Changes in Algal Plankton Composition and Physico-Chemical Variables in a Shallow Oxbow Lake
Abstract
1. Introduction
2. Materials and Methods
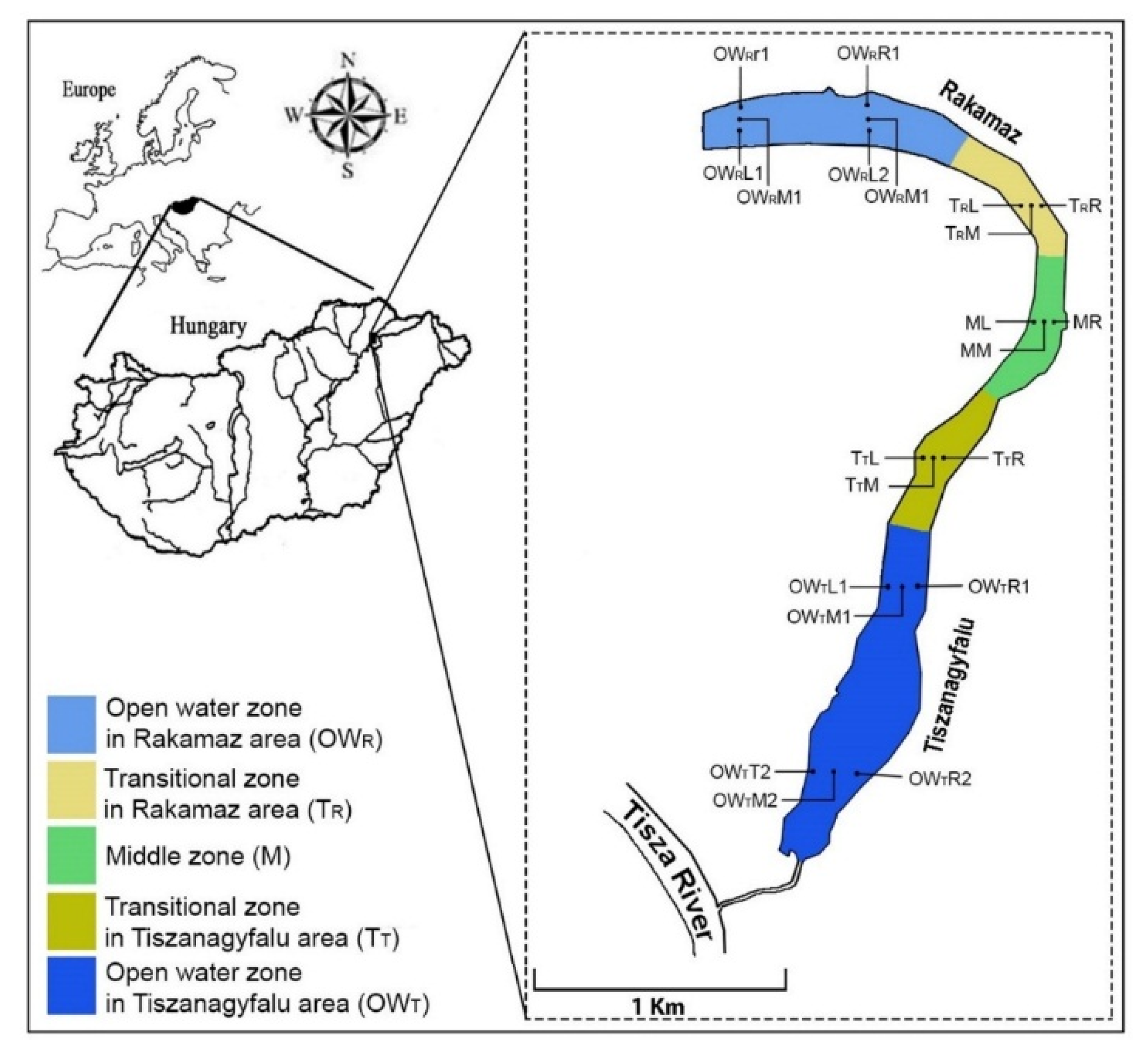
2.1. Study Site
2.2. Sample Collection
2.3. Data Analysis
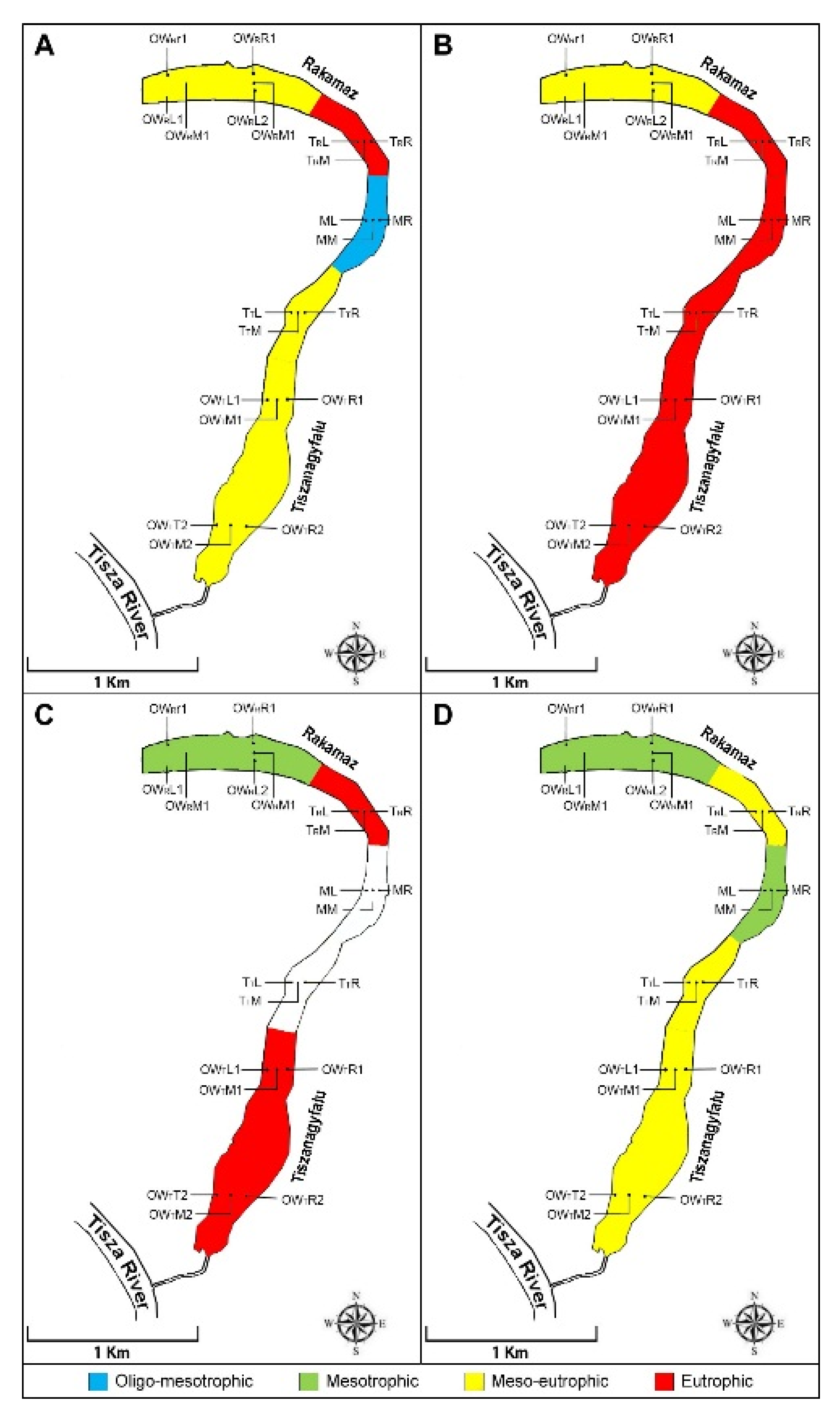
3. Results
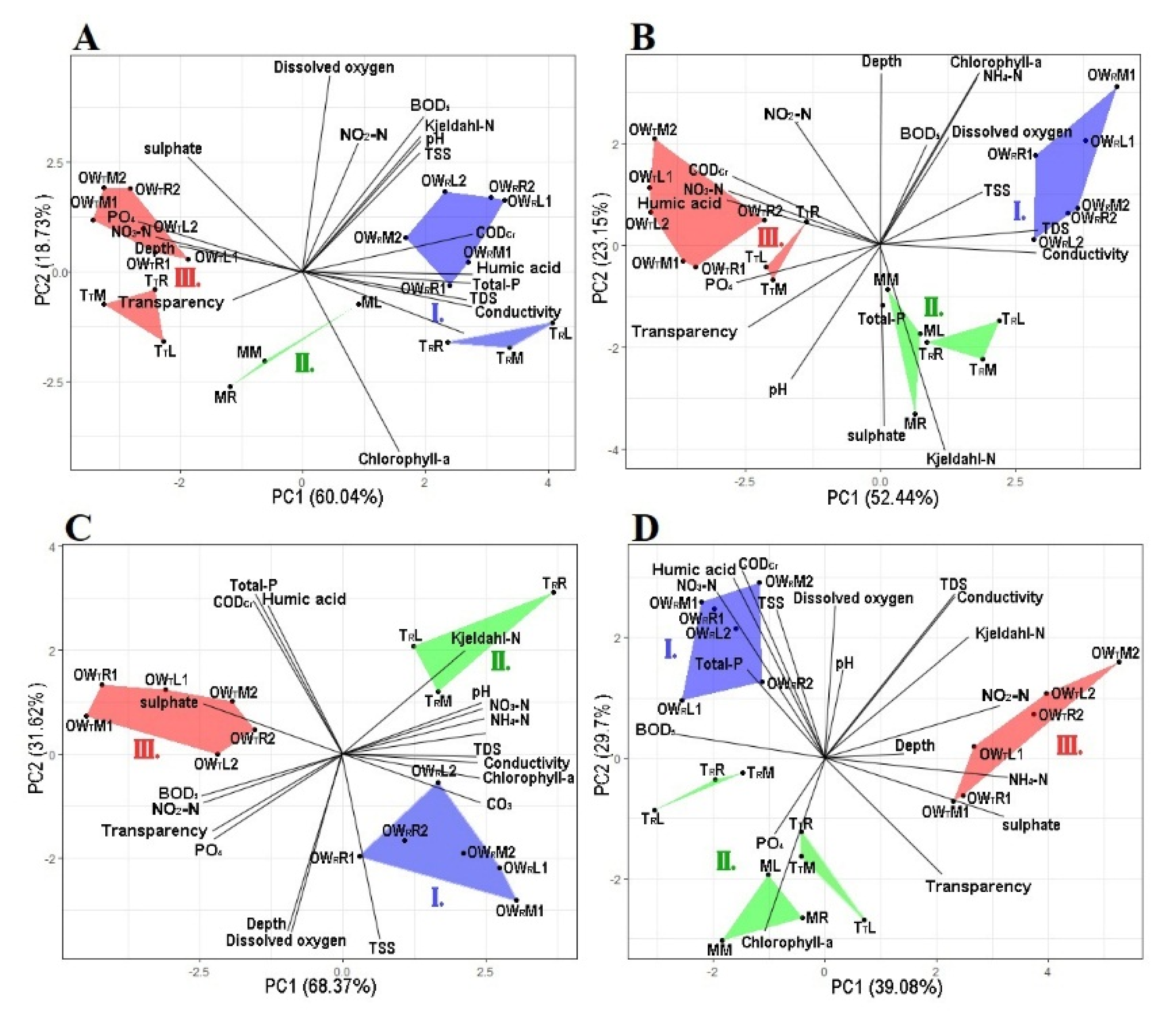
3.1. Principal Component Analysis of Physical and Chemical Variables
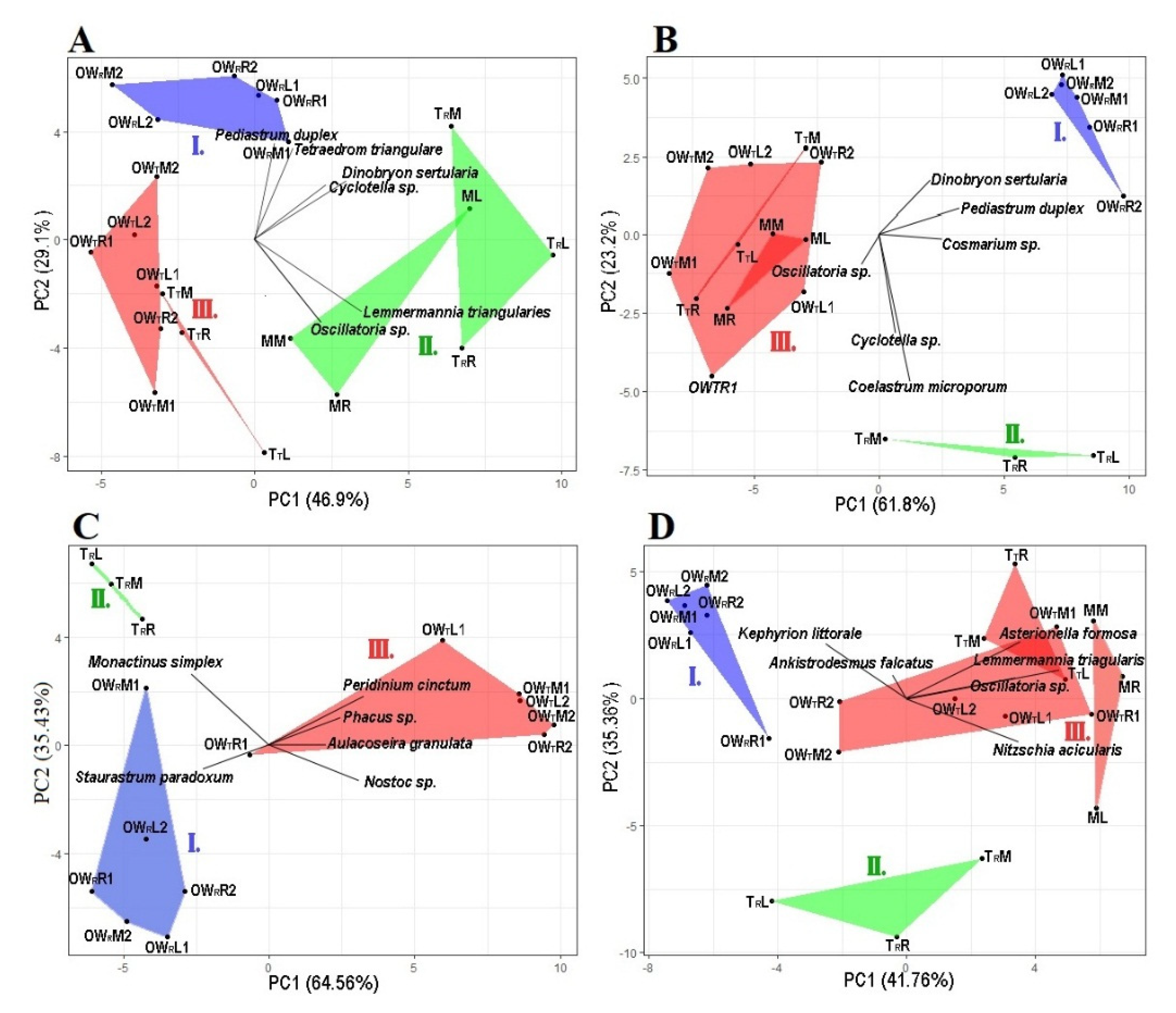
3.2. Principal Componant Analysis of the Algal Plankton
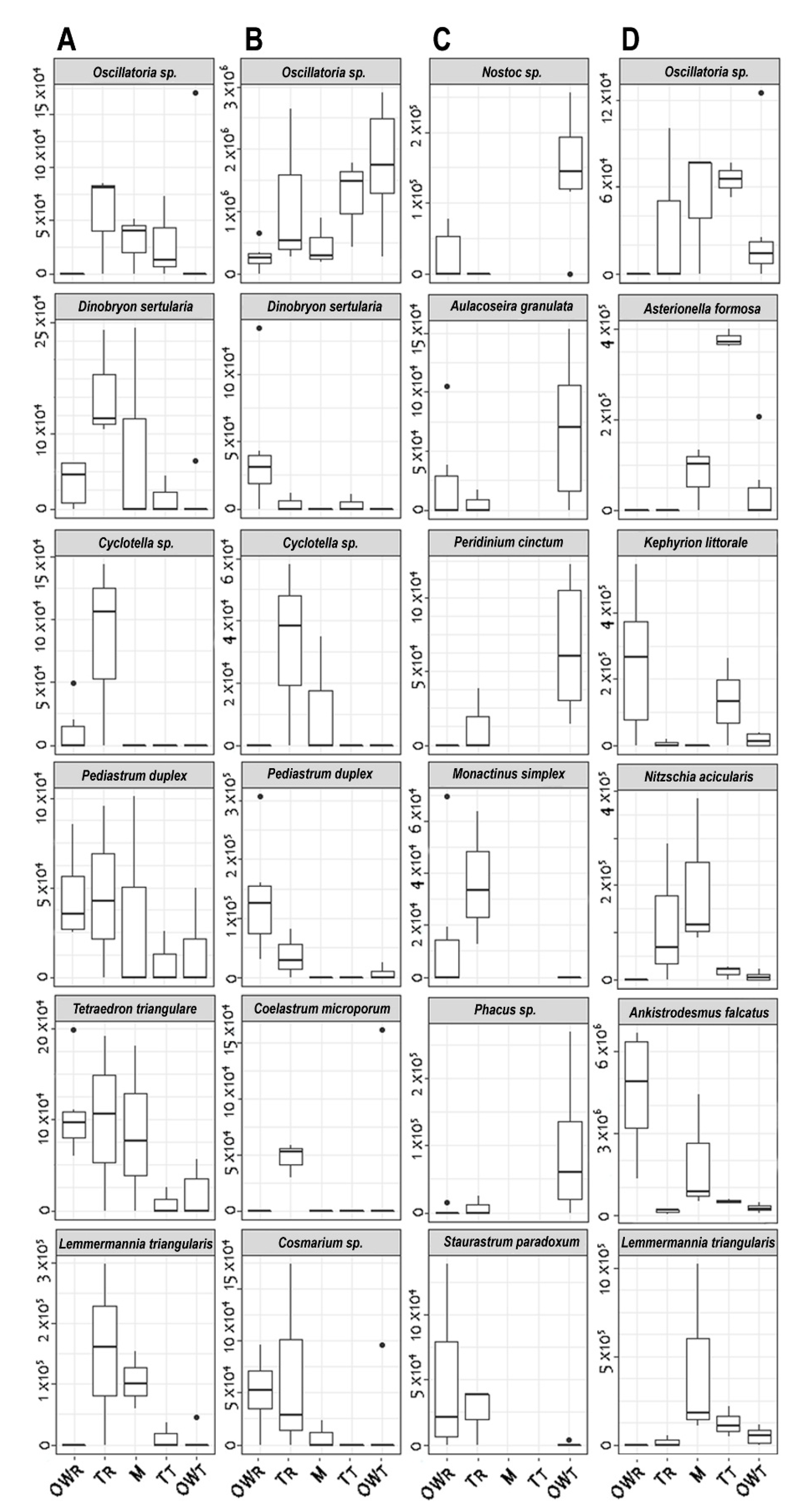
3.3. Occurrence of Dominant Algal Species in the Investigated Sampling Times
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berta, C.; Tóthmérész, B.; Wojewódka, M.; Augustyniuk, O.; Korponai, J.; Bertalan-Balázs, B.; Nagy, S.A.; Grigorszky, I.; Gyulai, I. Community response of Cladocera to trophic stress by biomanipulation in a shallow oxbow lake. Water 2019, 11, 929. [Google Scholar] [CrossRef]
- Vincent, W.F. Effects of climate change on lakes. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 55–60. [Google Scholar] [CrossRef]
- Nyeste, K.J.; Dobrocsi, P.; Czeglédi, I.; Czédli, H.; Harangi, S.; Baranyai, E.; Simon, E.; Nagy, S.A.; Antal, L. Age and diet-specific trace element accumulation patterns in different tissues of chub (Squalius cephalus): Juveniles are useful bioindicators of recent population. Ecol. Indic. 2019, 101, 1–10. [Google Scholar] [CrossRef]
- Meerhoff, M.; de Mello, T.F.; Kruk, C.; Alonso, C.; Bergonzoni, I.G.; Pacheco, J.P.; Lacerot, G.; Arim, M.; Beklioglu, M.; Balmana, S.B.; et al. Environmental warming in shallow lakes: A review of potential changes in community structure as evidenced from space-for-time substitution approaches. Adv. Ecol. Res. 2012, 46, 259–349. [Google Scholar] [CrossRef]
- Scheffer, M. Ecology of Shallow Lakes, 1st ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 1–357. [Google Scholar] [CrossRef]
- Moss, B.; Stephen, D.; Alvarez, C.; Becares, E.; Van De Bund, W.; Collings, S.E.; Van Donk, E.; De Eyto, E.; Feldmann, T.; Fernández-Aláez, C.; et al. The determination of ecological status in shallow lakes—A tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquat. Conserv. 2003, 13, 507–549. [Google Scholar] [CrossRef]
- Bhateria, R.; Disha, J. Water quality assessment of lake water: A review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef]
- Hillbricht-Ilkowska, A. Shallow lakes in lowland river systems: Role in transport and transformations of nutrients and in biological diversity. Hydrobiologia 1999, 408, 349–358. [Google Scholar] [CrossRef]
- Dembowska, E.A.; Mieszczankin, T.; Napiórkowski, P. Changes of the phytoplankton community as symptoms of deterioration of water quality in a shallow lake. Environ. Monit. Assess. 2018, 190, 95. [Google Scholar] [CrossRef] [PubMed]
- Van Donk, E.; van de Bund, W.J. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: Allelopathy versus other mechanisms. Aquat. Bot. 2002, 3–4, 261–274. [Google Scholar] [CrossRef]
- Song, Y.; Liew, J.H.; Sim, Z.H.; Mowe, M.A.D.; Mitrovic, S.M.; Tan, H.T.W.; Yeo, D.C.J. Effects of macrophytes on lake-water quality across latitudes: A meta-analysis. Oikos 2018, 128, 468–481. [Google Scholar] [CrossRef]
- Joniak, T.; Kuczyńska-Kippen, N. Habitat features and zooplankton community structure of oxbows int he limnophase: Reference to transitional phase between flooding and stabilization. Limnetica 2011, 29, 37–48. [Google Scholar]
- Borics, G.; Nagy, L.; Miron, S.; Grigorszky, I.; László-Nagy, Z.; Lukács, B.A.; G.-Tóth, L.; Várbíró, G. Which factors affect phytoplankton biomass in shallow eutrophic lakes? Hydrobiologia 2013, 714, 93–104. [Google Scholar] [CrossRef]
- Biggs, J.; von Fumetti, S.; Kelly-Quinn, M. The importance of small waterbodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 2017, 793, 3–39. [Google Scholar] [CrossRef]
- Ferencz, B.; Dawidek, J.; Toporowska, M. Instability of water quality of a shallow, polymictic, flow-through lake. Water Air Soil Pollut. 2018, 229, 141. [Google Scholar] [CrossRef]
- Somlyai, I.; Berta, C.; Nagy, S.A.; Dévai, G.; Ács, É.; Szabó, L.J.; Nagy, J.; Grigorszky, I. Heterogeneity and anthropogenic impacts on a small lowland stream. Water 2019, 11, 2002. [Google Scholar] [CrossRef]
- Choudhury, A.K.; Pal, R. Phytoplankton and nutrient dynamics of shallow coastal stations at Bay of Bengal, Eastern Indian coast. Aquat. Ecol. 2010, 44, 55–71. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Stanley, E.H.; Zanden, H.J.V. State of the World’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 2011, 36, 75–99. [Google Scholar] [CrossRef]
- Gligora, M.; Plenković-Moraj, A.; Kralj, K.; Grigorszky, I.; Peroš-Pucar, D. The relationship between phytoplankton species dominance and environmental variables in a shallow oxbow lake (Lake Vrana, Croatia). Hydrobiologia 2007, 584, 337–346. [Google Scholar] [CrossRef]
- Pereira, S.A.; Trindade, C.R.T.; Albertoni, E.F.; Palma-Silva, C. Aquatic macrophytes as indicators of water quality in subtropical shallow lakes, Southern Brazil. Acta Limnol. Bras. 2012, 1, 52–63. [Google Scholar] [CrossRef]
- Zingel, P.; Nõges, P.; Tuvikene, L.; Feldmann, T.; Järvalt, A.; Tõnno, I.; Agasild, H.; Tammert, H.; Luup, H.; Salujõe, J.; et al. Ecological processes in macrophytes- and phytoplankton-dominated shallow lakes. Proc. Est. Acad. Sci. Biol. Ecol. 2006, 55, 280–307. [Google Scholar]
- Carpenter, R.S.; Lodge, D.M. Effects of submerged macrophytes on ecosystem processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Väliranta, M.; Kultti, S.; Nyman, M.; Sarmaja-Korjonen, K. Holocene development of aquatic vegetation in shallow Lake Njargajavri, Finnish Laplan, with evidence of water-level fluctuations and drying. J. Paleolimnol. 2005, 34, 203–215. [Google Scholar] [CrossRef]
- Ozimek, T.; Gulati, R.D.; van Donk, E. Can macrophytes be useful in biomanipulation of lakes? The Lake Zwemlust example. Hydrobiologia 1990, 200, 399–407. [Google Scholar] [CrossRef]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Sakshaug, E. Phytoplankton Manual–Monography on Oceanographic Methodology. 6: A. Sournia; UNESCO: Paris, France, 1978; pp. 1–337. [Google Scholar] [CrossRef]
- Utermöhl, H. Neue Wege in der quantitativen Erfassung des Planktons (mit besonderer Berucksichtigung des Ultraplanktons). Verh. Int. Ver. Theor. Angew. Limnol. 1931, 5, 567–596. [Google Scholar] [CrossRef]
- Felföldy, L. A Biológiai Vízminősítés, 4th ed.; VGI: Budapest, Hungary, 1987; pp. 1–258. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Kozak, A.; Gołdyn, R.; Dondajewska, R.; Kowalczewska-Madura, K.; Holona, T. Changes in phytoplankton and water quality during sustainable restoration of an urban lake used for recreation and water supply. Water 2017, 9, 713. [Google Scholar] [CrossRef]
- Sige, D.C.; Dean, A.; Levado, E.; Tobin, M.J. Fourier-transform infrared spectroscopy of Pediastrum duplex: Characterization of a micro-population isolated from a eutrophic lake. Eur. J. Phycol. 2002, 37, 19–26. [Google Scholar] [CrossRef]
- Lenarczyk, J. Pediastrum Meyen sensu lato (Chlorophyceae) in the phytoplankton of lowland and upland water bodies of Central Europe (Poland). Fottea 2015, 15, 165–177. [Google Scholar] [CrossRef]
- Ramezanpour, Z.; Sukop, I.; Heteša, J. Phytoplankton diversity and their succession in water bodies of the Lednice Park during 2002 season. Acta Univ. Agric. Silvic. Mendel. Brun. 2004, 52, 83–96. [Google Scholar] [CrossRef]
- Willén, E. Planktonic green algae in an acidification gradient of nutrient-poor lakes. Arch. Protistenkd. 1992, 141, 47–64. [Google Scholar] [CrossRef]
- Celewicz-Gołdyn, S.; Kuczyńska-Kippen, N.; Nagengast, B. Phytoplankton community structure in two types (forest vs. field) of small water bodies. Steciana 2008, 12, 23–28. [Google Scholar]
- Watson, S.B.; Whitton, B.A.; Higgins, S.; Paerl, H.; Brooks, B.W.; Wehr, J.D. Harmful algal blooms. In Freshwater Algae of North America, 2nd ed.; Wehr, J., Sheath, R., Kociolek, J.P., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1–11. [Google Scholar]
- Pappas, J.L. Phytoplankton assemblages, environmental influences and trophic status using canonical correspondence analysis, fuzzy relations, and linguistic translation. Ecol. Inform. 2010, 5, 79–88. [Google Scholar] [CrossRef][Green Version]
- Dale, A.G. Colonies as Defence in the Freshwater Phytoplankton Genus Dinobryon (Chrysophyceae). Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1985. [Google Scholar]
- Celewicz-Gołdyn, S. Abundance of Dinobryon divergens Imhoff in the eutrophic Lake Rosnowskie Duze in 2002–2003. Steciana 2005, 9, 23–30. [Google Scholar]
- Mohamed, Z.A. Allelophatic activity of Spirogyra sp.: Stimulating bloom formation and toxin production by Oscillatoria agardhii in some irrigation canals, Egyp. J. Plankton Res. 2002, 24, 137–141. [Google Scholar] [CrossRef]
- Brittain, S.; Mohamed, Z.A.; Wang, J.; Lehmann, V.K.; Carmichael, W.W.; Rinehart, K.L. Isolation and characterization of microcystins from a river nile strain of Oscillatoria tenuis Agardh ex Gomont. Toxicon 2000, 38, 1759–1771. [Google Scholar] [CrossRef]
- Taş, B.; Gönülol, A.; Taş, E. Seasonal dynamics and biomass of mixotrophic flagellate Dinobryon sertularia Ehrenberg (Chrysophyceae) in Derbent reservoir (Samsun, Turkey). Turk. J. Fish. Aquat. Sci. 2010, 10, 305–313. [Google Scholar] [CrossRef]
- Stamenković, M.; Cvijan, M. High tolerance to water pollution in Cosmarium boitierense Kouwets and Staurastrum bloklandiae Coesel et Joosten, taxa recorded for the first time from the Balkan Peninsula. Algol. Stud. 2008, 127, 83–94. [Google Scholar] [CrossRef]
- Hutchinson, G.E. A treatise on limnology. In Introduction to Lake Biology and the Limnoplankton; John Wiley & Sons: New York, NY, USA, 1967; Volume II, p. 1115. [Google Scholar]
- Del Zamaloa, M.C.; Tell, G. The fossil record of freshwater micro-algae Pediastrum Meyen (Chlorophyceae) in Southern South America. J. Paleolimnol. 2005, 34, 433–444. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Bicudo, C.E.M. How important can the presence/absence of macrophytes be in determining phytoplankton strategies in two tropical shallow reservoirs with different trophic status? J. Plankton Res. 2010, 32, 31–46. [Google Scholar] [CrossRef]
- Stansbury, J.; Kozimor, L.; Admiraal, D.; Dove, E. Water quality modeling of the effects of macrophytes on dissolved oxygen in a shallow tailwater reservoir. Lake Reserv. Manag. 2008, 24, 339–348. [Google Scholar] [CrossRef]
- Wunsam, S.; Schmidt, R.; Klee, R. Cyclotella-taxa (Bacillariophyceae) in lakes of the Alpine region and their relationship to environmental variables. Aquat. Sci. 1995, 57, 360–386. [Google Scholar] [CrossRef]
- Bouterfas, R.; Belkoura, M.; Dauta, A. The effects of irradiance and photoperiod ont he growth rate of three freshwater green algae isolated from a eutrophic lake. Limnetica 2006, 25, 647–656. [Google Scholar]
- Lynch, D.L.; Fenwick, M.G.; Hansen, L.O. Heterotrophic nutrition in the genus Coelastrum Naeg. Trans. Am. Microsc. Soc. 1967, 86, 499–502. [Google Scholar] [CrossRef]
- Chang, X.; Eigemann, F.; Hilt, S. Do macrophytes support harmful cyanobacteria? Interactions with a green alga reverse the inhibiting effects of macrophyte allelochemicals on Microcystis aeruginosa. Harmful Algae 2012, 19, 76–84. [Google Scholar] [CrossRef]
- Fužinato, S.; Cvijan, M.; Krizamanić, J. A checklist of Desmids (Conjugatophyceae, Chlorophyta) of Serbia. III. Genus Staurastrum. Cryptogam. Algol. 2011, 32, 363–377. [Google Scholar] [CrossRef]
- Mihaljević, M.; Guncunski, D. Population dynamics of the Chlorococcal alga Pediastrum simplex Meyen in a carp fish pond. Acta Bot. Croat. 1994, 53, 61–67. [Google Scholar]
- Duangjan, K.; Wołowski, K.; Peerapornpisal, Y. New records of Phacus and Monomorphina taxa (Euglenophyta) for Thailand. Pol. Bot. J. 2014, 59, 235–247. [Google Scholar] [CrossRef][Green Version]
- Kokociński, M.; Soininen, J. New insights into the distribution of alien cyanobacterium Chrysosporum bergii (Nostocales, Cyanobacteria). Psychol. Res. 2019, 67, 208–214. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Lai, Z.; Tan, X.; Pang, S.; Yang, W. Seasonal variations of Aulacoseira granulate population abundance in the Pearl River Estuary. Estuar. Coast. Shelf. Sci. 2009, 85, 585–592. [Google Scholar] [CrossRef]
- Schweikert, M.; Meyer, B. Characterization of intracellular bacteria in the freshwater dinoflagellate Peridinium cinctum. Protoplasma 2001, 217, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Grigorszky, I.; Kiss, K.T.; Béres, V.; Bácsi, I.M.; Hamvas, M.; Máthé, C.; Vasas, G.; Padisák, J.; Borics, G.; Gligora, M.; et al. The effects of temperature, nitrogen, and phosphorus on the encystment of Peridinium cinctum, Stein (Dinophyta). Hydrobiologia 2006, 563, 527–535. [Google Scholar] [CrossRef]
- Prakash, A.; Rashid, M.A. Influence of humic substances on the growth of marine phytoplankton: Dinoflagellates. Limnol. Oceanogr. 1968, 13, 598–606. [Google Scholar] [CrossRef]
- Ptail, H.S. The role of Ankistrodesmus falcatus and Scenedesmus quadricauda in sewage purification. Bioresour. Technol. 1991, 37, 121–126. [Google Scholar] [CrossRef]
- Meyer, R.L. Notes on the algae of Arkansas. 1. Chrsyococcus, Kephyrion, Kephyriopsis, Pseudokephyrion and Stenokalyx. J. Ark. Acad. Sci. 1971, 25, 12. [Google Scholar]
- Jones, R.; Ilmavirta, V. Spatial heterogeneity of phytoplankton in the Lokka reservoir, Finnish Lapland. Ann. Bot. Fenn. 1983, 20, 391–397. [Google Scholar]
- Kling, H.J.; Mugidde, R.; Hecky, R.E. Recent changes in the phytoplankton community of Lake Victoria in response to eutrophication. In Great Lakes of the World: Food Webs, Health and Integrity; Munawar, M., Hecky, R.E., Eds.; Backhuys: Leiden, The Netherlands, 2001; pp. 47–66. [Google Scholar]
- Lund, J.W.G. Studies on Asterionella Formosa Hass: II. Nutrient depletion and the spring maximum. J. Ecol. 1950, 38, 15–35. [Google Scholar] [CrossRef]
- Nabout, J.C.; Nogueira, I.S.; Oliveira, L.G. Phytoplankton community of floodplain lakes of the Araguaia River, Brazil, in the rainy and dry seasons. J. Plankton Res. 2006, 28, 181–193. [Google Scholar] [CrossRef]
- Ganai, A.H.; Parveen, S. Effect of physico-chemical conditions on the structure and composition of the phytoplankton community in Wular Lake at Lankrishipora, Kashmir. Int. J. Biodivers. Conserv. 2014, 6, 71–84. [Google Scholar] [CrossRef]
- Welch, E.B.; Barbiero, R.P.; Bouchard, D.; Jones, C.A. Lake trophic state change and constant algal composition following dilution and diversion. Ecol. Eng. 1992, 1, 173–197. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Sivakumar, K. Studies on phytoplankton diversity in response to abiotic factors in Veeranam lake in the Cuddalore district of Tamil Nadu. J. Environ. Biol. 2008, 29, 747–752. [Google Scholar]
- Jargal, N.; Atique, U.; Mamun, M.; An, K.-G. Seasonal and long-term connections between trophic status, sestonic chlorophyll, nutrients, organic matter, and monsoon rainfall in a multipurpose reservoir. Water 2021, 13, 1720. [Google Scholar] [CrossRef]
| Variables | April | June | July | October |
|---|---|---|---|---|
| Min–Max Median | Min–Max Median | Min–Max Median | Min–Max Median | |
| BOD5 (mg/L) | 2.43–4.74 2.89 | 1.04–4.08 2 | 1.48–5.02 3.2 | 1.48–5.02 3.2 |
| Chlorophyll-a (mg/L) | 12.14–43.22 20.01 | 5.10–75.57 20.63 | 10.86–42.15 21.29 | 10.86–42.15 21.29 |
| CODCr (mg/L) | 11–43 23 | 15–47 28 | 13–29 35 | 27.1–47 35 |
| CODsMn (mg/L) | 5.3–8.02 7.04 | 7.01–11.73 8.38 | 11.36–22.10 16.88 | 11.36–22.10 16.88 |
| Conductivity (µS/cm−1) | 360.47–496.05 423.30 | 308.45–469.59 378.68 | 361.77–421.00 384.70 | 361.77–421.00 384.70 |
| Depth (cm) | 54–200 84 | 74–188 123 | 50–170 103 | 50–170 103 |
| PO43− (mg/L) | 0.000–0.0093 0.0019 | 0.0087–0.0902 0.0434 | 0.0000–0.1212 0.0071 | 0.0000–0.1212 0.0071 |
| H-CO3− (mg/L) | 18.8–164.55 84.63 | 111.33–199.47 157.72 | 143.81–231.5 176.28 | 143.81–231.5 176.28 |
| Humic acid (mg/L) | 0.1–2.71 1.58 | 1.65–4.39 2.81 | 1.5–5.00 2.30 | 1.5–5.00 2.30 |
| Kjeldahl-N (mg/L) | 0.59–4.07 1.67 | –1.16–5.36 2.74 | 0.58–5.78 1.94 | 0.58–5.78 1.94 |
| NO2-N (mg/L) | 0.0004–0.0033 0.0012 | 0.0000–0.0052 0.0004 | 0.0000–0.0008 0.0000 | 0.0000–0.0008 0.0000 |
| NO3-N (mg/L) | 0.0000–0.3056 0.1136 | 0.0454–0.1249 0.0795 | 0.2923–0.4463 0.3489 | 0.2923–0.4463 0.3489 |
| NH4-N (mg/L) | 0.0000–0.0000 0.0000 | 0.0000–0.1159 0.0000 | 0.0093–0.0432 0.0165 | 0.0093–0.0432 0.0165 |
| ODO (mg/L) | 7.68–14.83 9.62 | 0.12–8.77 5.27 | 6.63–9.85 8.39 | 6.63–9.85 8.39 |
| ORP (mV) | 174.9–227.6 194.6 | 172.4–235.6 211 | 429–505 462 | 429–505 462 |
| pH | 6.97–8.08 7.51 | 6.4–7.85 7.16 | 6.25–7 6.8 | 6.25–7 6.85 |
| Sulphate ion (mg/L) | 0.00–0.05 0.01 | 0.00–0.45 0.00 | 2.96–19.26 6.85 | 2.96–19.26 6.85 |
| TDS (mg/L) | 268.00–371.67 327.33 | 206.33–301.67 257.00 | 318.33–363.00 334.33 | 318.33–363.00 334.33 |
| Total-P (mg/L) | 0.07–0.15 0.10 | 0.07–0.51 0.21 | 0.30–1.01 0.36 | 0.30–1.01 0.36 |
| Transparency (cm) | 40–100 57 | 51–101 78 | 50–120 80 | 50–120 80 |
| TSS (mg/L) | 2.00–19.50 10.50 | 2.00–41.00 13.00 | 3.00–15.00 6.00 | 3.00–15.00 6.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaqoob, M.M.; Berta, C.; Szabó, L.J.; Dévai, G.; Szabó, S.; Nagy, S.A.; Bácsi, I.; Simon, A.; Nagy, J.; Somlyai, I.; et al. Changes in Algal Plankton Composition and Physico-Chemical Variables in a Shallow Oxbow Lake. Water 2021, 13, 2339. https://doi.org/10.3390/w13172339
Yaqoob MM, Berta C, Szabó LJ, Dévai G, Szabó S, Nagy SA, Bácsi I, Simon A, Nagy J, Somlyai I, et al. Changes in Algal Plankton Composition and Physico-Chemical Variables in a Shallow Oxbow Lake. Water. 2021; 13(17):2339. https://doi.org/10.3390/w13172339
Chicago/Turabian StyleYaqoob, Majd Muwafaq, Csaba Berta, László József Szabó, György Dévai, Szilárd Szabó, Sándor Alex Nagy, István Bácsi, Alexandra Simon, János Nagy, Imre Somlyai, and et al. 2021. "Changes in Algal Plankton Composition and Physico-Chemical Variables in a Shallow Oxbow Lake" Water 13, no. 17: 2339. https://doi.org/10.3390/w13172339
APA StyleYaqoob, M. M., Berta, C., Szabó, L. J., Dévai, G., Szabó, S., Nagy, S. A., Bácsi, I., Simon, A., Nagy, J., Somlyai, I., Ács, É., & Grigorszky, I. (2021). Changes in Algal Plankton Composition and Physico-Chemical Variables in a Shallow Oxbow Lake. Water, 13(17), 2339. https://doi.org/10.3390/w13172339












