Dynamic Changes of Microbiome with the Utilization of Volatile Fatty Acids as Electron Donors for Denitrification
Abstract
1. Introduction
2. Materials and Methods
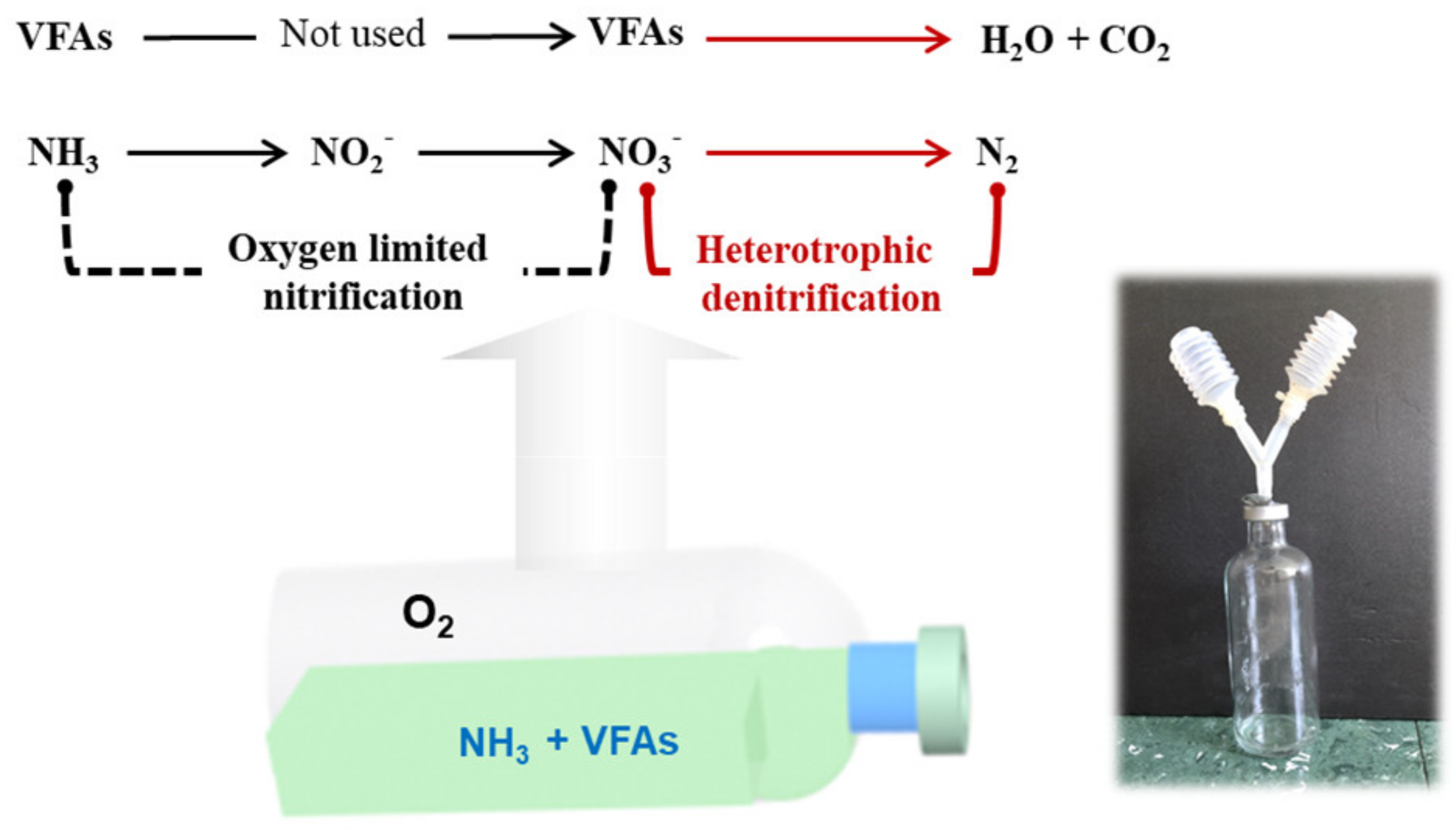
2.1. Nitrification and Denitrification Experiments
2.2. Analysis of Nitrogenous Compounds and Chemical Oxygen Demand (COD)
2.3. Volatile Fatty Acids Analysis
2.4. Gas Analysis
2.5. Microbial Community Analysis Using Pyrosequencing
3. Results
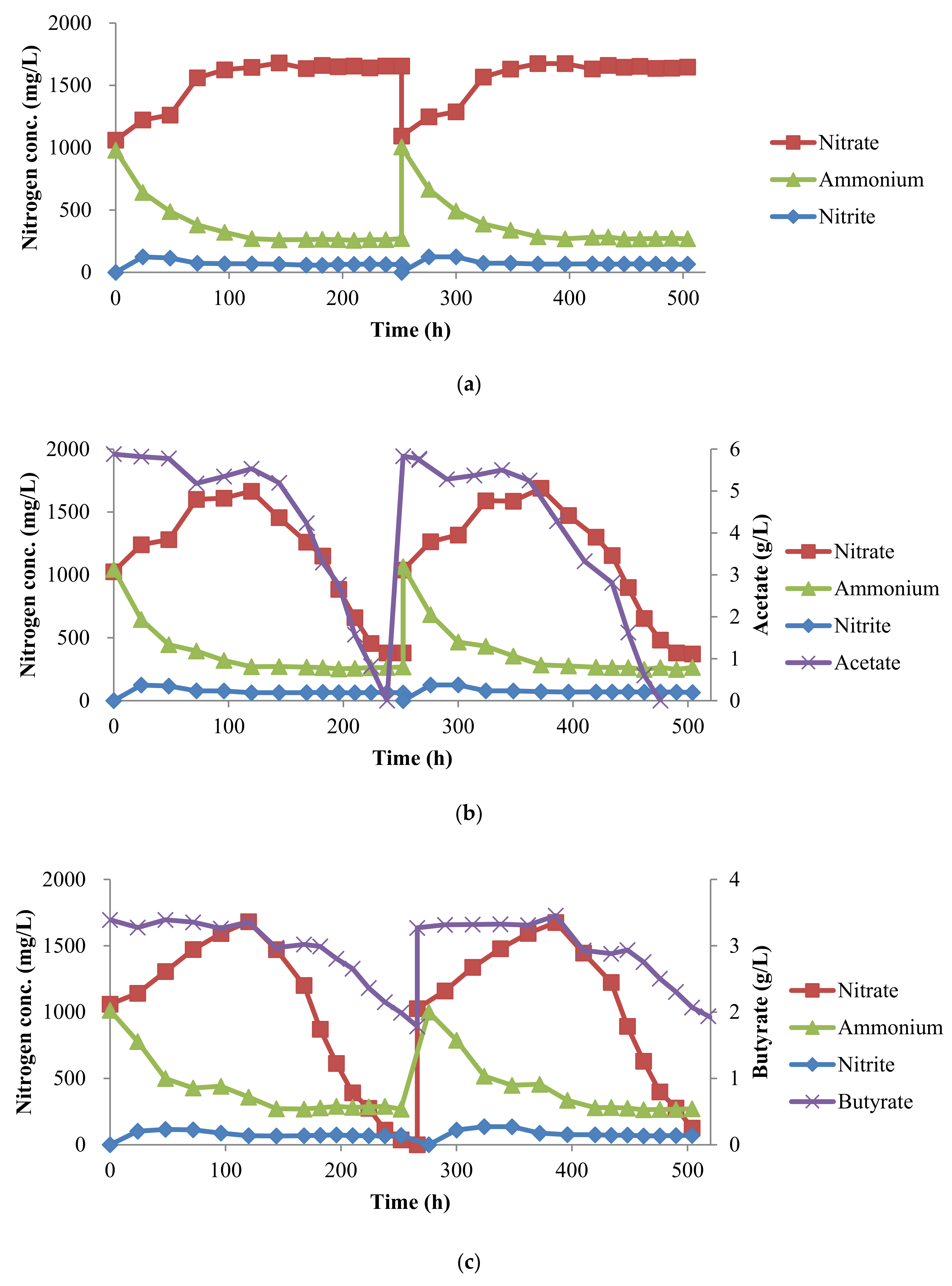
3.1. Oxygen-Limited Nitrification
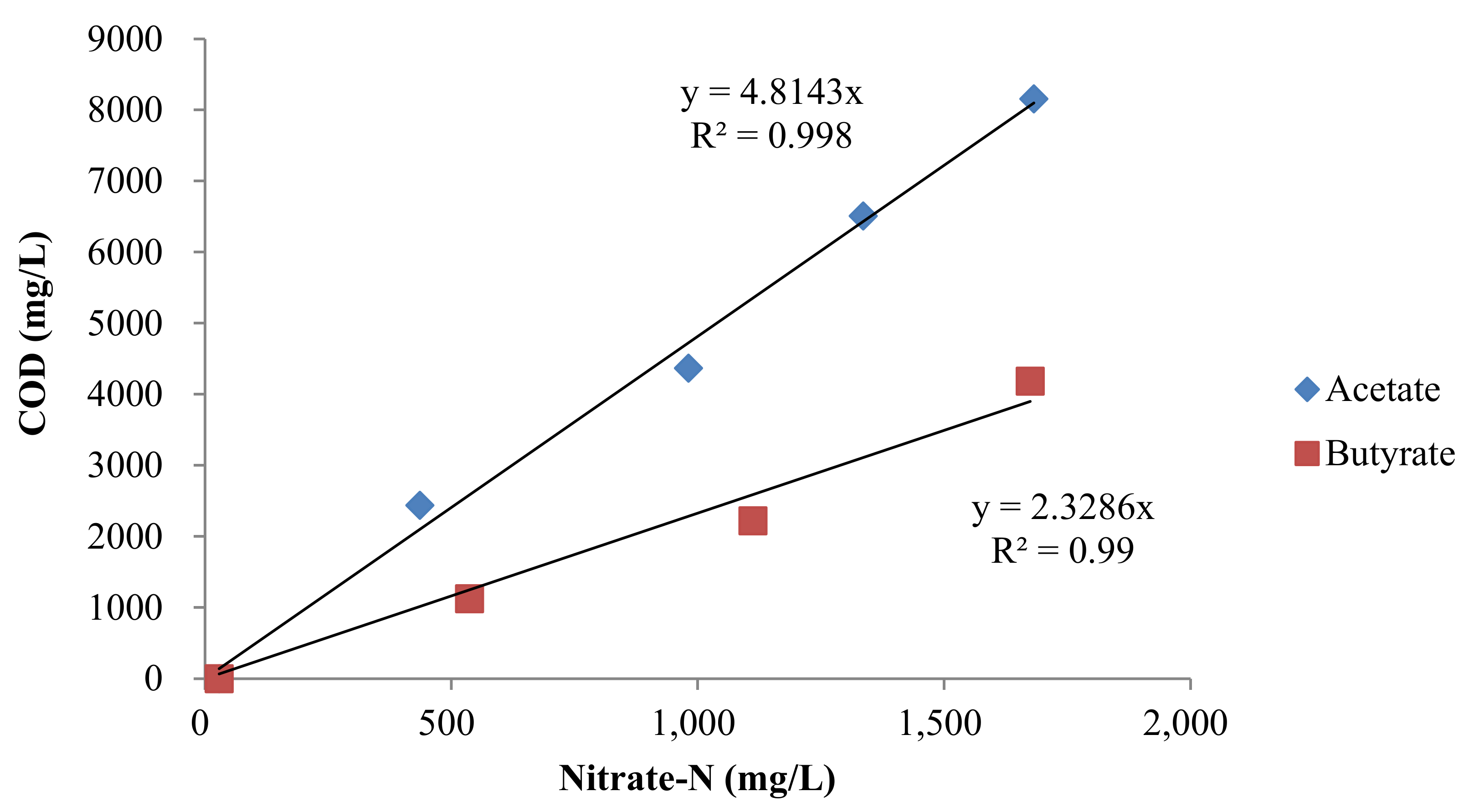
3.2. Denitrification with Volatile Fatty Acids
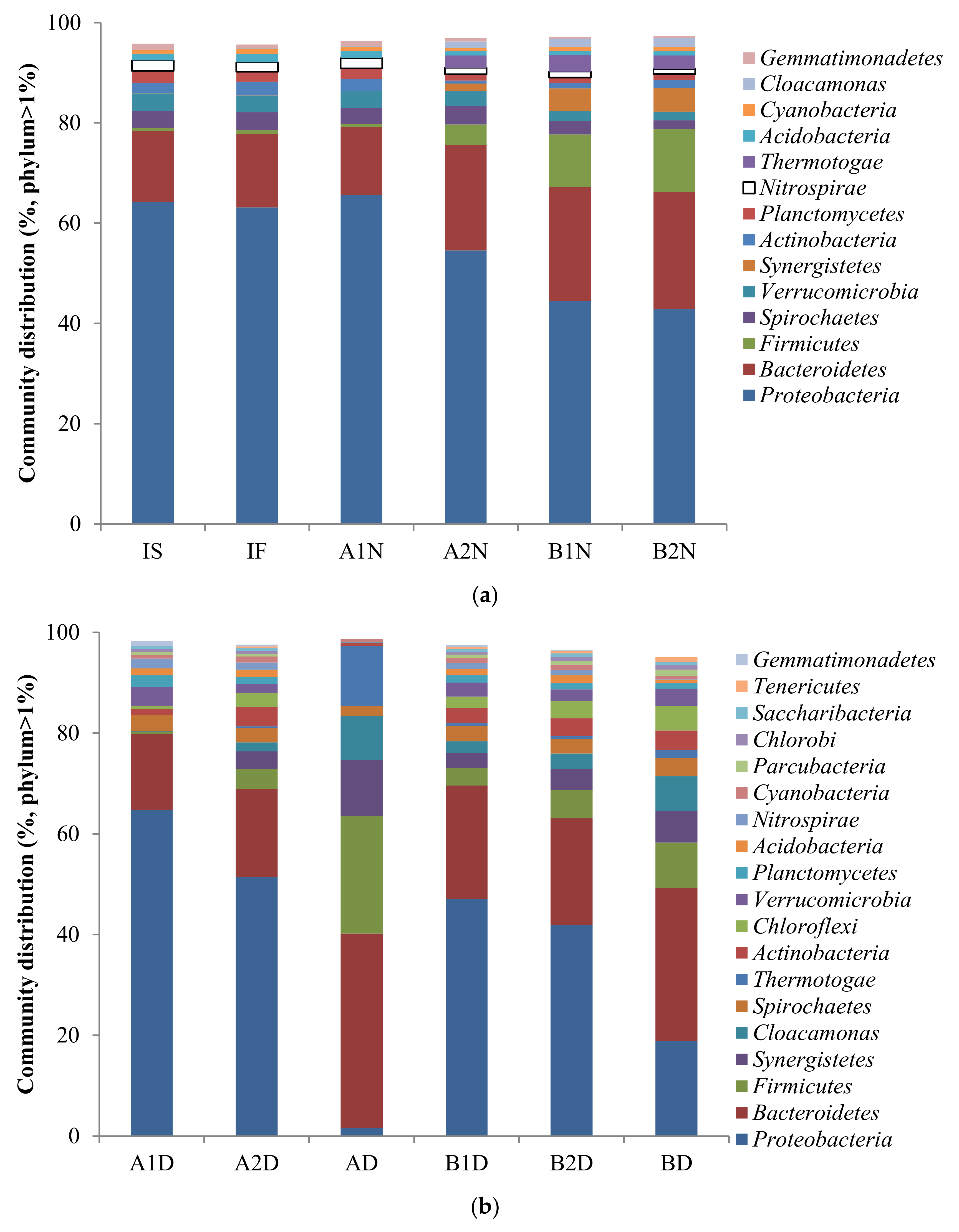
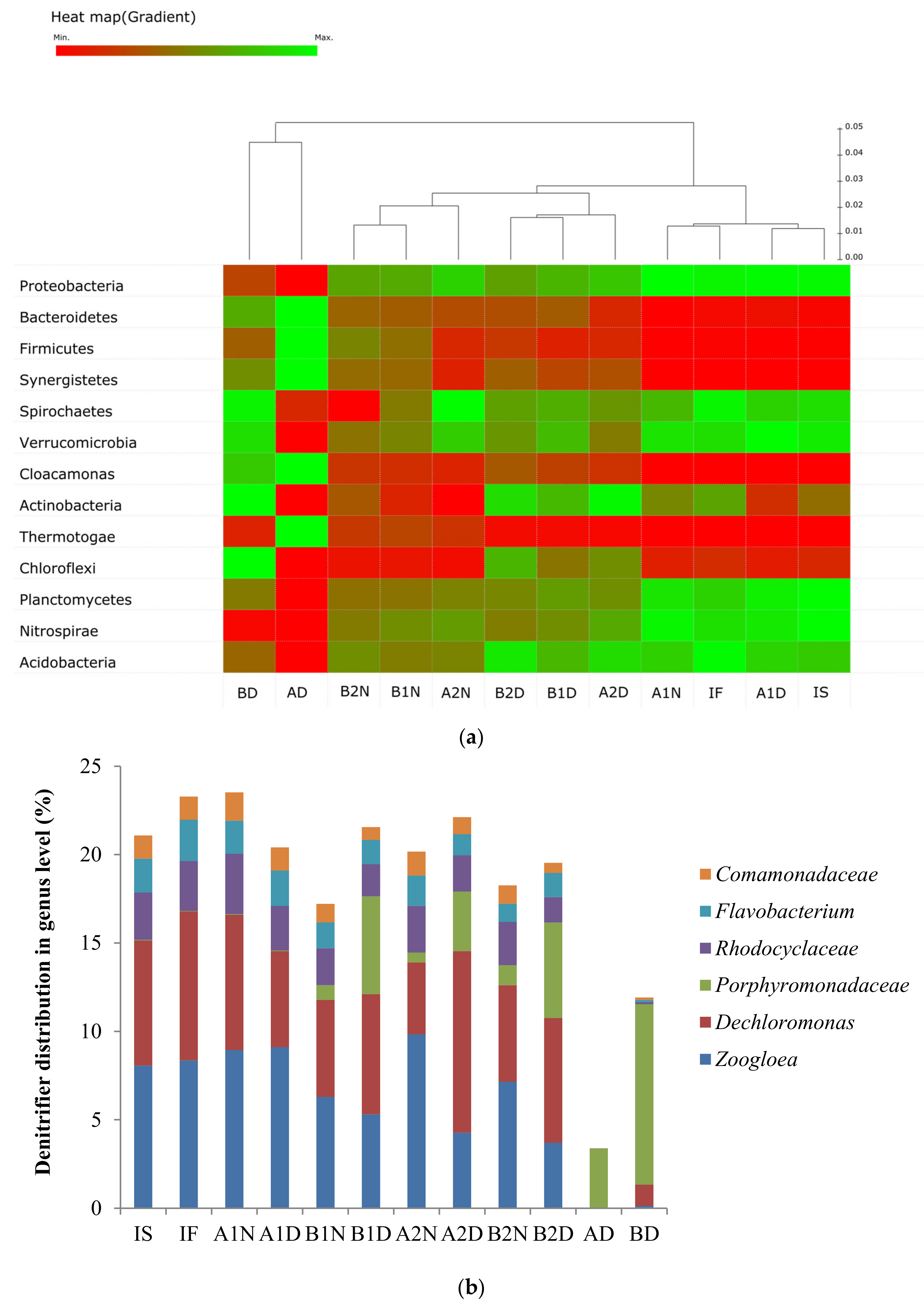
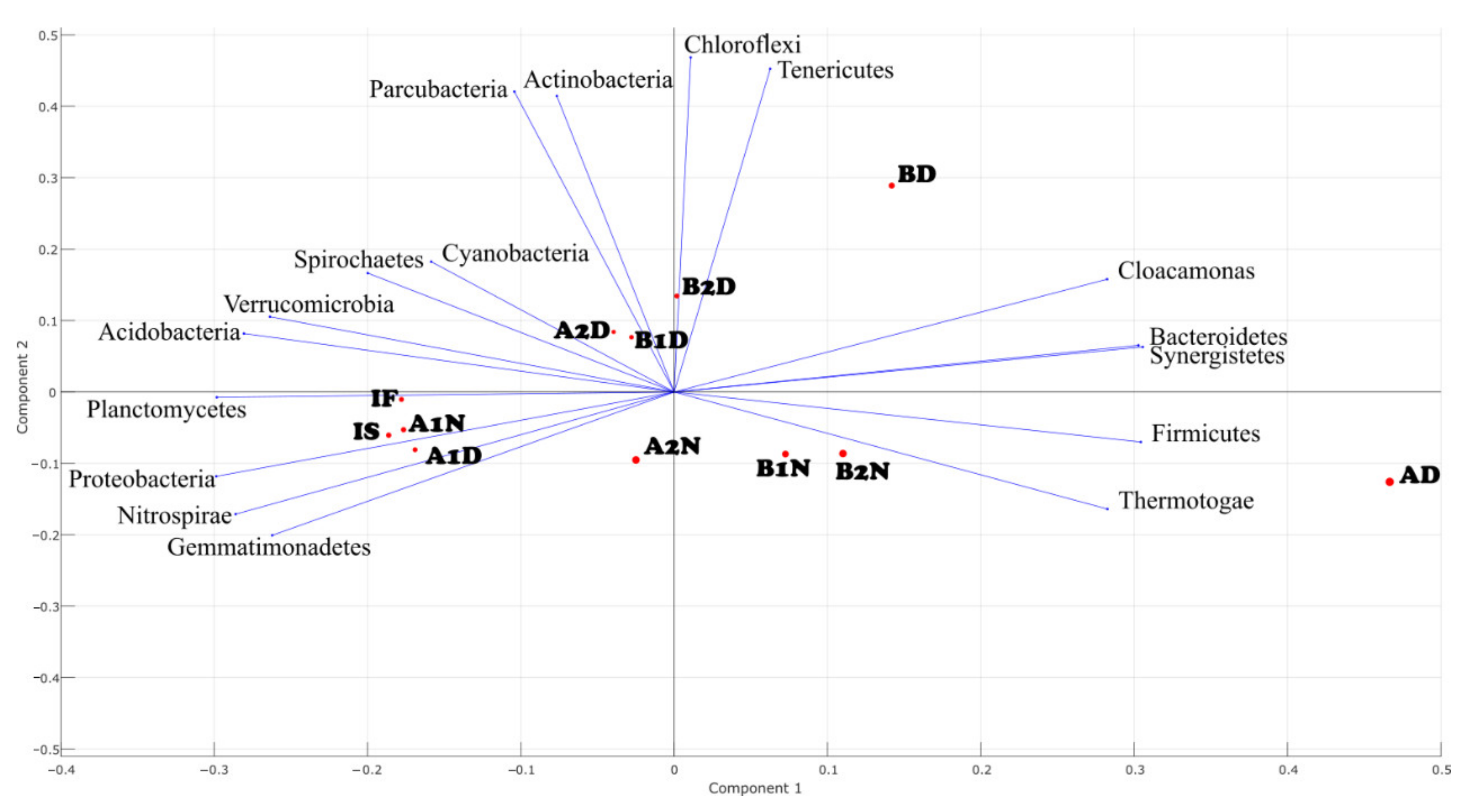
3.3. Change of the Microbial Community under Consecutive Aerobic–Anaerobic Conditions for Nitrification and Denitrification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bock, E.; Schmidt, I.; Stüven, R.; Zart, D. Nitrogen loss caused by denitrifying nitrosomonas cells using ammonium or hydrogen as electron donors and nitrite as electron acceptor. Arch. Microbiol. 1995, 163, 16–20. [Google Scholar] [CrossRef]
- Chung, J.; Amin, K.; Kim, S.; Yoon, S.; Kwon, K.; Bae, W. Autotrophic denitrification of nitrate and nitrite using thiosulfate as an electron donor. Water Res. 2014, 58, 169–178. [Google Scholar] [CrossRef]
- Wang, J.; Lu, H.; Chen, G.-H.; Lau, G.N.; Tsang, W.L.; van Loosdrecht, M.C.M. A novel sulfate reduction, autotrophic denitrification, nitrification integrated (sani) process for saline wastewater treatment. Water Res. 2009, 43, 2363–2372. [Google Scholar] [CrossRef]
- Park, H.I.; Kim, D.k.; Choi, Y.-J.; Pak, D. Nitrate reduction using an electrode as direct electron donor in a biofilm-electrode reactor. Proc. Biochem. 2005, 40, 3383–3388. [Google Scholar] [CrossRef]
- Till, B.A.; Weathers, L.J.; Alvarez, P.J.J. Fe(0)-supported autotrophic denitrification. Environ. Sci. Technol. 1998, 32, 634–639. [Google Scholar] [CrossRef]
- Third, K.A.; Burnett, N.; Cord-Ruwisch, R. Simultaneous nitrification and denitrification using stored substrate (phb) as the electron donor in an sbr. Biotechnol. Bioeng. 2003, 83, 706–720. [Google Scholar] [CrossRef]
- Lu, H.; Chandran, K. Factors promoting emissions of nitrous oxide and nitric oxide from denitrifying sequencing batch reactors operated with methanol and ethanol as electron donors. Biotechnol. Bioeng. 2010, 106, 390–398. [Google Scholar] [CrossRef]
- Hallin, S.; Throbäck, I.N.; Dicksved, J.; Pell, M. Metabolic profiles and genetic diversity of denitrifying communities in activated sludge after addition of methanol or ethanol. Appl. Environ. Microbiol. 2006, 72, 5445–5452. [Google Scholar] [CrossRef]
- Hallin, S.; Pell, M. Metabolic properties of denitrifying bacteria adapting to methanol and ethanol in activated sludge. Water Res. 1998, 32, 13–18. [Google Scholar] [CrossRef]
- Thalasso, F.; Vallecillo, A.; García-Encina, P.; Fdz-Polanco, F. The use of methane as a sole carbon source for wastewater denitrification. Water Res. 1997, 31, 55–60. [Google Scholar] [CrossRef]
- Modin, O.; Fukushi, K.; Yamamoto, K. Denitrification with methane as external carbon source. Water Res. 2007, 41, 2726–2738. [Google Scholar] [CrossRef]
- Elefsiniotis, P.; Wareham, D.G.; Smith, M.O. Use of volatile fatty acids from an acid-phase digester for denitrification. J. Biotechnol. 2004, 114, 289–297. [Google Scholar] [CrossRef]
- Ginige, M.P.; Keller, J.; Blackall, L.L. Investigation of an acetate-fed denitrifying microbial community by stable isotope probing, full-cycle rrna analysis, and fluorescent in situ hybridization-microautoradiography. Appl. Environ. Microbiol. 2005, 71, 8683–8691. [Google Scholar] [CrossRef]
- Liu, F.; Tian, Y.; Ding, Y.; Li, Z. The use of fermentation liquid of wastewater primary sedimentation sludge as supplemental carbon source for denitrification based on enhanced anaerobic fermentation. Bioresour. Technol. 2016, 219, 6–13. [Google Scholar] [CrossRef]
- Lu, H.; Chandran, K.; Stensel, D. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 2014, 64, 237–254. [Google Scholar] [CrossRef]
- Ma, W.; Han, Y.; Ma, W.; Han, H.; Zhu, H.; Xu, C.; Li, K.; Wang, D. Enhanced nitrogen removal from coal gasification wastewater by simultaneous nitrification and denitrification (snd) in an oxygen-limited aeration sequencing batch biofilm reactor. Biores. Technol. 2017, 244, 84–91. [Google Scholar] [CrossRef]
- Lan, C.-J.; Kumar, M.; Wang, C.-C.; Lin, J.-G. Development of simultaneous partial nitrification, anammox and denitrification (snad) process in a sequential batch reactor. Biores. Technol. 2011, 102, 5514–5519. [Google Scholar] [CrossRef]
- Shin, J.-H.; Sang, B.-I.; Chung, Y.-C.; Choung, Y.-k. The removal of nitrogen using an autotrophic hybrid hollow-fiber membrane biofilm reactor. Desalination 2005, 183, 447–454. [Google Scholar] [CrossRef]
- APHA. Standard Method for Examination of Water and Wastewater, 21th ed.; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Kim, O.-S.; Cho, Y.-J.; Lee, K.; Yoon, S.-H.; Kim, M.; Na, H.; Park, S.-C.; Jeon, Y.S.; Lee, J.-H.; Yi, H.; et al. Introducing eztaxon-e: A prokaryotic 16s rrna gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Hamady, M.; Lozupone, C.; Knight, R. Fast unifrac: Facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and phylochip data. ISME J. 2010, 4, 17–27. [Google Scholar] [CrossRef]
- Li, C.; Cao, J.; Ren, H.; Li, Y.; Tang, S. Comparison on kinetics and microbial community among denitrification process fed by different kinds of volatile fatty acids. Process Biochem. 2015, 50, 447–455. [Google Scholar] [CrossRef]
- Chen, K.-C.; Lin, Y.-F. The relationship between denitrifying bacteria and methanogenic bacteria in a mixed culture system of acclimated sludges. Water Res. 1993, 27, 1749–1759. [Google Scholar] [CrossRef]
- Yatong, X. Volatile fatty acids carbon source for biological denitrification. J. Environ. Sci. 1996, 8, 257–269. [Google Scholar]
- Xie, L.; Chen, J.; Wang, R.; Zhou, Q. Effect of carbon source and cod/no3−–n ratio on anaerobic simultaneous denitrification and methanogenesis for high-strength wastewater treatment. J. Biosci. Bioeng. 2012, 113, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, F.; Feng, X.; Liu, Y.; Yan, X.; Zhang, X.; Wang, L.; Zhao, L. Thauera and azoarcus as functionally important genera in a denitrifying quinoline-removal bioreactor as revealed by microbial community structure comparison. FEMS Microbiol. Ecol. 2006, 55, 274–286. [Google Scholar] [CrossRef]
- Fass, S.; Ganaye, V.; Urbain, V.; Manem, J.; Block, J. Volatile fatty acids as organic carbon sources in denitrification. Environ. Technol. 1994, 15, 459–467. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J. Long-term low do enriches and shifts nitrifier community in activated sludge. Environ. Sci. Technol. 2013, 47, 5109–5117. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, S.; Wang, P.; Wang, C.; Guo, C.; Addo, F.G.; Li, Y. Responses of bacterial community structure and denitrifying bacteria in biofilm to submerged macrophytes and nitrate. Sci. Rep. 2016, 6, 36178. [Google Scholar] [CrossRef]
- Li, E.; Lu, S. Denitrification processes and microbial communities in a sequencing batch reactor treating nanofiltration (nf) concentrate from coking wastewater. Water Sci. Technol. 2017, 76, 3289–3298. [Google Scholar] [CrossRef]
- Shen, Z.; Zhou, Y.; Hu, J.; Wang, J. Denitrification performance and microbial diversity in a packed-bed bioreactor using biodegradable polymer as carbon source and biofilm support. J. Hazard. Mater. 2013, 250–251, 431–438. [Google Scholar] [CrossRef]
- Liu, A.-C.; Chou, C.-Y.; Chen, L.-L.; Kuo, C.-H. Bacterial community dynamics in a swine wastewater anaerobic reactor revealed by 16s rdna sequence analysis. J. Biotechnol. 2015, 194, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, H.J.; Lee, D.S.; Jeon, C.O. Characterization of the denitrification-associated phosphorus uptake properties of “candidatus accumulibacter phosphatis” clades in sludge subjected to enhanced biological phosphorus removal. Appl. Environ. Microbiol. 2013, 79, 1969–1979. [Google Scholar] [CrossRef]
- Campanaro, S.; Treu, L.; Kougias, P.G.; De Francisci, D.; Valle, G.; Angelidaki, I. Metagenomic analysis and functional characterization of the biogas microbiome using high throughput shotgun sequencing and a novel binning strategy. Biotechnol. Biofuels 2016, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, F.J.; Duyts, H.; Laanbroek, H.J. Competition for ammonium between nitrifying and heterotrophic bacteria in continuously percolated soil columns. Appl. Environ. Microbiol. 1992, 58, 3303–3311. [Google Scholar] [CrossRef]
- Hanaki, K.; Wantawin, C.; Ohgaki, S. Effects of the activity of heterotrophs on nitrification in a suspended-growth reactor. Water Res. 1990, 24, 289–296. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Bio. Rev. 1997, 61, 533. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Shin, S.G.; Hwang, S.; Lee, C. Continuous fermentation of food waste leachate for the production of volatile fatty acids and potential as a denitrification carbon source. Biores. Technol. 2016, 207, 440–445. [Google Scholar] [CrossRef]
- Bories, A.; Guillot, J.-M.; Sire, Y.; Couderc, M.; Lemaire, S.-A.; Kreim, V.; Roux, J.-C. Prevention of volatile fatty acids production and limitation of odours from winery wastewaters by denitrification. Water Res. 2007, 41, 2987–2995. [Google Scholar] [CrossRef]
- Schellenberger, S.; Kolb, S.; Drake, H.L. Metabolic responses of novel cellulolytic and saccharolytic agricultural soil bacteria to oxygen. Environ. Microbiol. 2010, 12, 845–861. [Google Scholar] [CrossRef]
- Roest, K.; Heilig, H.G.H.J.; Smidt, H.; de Vos, W.M.; Stams, A.J.M.; Akkermans, A.D.L. Community analysis of a full-scale anaerobic bioreactor treating paper mill wastewater. Syst. Appl. Microbiol. 2005, 28, 175–185. [Google Scholar] [CrossRef] [PubMed]
| COD/N Ratio | NH4+-N Removal (%) | NH4+-N Accumulation (ppm) | NO2−-N Accumulation (ppm) | NO3−-N Removal (%) | NO3−-N Removal Rate (ppm/h) |
|---|---|---|---|---|---|
| 0 | 72.4 ± 0.04 | 270.5 ± 0.5 | 60 ± 0 | 0 | 0 |
| Acetate | |||||
| 1 | 73.1 ± 0.47 | 259.5 ± 4.5 | 64.5 ± 0.5 | 27.3 | 6.35 ± 0.05 |
| 2 | 73.5 ± 0.08 | 266 ± 0 | 66 ± 4 | 56.5 | 9.05 ± 0.05 |
| 3 | 74.4 ± 0.70 | 268 ± 6 | 63 ± 1 | 79.9 | 11 ± 0.01 |
| 4 | 74.0 ± 1.29 | 255 ± 11 | 65 ± 0 | 100.0 | 11.45 ± 0.25 |
| Average (s.d.) | 73.8 ± 0.5 | 262.1 ± 5.2 | 64.6 ± 1.1 | ||
| Butyrate | |||||
| 0.5 | 73.7 ± 0.56 | 274.5 ± 2.5 | 65.5 ± 1.5 | 30.3 | 5.5 ± 0 |
| 1 | 70.9 ± 0.33 | 286.5 ± 7.5 | 68.5 ± 0.5 | 66.3 | 9.3 ± 0 |
| 2 | 70.7 ± 0.33 | 282 ± 9 | 64 ± 0 | 100 | 11.2 ± 0.8 |
| 3 | 73.3 ± 0.26 | 271 ± 4 | 68.5 ± 0.5 | 100 | 11.5 ± 0 |
| Average (s.d.) | 72.2 ± 1.4 | 278.5 ± 6.1 | 66.6 ± 1.9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, O.; Cha, S.-j.; Kim, H.; Kim, H.; Sang, B.-I. Dynamic Changes of Microbiome with the Utilization of Volatile Fatty Acids as Electron Donors for Denitrification. Water 2021, 13, 1556. https://doi.org/10.3390/w13111556
Choi O, Cha S-j, Kim H, Kim H, Sang B-I. Dynamic Changes of Microbiome with the Utilization of Volatile Fatty Acids as Electron Donors for Denitrification. Water. 2021; 13(11):1556. https://doi.org/10.3390/w13111556
Chicago/Turabian StyleChoi, Okkyoung, Se-jin Cha, Hyunjin Kim, Hyunook Kim, and Byoung-In Sang. 2021. "Dynamic Changes of Microbiome with the Utilization of Volatile Fatty Acids as Electron Donors for Denitrification" Water 13, no. 11: 1556. https://doi.org/10.3390/w13111556
APA StyleChoi, O., Cha, S.-j., Kim, H., Kim, H., & Sang, B.-I. (2021). Dynamic Changes of Microbiome with the Utilization of Volatile Fatty Acids as Electron Donors for Denitrification. Water, 13(11), 1556. https://doi.org/10.3390/w13111556








