najjar, R.G. A new coupled, one-dimensional biological-physical model for the upper ocean: Applications to the JGOFS Bermuda Atlantic Time-series Study (BATS) site. Deep Sea Res. Part II 1996, 43, 591–624. [Google Scholar] [CrossRef]
- Laws, E.A.; Falkowski, P.G.; Smith, W.O.J.; Ducklow, H.; McCarthy, J.J. Temperature effects on export production in the open ocean. Glob. Biogeochem. Cycles 2000, 14, 1231–1246. [Google Scholar] [CrossRef]
- Palmer, J.R.; Totterdell, I.J. Production and export in a global ocean ecosystem model. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 1169–1198. [Google Scholar] [CrossRef]
- Taucher, J.; Oschlies, A. Can we predict the direction of marine primary production change under global warming? Geophys. Res. Lett. 2011, 38, 6. [Google Scholar] [CrossRef]
- Toseland, A.; Daines, S.J.; Clark, C.R.; Kirkham, A.; Strauss, J.; Uhlig, C.; Lenton, T.M.; Valentin, K.; Pearson, G.A.; Moulton, V.; et al. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Chang. 2013, 3, 979–984. [Google Scholar] [CrossRef]
- Stock, C.A.; Dunne, J.P.; John, J.G. Global-scale carbon and energy flows through the marine planktonic food web: An analysis with a coupled physical-biological model. Prog. Oceanogr. 2014, 120, 1–28. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulsifer, J.; Laws, E. Temperature Dependence of Freshwater Phytoplankton Growth Rates and Zooplankton Grazing Rates. Water 2021, 13, 1591. https://doi.org/10.3390/w13111591
Pulsifer J, Laws E. Temperature Dependence of Freshwater Phytoplankton Growth Rates and Zooplankton Grazing Rates. Water. 2021; 13(11):1591. https://doi.org/10.3390/w13111591
Chicago/Turabian StylePulsifer, Jennifer, and Edward Laws. 2021. "Temperature Dependence of Freshwater Phytoplankton Growth Rates and Zooplankton Grazing Rates" Water 13, no. 11: 1591. https://doi.org/10.3390/w13111591
APA StylePulsifer, J., & Laws, E. (2021). Temperature Dependence of Freshwater Phytoplankton Growth Rates and Zooplankton Grazing Rates. Water, 13(11), 1591. https://doi.org/10.3390/w13111591







