Ecotoxicological Assessment of Microplastics in Freshwater Sources—A Review
Abstract
:1. Microplastics as an Emerging Environmental Problem
2. Microplastics Types and Properties
3. Sources of Microplastics in the Environment
4. Characterization Methods for Microplastics in the Environment
4.1. Microscopy Based Methods
4.2. Spectroscopy-Based Methods
4.3. Chromatography-Based Methods
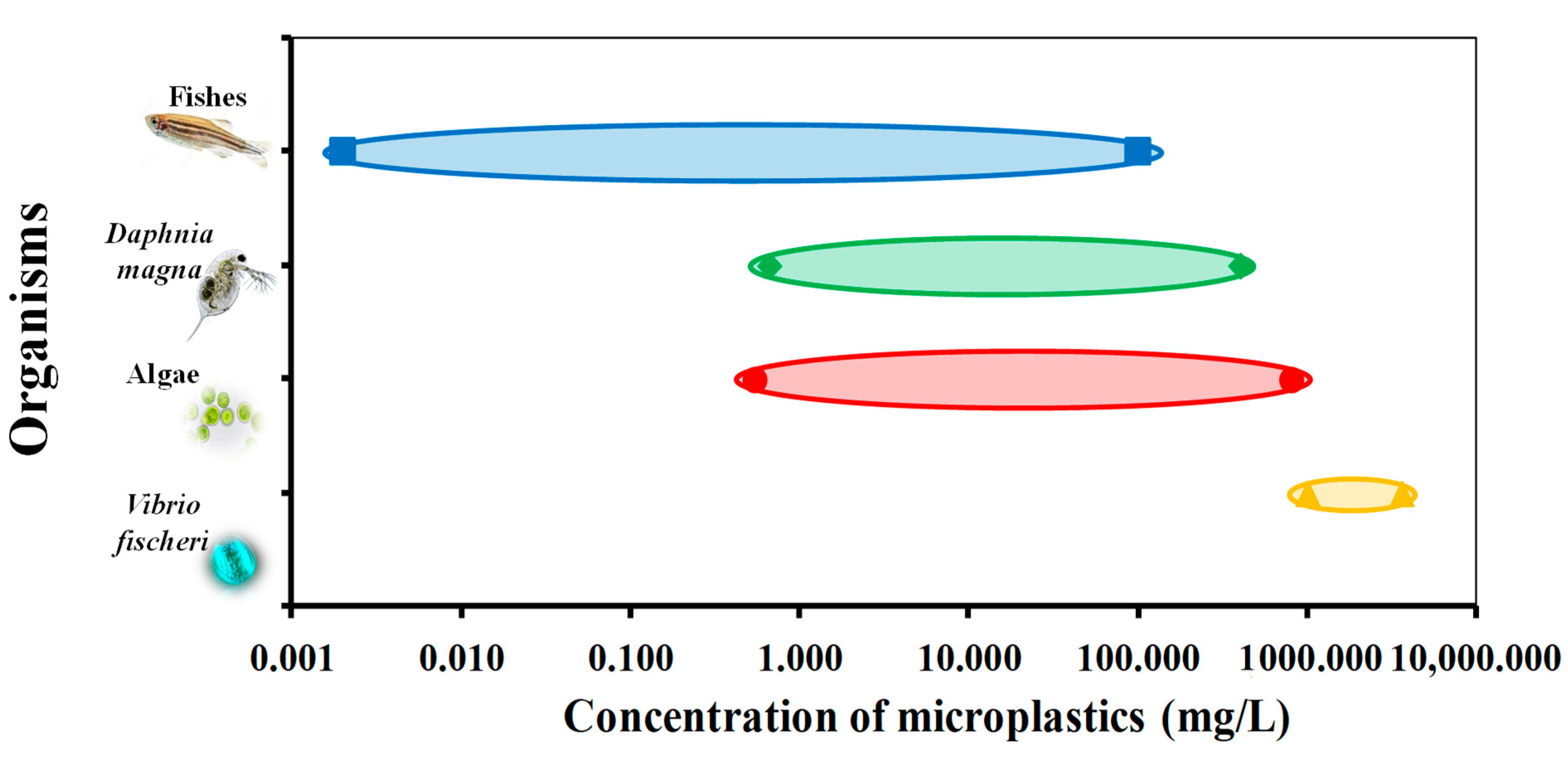
5. Ecotoxicological Effects on Microplastics
5.1. Ecotoxicological Effects on Vibrio fischeri
5.2. Ecotoxicological Effects on Algae
5.3. Ecotoxicological Effects on Daphnids
5.4. Ecotoxicological Effects on Fish
5.5. Cumulative Effects of MPs and Anthropogenic Molecules
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chalmin, P. The history of plastics: From the capitol to the Tarpeian Rock. Field Actions Sci. Rep. 2019, 19, 6–11. [Google Scholar]
- PlasticsEurope, Plastics–the Facts 2019. An analysis of European plastics production, demand and waste data. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 25 October 2020).
- Rodrigues, M.O.; Gonçalves, A.M.M.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Abrantes, N. Effectiveness of a methodology of microplastics isolation for environmental monitoring in freshwater systems. Ecol. Indic. 2017, 89, 488–495. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the Environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef]
- Bond, T.; Ferrandiz-Mas, V.; Felipe-Sotelo, M.; van Sebille, E. The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 685–722. [Google Scholar] [CrossRef]
- Gong, J.; Kong, T.; Li, Y.; Li, Q.; Li, Z.; Zhang, J. Biodegradation of microplastic derived from poly(ethylene terephthalate) with bacterial whole-cell biocatalysts. Polymers 2018, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.P.; Sapp, M.; Schratzberger, M.; Mark Osborn, A. Interactions between microorganisms and marine micro plastics: A call for research. Mar. Technol. Soc. J. 2011, 45, 12–20. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Microplastics are Contaminants of Emerging Concern in Freshwater Environments: An Overview; Wagner, M., Lambert, S., Eds.; Freshwater Microplastics; Springer Open: Cham, Switzerland, 2018; pp. 1–24. [Google Scholar]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Gajšt, T.; Bizjak, T.; Palatinus, A.; Liubartseva, S.; Kržan, A. Sea surface microplastics in Slovenian part of the Northern Adriatic. Mar. Pollut. Bull. 2016, 113, 392–399. [Google Scholar] [CrossRef]
- Jiang, J.Q. Occurrence of microplastics and its pollution in the environment: A review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC-Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Shan, J.; Zhao, J.; Liu, L.; Zhang, Y.; Wang, X.; Wu, F. A novel way to rapidly monitor microplastics in soil by hyperspectral imaging technology and chemometrics. Environ. Pollut. 2018, 238, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Birch, Q.T.; Potter, P.M.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Sources, transport, measurement and impact of nano and microplastics in urban watersheds. Rev. Environ. Sci. Biotechnol. 2020, 19, 275–336. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Ulke, J.; Fonta, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rochad, T.L.; Futtera, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Zou, H.; Zhang, Y.; Zhu, R. Occurrence, source and ecotoxicological effect of microplastics in freshwater environment. Ecol. Environ. Sci. 2017, 26, 1619–1626. [Google Scholar]
- Naji, A.; Nuri, M.; Vethaak, A.D. Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ. Pollut. 2018, 235, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 2136–2140. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Bai, H.; Chen, B.; Sun, X.; Qu, K.; Xia, B. Microplastic pollution in North Yellow Sea, China: Observations on occurrence, distribution and identification. Sci. Total Environ. 2018, 636, 20–29. [Google Scholar] [CrossRef]
- EUROPEAN CHEMICALS AGENCY(ECHA). Available online: https://echa.europa.eu/documents/10162/12414bc7-6bb2-17e7-c9ec-652a20fa43fc (accessed on 27 October 2020).
- D’Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Sci. Rep. 2019, 19, 12–21. [Google Scholar]
- EC. European Strategy for Plastics in a Circular Economy. COM 2018, 28. Available online: https://ec.europa.eu/environment/circular-economy/pdf/plastics-strategy.pdf (accessed on 22 December 2020).
- Gutiérrez, C.; de Haro, J.C.; García, M.T.; Gracia, I.; de Lucas, A.; Rodríguez, J.F. Polystyrene Wastes: Threat or Opportunity? In Environment, Energy and Climate Change I: Environmental Chemistry of Pollutants and Wastes; Jiménez, E., Cabañas, B., Lefebvre, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 261–286. [Google Scholar]
- Rezania, S.; Park, J.; Din, M.F.M.; Taib, S.M.; Talaiekhozani, A.; Yadav, K.K.; Kamyab, H. Microplastics pollution in different aquatic environments and biota: A review of recent studies. Mar. Pollut. Bull. 2018, 133, 191–208. [Google Scholar] [CrossRef]
- Teck Kim, Y.; Min, B.; Won Kim, K. General characteristics of packaging materials for food system. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2014; pp. 13–35. [Google Scholar]
- Glaser, J.A. Biological degradation of polymers in the environment. In Plastics in the Environment; Gomiero, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- McLain, V.C. Final report on the safety assessment of polyethylene. Int. J. Toxicol. 2007, 26, 115–127. [Google Scholar]
- Summers, J.W. The melting temperature (or not melting) of poly(vinyl chloride). J. Vinyl. Addit. Techn. 2008, 14, 105–109. [Google Scholar] [CrossRef]
- Görtz, H.H. Polystyrene: Syndiotactic. In Encyclopedia of Materials: Science and Technology; Jürgen Buschow, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 7742–7744. [Google Scholar]
- Beyler, C.L.; Hirschler, M.M. Thermal decomposition of polymers. In SFPE Handbook of Fire Protection Engineering, 4th ed.; DiNenno, P.J., Ed.; National Fire Protection Association: Quincy, MA, USA, 2002; pp. 112–143. [Google Scholar]
- Ehrenstein, G.W. Polymeric Materials: Structure, Properties, Applications; Hanser Gardner Publications: München, Germany, 2012. [Google Scholar]
- Hrnjak-Murgić, Z.; Rešček, A.; Ptiček Siročić, A.; Kratofil Krehula Lj Katančić, Z. Nanoparticles in Active Polymer Food Packaing; Smithers Pira: Surrey, UK, 2015. [Google Scholar]
- Sangeetha Devi, R.; Rajesh Kannan, V.; Natarajan, K.; Nivas, D.; Kannan, K.; Chandru, S.; Robert Anthony, A. The Role of Microbes in Plastic Degradation, in R. Chandra: Environmental Waste Management; CRC Press: Boca Raton, FL, USA, 2015; pp. 341–370. [Google Scholar]
- Dutta Laha, S.; Dutta, K.; Paban Kundu, P. Biodegradation of low density polyethylene films. In Handbook of Research on Microbial Tools for Environmental Waste Management; Pathak, V.M., Navneet, Eds.; IGI Global: Hershey, PA, USA, 2018; pp. 282–318. [Google Scholar]
- Krueger, M.C.; Harms, H.; Schlosser, D. Prospects for microbiological solutions to environmental pollution with plastics. Appl. Microbiol. Biotechnol. 2015, 99, 8857–8874. [Google Scholar] [CrossRef] [PubMed]
- Arutchelvi, J.; Sudhakar, M.; Arkatkar, A.; Doble, M.; Bhaduri, S.; Uppara, P.V. Biodegradation of polyethylene and polypropylene. Indian J. Biotechnol. 2008, 7, 9–22. [Google Scholar]
- Artham, T.; Doble, M. Biodegradation of aliphatic and aromatic polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef]
- Vasile, C. Degradation and decomposition. In Handbook of Polyolefins Synthesis and Properties; Vasile, C., Seymour, R.B., Eds.; Marcel Dekker Inc: New York, NY, USA, 1993; pp. 479–506. [Google Scholar]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef]
- Mor, R.; Sivan, A. Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber: Biodegradation of polystyrene. Biodegradation 2008, 19, 851–858. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to poly (ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.D. Enzymatic degradation of poly(ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. Fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC-Trend. Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 1–16. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Do, T.C.V.; Scherer, H.W. Compost and biogas residues as basic materials for potting substrates. Plant. Soil. Environ. 2012, 58, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, S.; Sinclair, C.J.; Boxall, A.B. Occurrence, degradation and effect of polymer-based materials in the environment. Rev. Environ. Contamin. Toxicol. 2014, 227, 1–53. [Google Scholar]
- Elert, A.M.; Becker, R.; Duemichen, E.; Eisentraut, P.; Falkenhhagen, J.; Sturm, H.; Braun, U. Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 2017, 231, 1256–1264. [Google Scholar] [CrossRef]
- Industrievereinigung Chemiefaser e.V (2015) Production since 1975. Available online: https://www.ivc-ev.de/live/index.php?page_id=87 (accessed on 30 October 2020).
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Raschid-Sally, L.; Thebo, A. Global wastewater and sludge production: Treatment and use. In Wastewater: Economic Asset in an Urbanizing World; Drechsel, P., Qadir, M., Wichelns, D., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 15–38. [Google Scholar]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senathirajah, K.; Subashchandrabose, S.R.; Evans, G.; Thavamani, P. Transport and fate of microplastics in wastewater treatment plants: Implications to environmental health. Rev. Environ. Sci. Biotechnol. 2018, 17, 637–653. [Google Scholar] [CrossRef]
- EU. Council Directive of 21 May 1991 concerning urban waste water treatment (91/271/EEC). Off. J. Eur. Communities L135 1991, 40–91. [Google Scholar]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, J.C.; Frias, J.G.L.; Micaelo, A.C.; Sobral, P. Resin pellets from beaches of the Portuguese coast and adsorbed persistent organic pollutants. Estuar. Coast. Shelf. Sci. 2013, 130, 62–69. [Google Scholar] [CrossRef]
- Imhof, H.K.; Laforsch, C.; Wiesheu, A.C.; Schmid, J.; Anger, P.M.; Niessner, R.; Ivleva, N.P. Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Res. 2011, 98, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 2015, 175, 39–46. [Google Scholar] [CrossRef]
- Ivleva, N.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. 2017, 56, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.-P.W.; Galbraith, M.; Ross, P.S. Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 320–330. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Kooi, M.; Besseling, E.; Kroeze, C.; van Wezel, A.P.; Koelmans, A.A. Modeling the Fate and Transport of Plastic Debris in Fresh-waters: Review and Guidance. In Freshwater Microplastics: Emerging Environmental Contaminants; Wagner, S.M., Ed.; Lambert Springer Open: Cham, Switzerland, 2018; pp. 125–152. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Statement on the presence of microplastics and nano-plastics in food, with particular focus on seafood. EFSA J. 2016, 14, 4501. [Google Scholar]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Chal-lenges in analytical chemistry-A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef]
- Qiu, Q.; Tan, Z.; Wang, J.; Peng, J.; Li, M.; Zhan, Z. Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuarine Coast. Shelf Sci. 2016, 176, 102–109. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.; Song, Y.K.; Hong, S.H.; Jang, Y.C.; Jang, M.; Heo, N.W.; Han, G.M.; Lee, M.J.; Kang, D.; et al. Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pollut. Bull. 2013, 77, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sierra, I.; Chialanza, M.R.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of microplastics in wastewater samples by means of polarized light optical microscopy. Environ. Sci. Pollut. Res. 2019, 27, 7409–7419. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, C. Recent advance in the analysis methodologies for microplastics in aquatic organisms: Current knowledge and research challenges. Anal. Methods 2020, 12, 1–37. [Google Scholar] [CrossRef]
- Klein, S.; Dimzon, I.K.; Eubeler, J.; Knepper, T.P. Analysis, Occurrence, and Degradation of Microplastics in the Aqueous Envi-ronment. In Freshwater Microplastics: Emerging Environmental Contaminants; Wagner, M., Lambert, S., Eds.; Springer Open: Cham, Switzerland, 2018; pp. 51–68. [Google Scholar]
- Girão, A.V.; Caputo, G.; Ferro, M. Application of Scanning Electron Microscopy–Energy Dispersive X-Ray Spectroscopy (SEM-EDS). In Arsenic Speciation in Algae; Elsevier: Amsterdam, The Netherlands, 2017; Volume 75, pp. 153–168. [Google Scholar]
- Mehdinia, A.; Dehbandi, R.; Hamzehpour, A.; Rahnama, R. Identification of microplastics in the sediments of southern coasts of the Caspian Sea, north of Iran. Environ. Pollut. 2020, 258, 113738. [Google Scholar] [CrossRef]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer Open: Cham, Switzerland, 2009. [Google Scholar]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.; Ribeiro-Claro, P.J. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- PAPER WP53077, Guide to the identification of microplastics by FTIR and Raman spectroscopy, Thermofisher Scientific. 2018. Available online: https://assets.thermofisher.com/TFS-Assets/MSD/Application-Notes/WP53077-microplastics-identification-ftir-raman-guide.pdf (accessed on 2 November 2020).
- Mohan, A.J.; Sekhar, V.C.; Bhaskar, T.; Nampoothiri, K.M. Microbial assisted High Impact Polystyrene (HIPS) degradation. Bioresour. Technol. 2016, 213, 204–207. [Google Scholar] [CrossRef]
- Leslie, H.; Brandsma, S.H.; Van Velzen, M.; Vethaak, A. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.C.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Noda, I.; Dowrey, A.E.; Haynes, J.L.; Marcott, C. Group Frequency Assignments for Major Infrared Bands Observed in Common Synthetic Polymers. In Physical Properties of Polymers Handbook; Springer: Berlin/Heidelberg, Germany, 2007; pp. 395–406. [Google Scholar]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Mecozzi, M.; Nisini, L. The differentiation of biodegradable and non-biodegradable polyethylene terephthalate (PET) samples by FTIR spectroscopy: A potential support for the structural differentiation of PET in environmental analysis. Infrared Phys. Technol. 2019, 101, 119–126. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, M.; Scholz-Böttcher, B.M.; Oberbeckmann, S.; Labrenz, M.; Fischer, D.; Eichhorn, K.-J.; Voit, B. Comparison of μ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal. Bioanal. Chem. 2018, 410, 5313–5327. [Google Scholar] [CrossRef] [PubMed]
- Bart, J.C.J. Plastics Additives: Advanced Industrial Analysis; IOS Press: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro-Claro, P.; Nolasco, M.M.; Araujo, C. Characterization of Microplastics by Raman Spectroscopy. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 75, pp. 119–151. [Google Scholar]
- Zada, L.; Leslie, H.A.; Vethaak, A.D.; Tinnevelt, G.; Janssen, J.; Boer, J.F.; de Ariese, F. Fast microplastics identification with stim-ulated Raman scattering microscopy. J. Raman Spectrosc. 2018, 49, 1136–1144. [Google Scholar] [CrossRef] [Green Version]
- De Tender, C.A.; Devriese, L.I.; Haegeman, A.; Maes, S.; Ruttink, T.; Dawyndt, P. Bacterial Community Profiling of Plastic Litter in the Belgian Part of the North Sea. Environ. Sci. Technol. 2015, 49, 9629–9638. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhang, K.; Chen, X.; Shi, H.; Luo, Z.; Wu, C. Sources and distribution of microplastics in China’s largest inland lake-Qinghai Lake. Environ. Pollut. 2018, 235, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, K.C.; Rajwa, B.; Li, J.; Yang, S.; Lin, H.; Liao, C.-S.; Eakins, G.; Kuang, S.; Patsekin, V.; et al. Stimulated Raman scattering flow cytometry for label-free single-particle analysis. Optica 2017, 4, 103–109. [Google Scholar] [CrossRef]
- Ghosal, S.; Chen, M.; Wagner, J.; Wang, Z.M.; Wall, S. Molecular identification of polymers and anthropogenic particles extracted from oceanic water and fish stomach–A Raman micro-spectroscopy study. Environ. Pollut. 2018, 233, 1113–1124. [Google Scholar] [CrossRef]
- Duemichen, E.; Eisentraut, P.; Celina, M.; Braun, U. Automated thermal extraction-desorption gas chromatography mass spec-trometry: A multifunctional tool for comprehensive characterization of polymers and their degradation products. J. Chromatogr. A 2019, 1592, 133–142. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine CopepodCalanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- De Sá, L.C.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Piazza, V.; Albentosa, M.; Bebianno, M.J.; Cardoso, C.; Faimali, M.; Garaventa, F.; Garrido, S.; González, S.; Pérez, S.; et al. Microplastics do not affect standard ecotoxicological endpoints in marine unicellular organisms. Mar. Pollut. Bull. 2019, 143, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Frydkjær, C.K.; Iversen, N.; Roslev, P. Ingestion and Egestion of Microplastics by the Cladoceran Daphnia magna: Effects of Regular and Irregular Shaped Plastic and Sorbed Phenanthrene. Bull. Environ. Contam. Toxicol. 2017, 99, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A.; et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigurnjak, M.; Ukić, Š.; Cvetnić, M.; Markić, M.; Novak Stankov, M.; Rasulev, B.; Kušić, H.; Lončarić Božić, A.; Rogošić, M.; Bolanča, T. Combined toxicities of binary mixtures of alachlor, chlorfenvinphos, diuron and isoproturon. Chemosphere 2020, 240, 124973. [Google Scholar] [CrossRef]
- Gagné, F. Toxicity and disruption of quorum sensing in Aliivibrio fisheri by environmental chemicals: Impacts of selected contami-nants and microplastics. J. Xenobiot. 2017, 7, 15–20. [Google Scholar] [CrossRef]
- Casado, M.P.; Macken, A.; Byrne, H.J. Ecotoxicological assessment of silica and polystyrene nanoparticles assessed by a multitrophic test battery. Environ. Int. 2013, 51, 97–105. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical Adsorption of Charged Plastic Nanoparticles Affects Algal Photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton response to polystyrene mi-croplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, P.; Zhang, X.; Zhang, Y.; Xie, S.; Deng, J. Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazard. Mater. 2019, 374, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic interactions with freshwater microalgae: Het-ero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yan, Z.; Lu, G.; Ji, Y. Single and combined effects of microplastics and roxithromycin on Daphnia magna. Environ. Sci. Pollut. Res. 2019, 26, 17010–17020. [Google Scholar] [CrossRef]
- Rehse, S.; Kloas, W.; Zarfl, C. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 2016, 153, 91–99. [Google Scholar] [CrossRef]
- Jaikumar, G.; Baas, J.; Brun, N.R.; Vijver, M.G.; Bosker, T. Acute sensitivity of three Cladoceran species to different types of mi-croplastics in combination with thermal stress. Environ. Pollut. 2018, 239, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Kokalj, A.J.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef]
- Jemec Kokalj, A.; Kuehnel DPuntar, B.; Žgajnar Gotvajn, A.; Kalčikova, G. An exploratory ecotoxicity study of primary micro-plastics versus aged in natural waters and wastewaters. Environ. Pollut. 2019, 254, 112980. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 1–8. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of micro-plastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584, 1022–1031. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H.-Q. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pegado, T.S.S.; Schmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First evidence of microplastic inges-tion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Adil, M.; Ehtisham-Ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; McNerny, D.Q.; Baker, J.R.; Orr, B.G.; Banaszak Holl, M.M. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayersnls. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef]
- Canniff, P.M.; Hoang, T.C. Microplastic ingestion by Daphnia magna and its enhancement on algal growth. Sci. Total Environ. 2018, 633, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Huang, A.; Cao, S.; Sun, F.; Wang, L.; Guo, H.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef]
- Ogonowski, M.; Schür, C.; Jarsén, Å.; Gorokhova, E. The Effects of Natural and Anthropogenic Microparticles on Individual Fitness in Daphnia magna. PLoS ONE 2016, 11, e0155063. [Google Scholar] [CrossRef]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Nanoplastic Affects Growth of S. obliquus and Reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef]
- Sanchez, W.; Bender, C.; Porcher, J.M. Wild gudgeons (Gobio gobio) from French rivers are contaminated by microplastics: Pre-liminary study and first evidence. Environ. Res. 2014, 128, 98–100. [Google Scholar] [CrossRef]
- Campbell, S.H.; Williamson, P.R.; Hall, B.D. Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. Facet 2017, 2, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.; Barletta, M.; Costa, M. Ingestion of nylon threads by Gerreidae while using a tropical estuary as foraging grounds. Aquat. Biol. 2012, 17, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.C.L.; Ribeiro, A.L.P.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Lönnstedt, O.M.; Eklöv, P. Environmentally relevant concentrations of microplastic particles influence larval fish ecology. Science 2016, 352, 1213–1216. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Saley, A.; Smart, A.; Bezerra, M.; Burnham, T.; Capece, L.; Lima, L.; Carsh, A.; Williams, S.; Morgan, S. Microplastic accumulation and biomagnification in a coastal marine reserve situated in a sparsely populated area. Mar. Pollut. Bull. 2019, 146, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Pazos, R.S.; Maiztegui, T.; Colautti, D.C.; Paracampo, A.H.; Gómez, N. Microplastics in gut contents of coastal freshwater fish from Río de la Plata estuary. Mar. Pollut. Bull. 2017, 122, 85–90. [Google Scholar] [CrossRef]
- Veneman, W.J.; Spaink, H.P.; Brun, N.R.; Bosker, T.; Vijver, M.G. Pathway analysis of systemic transcriptome responses to injected polystyrene particles in zebrafish larvae. Aquat. Toxicol. 2017, 190, 112–120. [Google Scholar] [CrossRef]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physio-logical, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2019, 710, 1–34. [Google Scholar]
- Lee, W.S.; Cho, H.J.; Kim, E.; Huh, Y.H.; Kim, H.J.; Kim, B.; Kang, T.; Lee, J.S.; Jeong, J. Bioaccumulation of polystyrene nano-plastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019. [Google Scholar] [CrossRef]
- Parenti, C.C.; Ghilardi, A.; Della Torre, C.; Magni, S.; Del Giacco, L.; Binelli, A. Evaluation of the infiltration of polystyrene nano-beads in zebrafish embryo tissues after short-term exposure and the related biochemical and behavioural effects. Environ. Pollut. 2019, 254, 112947. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yu, W.; Li, C.; Liu, Y.; Qiu, J.; Kong, F. Adsorption behavior of three triazole fungicides on polystyrene microplastics. Sci. Total Environ. 2019, 691, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Lavorante, B.R.B.O.; Montenegrod, B.S.M.; Guilhermino, L. Influence of microplastics on the toxicity of the pharma-ceuticals procainamide and doxycycline on the marine microalgae Tetraselmis chuii. Aquat. Toxicol. 2018, 197, 143–152. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J.; Alcolea, A.; Brebbia, C.A.; Itoh, H. Microplastics and microfibers in the sludge of a municipal wastewater treatment plant. Int. J. Sustain. Dev. Plan. 2016, 11, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, Q.; Lu, T.; Ke, M.; Zhu, Y.; Zhang, M.; Zhang, Z.; Du, B.; Pan, X.; Sun, L.; et al. The combined toxicity effect of nanoplastics and glyphosate on Microcystis aeruginosa growth. Environ. Pollut. 2018, 243, 1106–1112. [Google Scholar] [CrossRef]
- Sleight, V.A.; Bakir, A.; Thompson, R.C.; Henry, T.B. Assessment of microplastic-sorbed contaminant bioavailability through analysis of biomarker gene expression in larval zebrafish. Mar. Pollut. Bull. 2017, 116, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Luís, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [CrossRef]
- Besseling, E.; Wegner, A.; Foekema, E.M.; Heuvel-Greve, M.J.V.D.; Koelmans, A.A. Effects of Microplastic on Fitness and PCB Bioaccumulation by the Lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.I.; De Witte, B.; Vethaak, A.D.; Hostens, K.; Leslie, H. Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): An experimental study. Chemosphere 2017, 186, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Vijver, M.G.; Lahive, E.; Spurgeon, D.J.; Svendsen, C.; Heutink, R.; Van Bodegom, P.M.; Baas, J. Acute toxicity of organic pesticides to Daphnia magna is unchanged by co-exposure to polystyrene microplastics. Ecotoxicol. Environ. Saf. 2018, 166, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EU. Directive 2013/39/EU of the European Parliament and of the Council amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Commun. 2013, 226, 1–17. [Google Scholar]
- JCR. Technical Report: Review of the 1st Watch List under the Water Framework Directive and recommendations for the 2nd Watch List. 2018. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC111198/wl_report_jrc_2018_04_26_final_online.pdf (accessed on 23 October 2020).
- Alavanja, M.C.R. Pesticides use and exposure extensive worldwide. Rev. Environ. Health 2009, 24, 303–309. [Google Scholar] [CrossRef]
- Astner, A.; Hayes, D.G.; O’Neill, H.; Evans, B.; Pingali, S.; Urban, V.; Young, T. Mechanical formation of micro- and nano-plastic materials for environmental studies in agricultural ecosystems. Sci. Total Environ. 2019, 685, 1097–1106. [Google Scholar] [CrossRef]
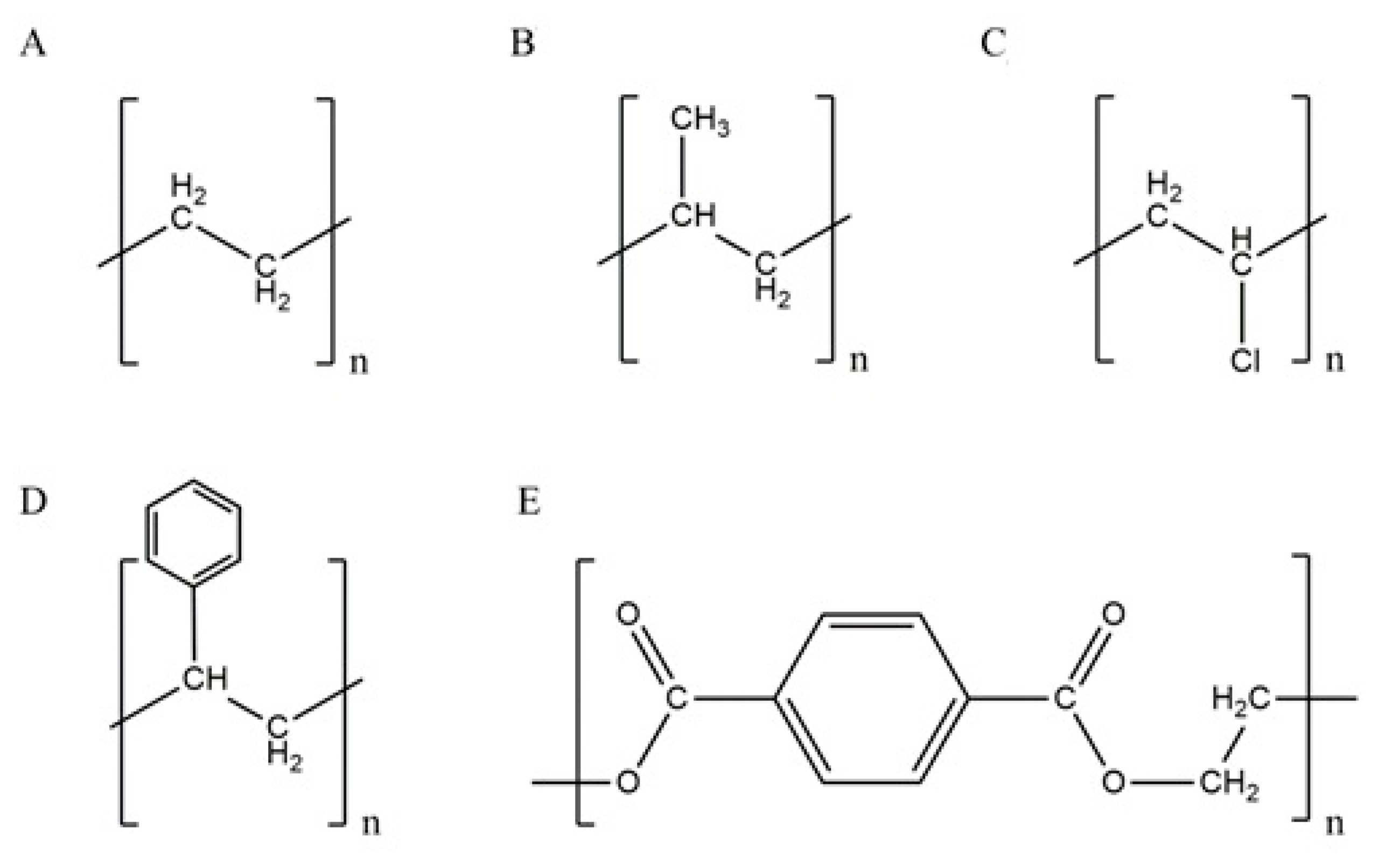



| Types of Plastic | PE | PP | PVC | PS | PET |
|---|---|---|---|---|---|
| Density (g cm−3) | 0.91–0.97 [9,36], 0.91–0.92 (23/4 °C) [37] | 0.85–0.94 [9] 0.89–0.92 [36], 0.94–0.97 (23/4 °C) [37] | 1.38 [9], 1.30–1.58 [36], 1.35–1.45 (23/4 °C) [37] | 0.96–1.05 [9], 1.04–1.05 [36], 0.90–0.91 (23/4 °C) [37] | 1.34–1.39 [9], 1.29 [36], 1.03–1.09 (23/4 °C) [37] |
| Melting point (°C) | 90–110 [38], 98–130 [36] | 168–175 [36] | 115–245 [39] | none (atactic), 240 (isotactic), 270 (syndiotactic), [40] | 245 [36] |
| Glass transition temperature (°C) | −25 [36] | −10 [36], −20 [41] | 75–105 [36] 80–85 [41] | 74–105 [36], > 80 [41] | 73 [36], 70 [41] |
| Tensile strength (MPa) | 8–32 [36] | 31–41 [36] | 41–52 [36] | 36–52 [36] | 48 [36] |
| Crystallinity (%) | 45–95 [9], 50 [37] | 50–80 [9], 65 [41], 50 [37] | High [9], 5–15 [41], 0 [37] | Low [9], 0 [37] | High [41], 30–40 [42], 0–50 [37] |
| Lifespan (year) | 10–600 [37] | 10–600 [37] | 50–100 [37] | 50–80 [37] | 450 [37] |
| Polymer | Functional Group | Wavenumber (cm−1) |
|---|---|---|
| PE | CH2 symmetric stretch | 2918; 2850 |
| CH2 asymmetric stretch | 2919 | |
| CH2 bend | 1473; 1463 | |
| CH3 symmetric stretch | 1377 | |
| CH2 wag | 1366; 1351;1176 | |
| CH2 twist | 1050 | |
| CH2 rock | 731–720 | |
| PP | CH2 asymmetric stretch | 2919 |
| CH3 asymmetric stretch | 2951 | |
| CH3 symmetric stretch | 2868 | |
| CH2 symmetric stretch | 2837 | |
| CH2 scissors | 1458 | |
| CH3 symmetric bend, CH2 wag | 1377 | |
| CH bend, CH2 twist, CH3 rock | 1256 | |
| CH2 twist, C-C stretch, C-H bend | 1220 | |
| CH bend, CH3 rock, C-C stretch | 1168 | |
| C-C stretch, CH3 rock, CH2 wag, CH twist, CH bend | 1104 | |
| C-CH3 stretch, C-C stretch, CH bend | 1045 | |
| CH3 rock, CH3 wag, CH bend | 998 | |
| CH3 rock, C-C stretch | 941 | |
| CH3 rock | 937 | |
| CH3 rock, CH3 rock, CH bend | 899 | |
| CH2 rock, C-CH3 stretch | 841 | |
| CH2 rock, C-C stretch, C-CH stretch | 809 | |
| CH2 rock | 730; 720 | |
| PVC | CH2 asymmetric stretch | 2904 |
| CH2 symmetric stretch | 2837 | |
| CH2 wag | 1354 | |
| CH bend | 1333; 1254; 1243 | |
| C-C stretch | 1099 | |
| CH2 rock | 970; 957 | |
| C-Cl stretch | 603 | |
| PET | CH2 asymmetric stretch | 2969 |
| C=O stretch | 1740–1710 | |
| CH2 bend | 1470 | |
| CH2 wag | 1370–1340 | |
| C-C-O asymmetric stretch bonded to aromatic ring | 1250 | |
| C-O stretch | 1260 | |
| C-O-C stretch | 1100 | |
| aromatic in-plane CH bend | 1019 | |
| oxy-methylene group bend | 973–898 | |
| aromatic out-of-plane CH wag | 875 | |
| C=O rock, C-O deformation | 795 | |
| aromatic in-phase CH wag | 730 | |
| PS | CH2 asymmetric stretch | 2924 |
| CH2 symmetric stretch | 2850 | |
| CH2 bend | 1451 | |
| aromatic CH stretch | 3024 | |
| aromatic ring stretch | 1604; 1492 | |
| aromatic CH bend | 1027 |
| Microorganism/Organism | Type of MPs | Size of MPs | Concentration of MPs | Parameters Value | Effects | References |
|---|---|---|---|---|---|---|
| Vibrio fischeri | PE | 1.00–3.00 µm | 1000.0 mg/L | EC20 = 3600.0 mg/L (5 min) EC20 = 2600.0 mg/L (30 min) | decrease of bioluminescence | [112] |
| PS-PEI a | 0.06 µm | 3.0–1000.0 mg/L | EC50 = ≥ 1000.0 mg/L (30 min) | [113] | ||
| 0.11 µm | EC50 = ≥ 1000.0 mg/L (30 min) | |||||
| Algae Pseudokirchneriella subcapitata | PS-PEI a | 0.06 µm | 0.1–1.0 mg/L | EC50 = 0.58 ± 0.04 mg/L (72 h) | inhibition of algal growth | [113] |
| 0.11 µm | 0.1–0.8 mg/L | EC50 = 0.54 ± 0.06 mg/L (72 h) | ||||
| Chlorella sp. | PS | 0.02 µm | 80.0–800.0 mg/L | kb = 3918.0 (mg/L)1−n | adsorption of particles on algae, reduction of photosynthesis, oxidative stress | [114] |
| Scenedesmus sp. | kb = 4309.0 (mg/L)1−n | |||||
| Scenedesmus sp. | PS | 0.10 µm | 10.0, 50.0 and 100.0 mg/L | IR c = 21.0, 29.0, 38.5% | inhibition of algal growth, morphological changes, oxidative stress | [115] |
| 1.00 µm | IR c = 20.9, 28.4, 38.1% | |||||
| Chlorella pyrenoidosa | PP | 64.0–236.0 µm | 5.0, 10.0, 50.0, 100.0, 250.0, 500.0 mg/L | IRa d = 10.61, 15.86, 22.10, 31.08, 25.53, 24.57% | decrease of chlorophyll a content and photosynthetic activity | [116] |
| PVC | 111.0–216.0 µm | IRa d = 20.39, 37.67, 49.46, 48.49, 55.23, 55.17% | ||||
| Microcystis flos-aquae | PP | 64.00–236.00 µm | IRa d = 11.13, 1.29, 10.52, 13.13, 13.06, 16.92% | |||
| PVC | 111.0–216.0 µm | IRa d = 9.55, 24.92, 23.97, 18.61, 32.20, 46.93% | ||||
| Chlamydomas reinhardtii | PP | 400.0–1000.0 µm | 400.0 mg/L | IR c = ~18.0% (78 days) | inhibition of algal growth, formation of hetero-aggregates | [117] |
| Daphnia magna | PS-PEI a | 0.06 µm | 0.33–3.30 mg/L | EC50 = 0.77 ± 0.10 mg/L (48 h) | immobilization rate | [113] |
| 0.11 µm | EC50 = 0.66 ± 0.17 mg/L (48 h) | |||||
| PS | 1.0 µm | 0.1–600.0 mg/L | EC50 = 66.97 mg/L (48 h) LC50 = 87.83 mg/L (48 h) | immobilization rate, oxidative stress, mortality | [118] | |
| 10.0 µm | 0.01–40.0 mg/L | EC50 = 199.94 mg/L (48 h) LC50 = 291.69 mg/L (48 h) | ||||
| PE | 1.0 µm | 12.5, 25.0, 50.0, 100.0, 200.0 and 400.0 mg/L | ID e = 25.00% ± 1.91, 35.00% ± 1.00, 55.00% ± 1.00, 50.00% ± 2.58, 75.00% ± 1.00, 35.00% ± 1.91 (96 h) | immobilization rate, accumulation in gut | [119] | |
| PE | 1.00–5.00 µm | 3.0, 4.0, 5.0, 6.0 and 7.0 f particles/mL | LC50 f = 32.0 particles/mL (48 h, 18 °C) LC50 f = 18.0 particles/mL (96 h, 18 °C) LC50 f = 10.0 particles/mL (48 h, 22 °C) LC50 f = 5.8 particles/mL (96 h, 22 °C) LC50 f = 8.0 particles/mL (48 h, 26 °C) LC50 f = 4.0 particles/mL (96 h, 26 °C) | immobilization rate, mortality | [120] | |
| PET | fibers of 20.0 µm thickness | 12.5–100.0 mg/L | EC50 = 1.34 mg/L (24 h) mortality was in the range 20.0–40.0% for 12.5–100.0 mg/L | immobility, accumulation in gut, mortality | [121] | |
| Fish Danio rerio | PE | 140.6 ± 80.0 µm | 100 mg/L | 75.0% deformed embryos (96 h, aged MP in WWTP effluent) EC50 ≤ 1.0% (96 h, aged MP in landfill leachate) | impact on development | [122] |
| PE, PP, PS, and PVC | 0.10, 1.0 and 5.0 µm | 0.001–10.0 mg/L | SP g = 73.0% ± 24.0 (PP, after 10 days, at 10.0 mg/L) SP g = 83.0% ± 24.0 (PVC, after 10 days, at 10.0 mg/L) | morphological deformations, damage of the intestine, mortality | [123] | |
| PS | 45.0 µm 0.05 µm | 1.0 mg/L | 22.0%, 6.1% suppressed locomotor ability, body length during exposure at 0.05 µm particles, respectively | suppressed locomotor activity, decrease of body length, deterioration of nervous and visual systems | [124] | |
| PS | 0.07 µm 5.0 µm | 0.002, 0.2 and 2.0 mg/L | 5.7 × 10−4, 1.25 × 10−3 and 8.9 × 10−4 mg/mg fish (5.0 µm particles accumulated in gill, liver, and gut, respectively) | accumulation of particles in fish gill, gut, and liver, deterioration of liver metabolism, oxidative stress | [125] | |
| 20.0 µm | - | |||||
| Cyprinus carpio Carassius auratus Hypophthalmichthys molitrix Pseudorasbora parva Megalobrama amblycephala Hemiculter bleekeri | 49.1% cellophane | 76.3% particles <5 mm | - | 2.5 ± 1.3 particles/fish 1.9 ± 1.0 particles/fish 3.8 ± 2.0 particles/fish 2.5 ± 1.8 particles/fish 1.8 ± 1.7 particles/fish 2.1 ± 1.1 particles/fish | accumulation in stomach and intestine | [126] |
| Bagre bagre Bagre marinus Caranx hippos Lutjanus analis Polydactylus oligodon Cynoscion leiarchus Sphyrna tiburo Trichiurus lepturus | 97.4% of polyamide | 0.38–4.16 mm | - | 12.8 particles/fish 7.8 particles/fish 30.7 particles/fish 1.0 particle/fish 3.0 particles/fish 2.0 particles/fish 9.0 particles/fish 2.0 particles/fish | accumulation in stomach and intestine | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miloloža, M.; Kučić Grgić, D.; Bolanča, T.; Ukić, Š.; Cvetnić, M.; Ocelić Bulatović, V.; Dionysiou, D.D.; Kušić, H. Ecotoxicological Assessment of Microplastics in Freshwater Sources—A Review. Water 2021, 13, 56. https://doi.org/10.3390/w13010056
Miloloža M, Kučić Grgić D, Bolanča T, Ukić Š, Cvetnić M, Ocelić Bulatović V, Dionysiou DD, Kušić H. Ecotoxicological Assessment of Microplastics in Freshwater Sources—A Review. Water. 2021; 13(1):56. https://doi.org/10.3390/w13010056
Chicago/Turabian StyleMiloloža, Martina, Dajana Kučić Grgić, Tomislav Bolanča, Šime Ukić, Matija Cvetnić, Vesna Ocelić Bulatović, Dionysios D. Dionysiou, and Hrvoje Kušić. 2021. "Ecotoxicological Assessment of Microplastics in Freshwater Sources—A Review" Water 13, no. 1: 56. https://doi.org/10.3390/w13010056
APA StyleMiloloža, M., Kučić Grgić, D., Bolanča, T., Ukić, Š., Cvetnić, M., Ocelić Bulatović, V., Dionysiou, D. D., & Kušić, H. (2021). Ecotoxicological Assessment of Microplastics in Freshwater Sources—A Review. Water, 13(1), 56. https://doi.org/10.3390/w13010056







_Dionysiou.jpg)


