Are Microplastics Impairing Marine Fish Larviculture?—Preliminary Results with Argyrosomus regius
Abstract
:1. Introduction
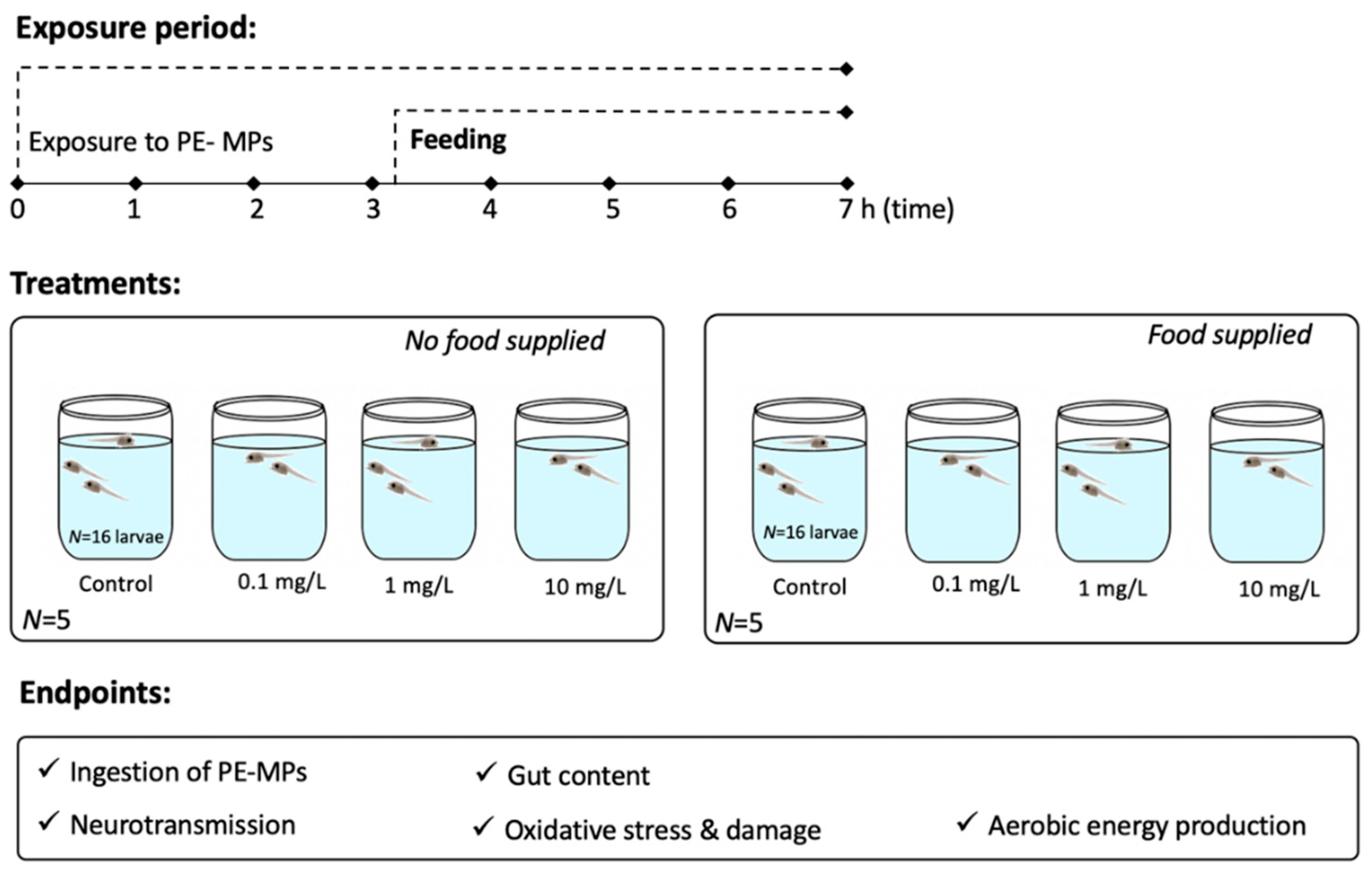
2. Materials and Methods
2.1. Organisms Culture Conditions
2.2. Microplastic Particles Used in the Assay and Spiking
2.3. Experimental Setup
2.4. Extraction and Quantification of Microplastics
2.5. Gut Content Analysis
2.6. Oxidative Stress, Aerobic Energy Production, and Neurotoxicity
2.7. Statistical Analysis
3. Results and Discussion
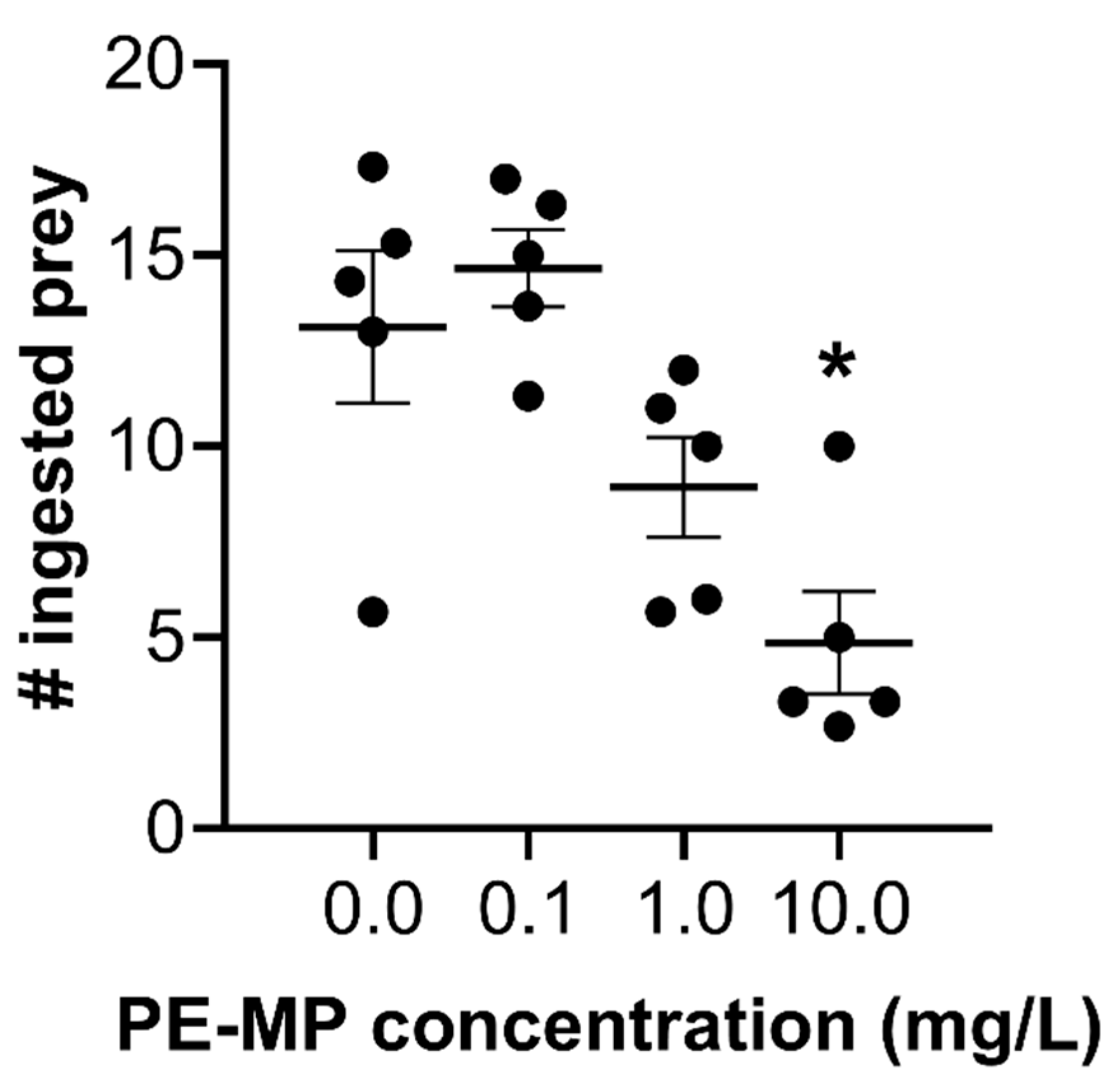
3.1. Ingestion of Polyethylene Microplastics (PE-MPs) and Prey Items by Meagre Fish Larvae
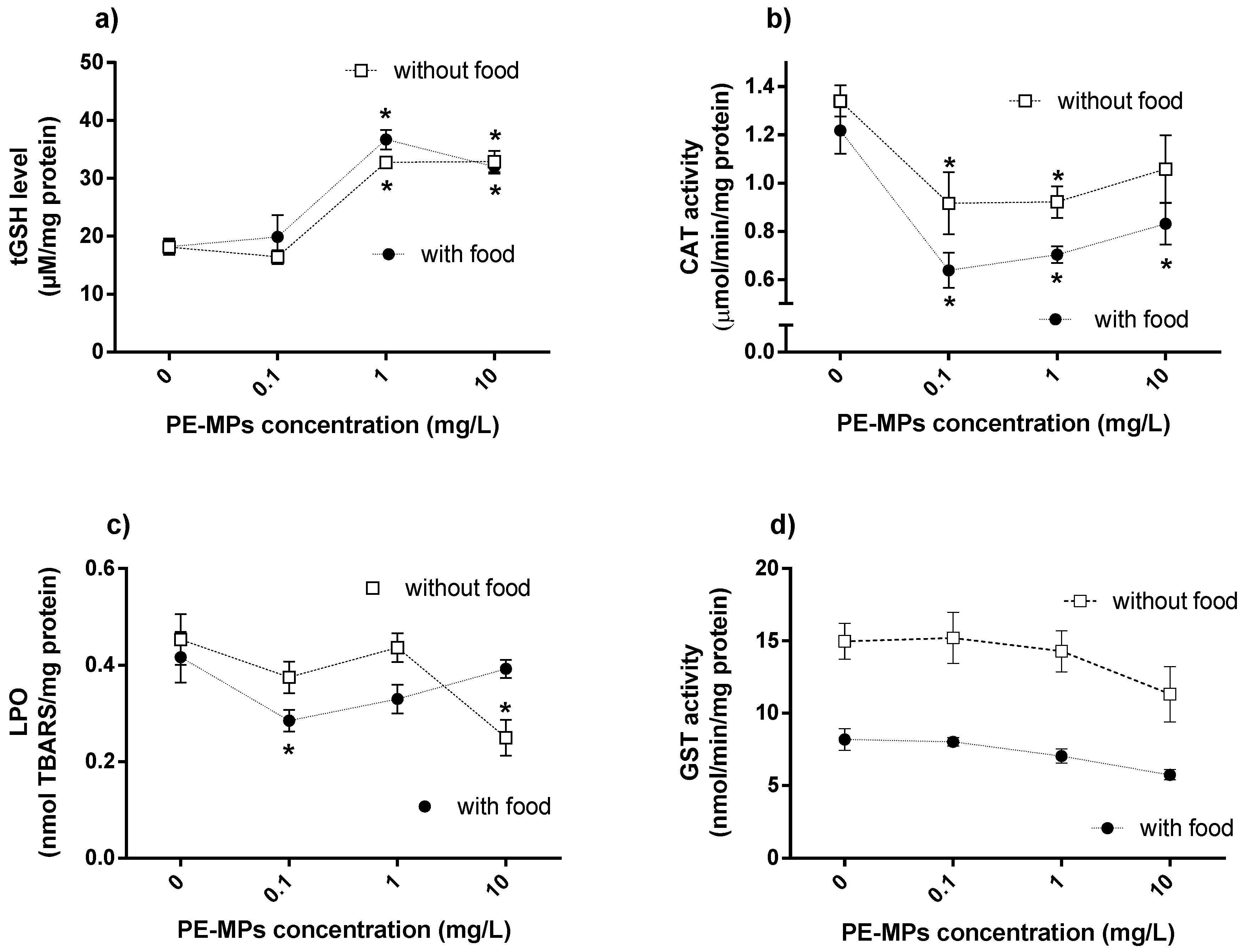
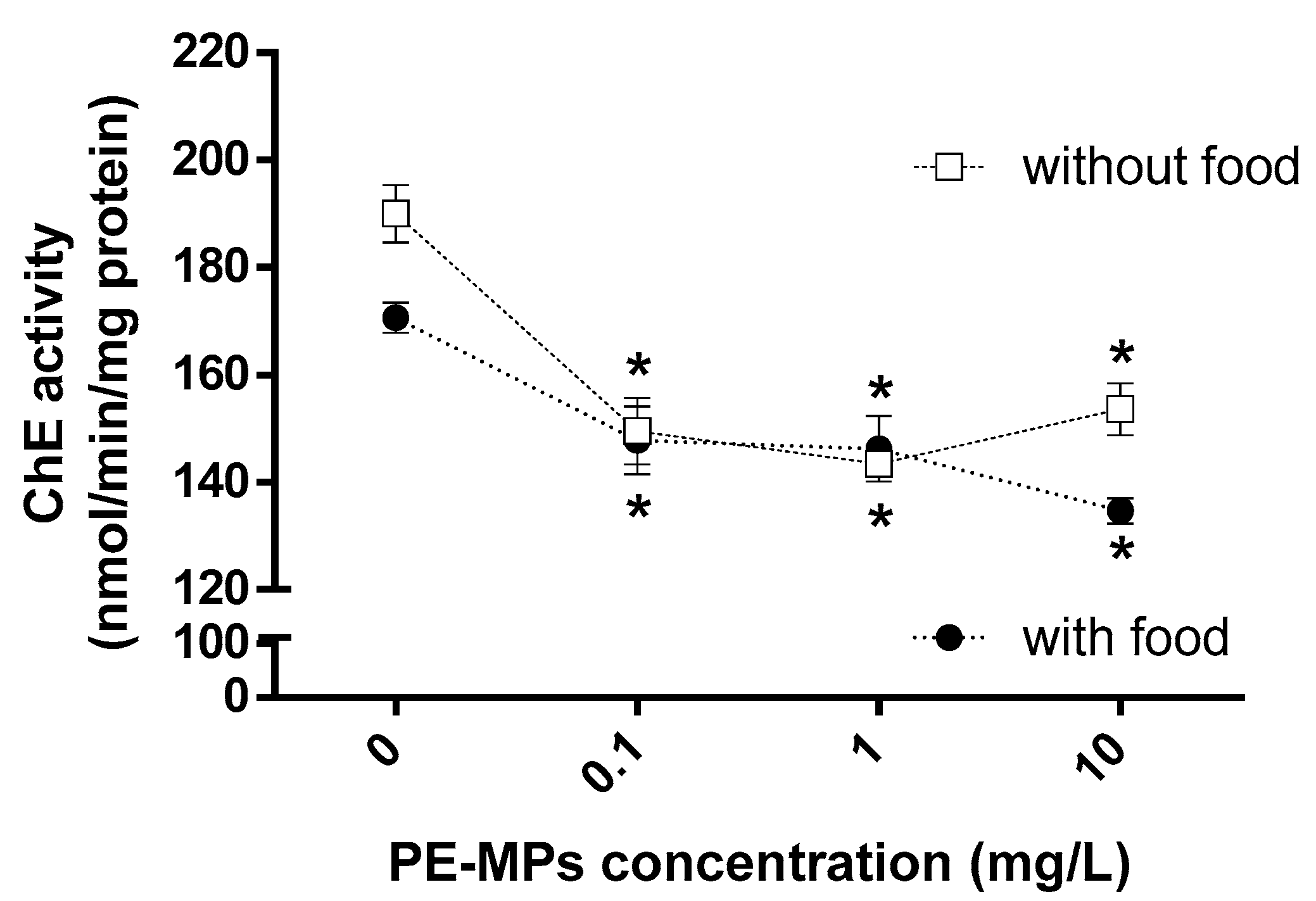
3.2. Effects of PE-MPs Exposure and Food Supply on the Biochemical Responses of Meagre Larvae
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Hamre, K.; Yúfera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.C.; Izquierdo, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquac. 2013, 5, S26–S58. [Google Scholar] [CrossRef] [Green Version]
- China, V.; Holzman, R. Hydrodynamic starvation in first-feeding larval fishes. Proc. Natl. Acad. Sci. USA 2014, 111, 8083–8088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoverde, C.; Rodriguez, C.; Perez, J.; Gisbert, E.; Estevez, A. Early weaning in meagreArgyrosomus regius: Effects on growth, survival, digestion and skeletal deformities. Aquac. Res. 2017, 48, 5289–5299. [Google Scholar] [CrossRef]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, J.; Yu, X.-Y. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mendozal, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical Paper 615; FAO: Rome, Italy, 2017; p. 147. [Google Scholar]
- Ma, J.; Niu, X.; Zhang, D.; Lu, L.; Ye, X.; Deng, W.; Li, Y.; Lin, Z. High levels of microplastic pollution in aquaculture water of fish ponds in the Pearl River Estuary of Guangzhou, China. Sci. Total Environ. 2020, 744, 140679. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jin, M.; Tao, P.; Wang, Z.; Xie, W.; Yu, X.; Wang, K. Assessment of microplastics derived from mariculture in Xiangshan Bay, China. Environ. Pollut. 2018, 242, 1146–1156. [Google Scholar] [CrossRef]
- Conkle, J.L.; Del Valle, C.D.B.; Turner, J.W. Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ. Manag. 2018, 61, 1–8. [Google Scholar] [CrossRef]
- Pabortsava, K.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef]
- Hanachi, P.; Karbalaei, S.; Walker, T.R.; Cole, M.; Hosseini, S.V. Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ. Sci. Pollut. Res. 2019, 26, 23777–23787. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, M.M.; Campoverde, C.; Öztürk, S.; Moreira, C.; Diaz, M.; Moyano, F.J.; Estévez, A.; Gisbert, E. Morphological and functional description of the development of the digestive system in meagre (Argyrosomus regius): An integrative approach. Aquaculture 2016, 464, 381–391. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Duan, L.; Zhang, Y.; Zhou, Y.; Sui, Q.; Xu, D.; Qu, H.; Yu, G. Occurrence and distribution of microplastics in domestic, industrial, agricultural and aquacultural wastewater sources: A case study in Changzhou, China. Water Res. 2020, 182, 115956. [Google Scholar] [CrossRef] [PubMed]
- Roo, J.; Hernandez-Cruz, C.M.; Borrero, C.; Schuchardt, D.; Fernández-Palacios, H. Effect of larval density and feeding sequence on meagre (Argyrosomus regius; Asso, 1801) larval rearing. Aquaculture 2010, 302, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.J.; Silva, A.L.P.; Gravato, C.; Pestana, J.L. Ingestion of small-sized and irregularly shaped polyethylene microplastics affect Chironomus riparius life-history traits. Sci. Total Environ. 2019, 672, 862–868. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Matos, J.T.; Da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Rocha, R.J.M.; Ribeiro, L.; Costa, R.; Dinis, M.T. Does the presence of microalgae influence fish larvae prey capture? Aquac. Res. 2008, 39, 362–369. [Google Scholar] [CrossRef]
- Abe, F.R.; Soares, A.M.V.M.; De Oliveira, D.P.; Gravato, C. Toxicity of dyes to zebrafish at the biochemical level: Cellular energy allocation and neurotoxicity. Environ. Pollut. 2018, 235, 255–262. [Google Scholar] [CrossRef]
- Campos, D.; Gravato, C.; Quintaneiro, C.; Soares, A.M.; Pestana, J.L.T. Responses of the aquatic midge Chironomus riparius to DEET exposure. Aquat. Toxicol. 2016, 172, 80–85. [Google Scholar] [CrossRef]
- Meireles, G.; Daam, M.A.; Sanches, A.L.M.; Zanoni, M.V.B.; Soares, A.M.; Gravato, C.; De Oliveira, D.P. Red disperse dyes (DR 60, DR 73 and DR 78) at environmentally realistic concentrations impact biochemical profile of early life stages of zebrafish (Danio rerio). Chem. Interact. 2018, 292, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.D.C.; Gravato, C.; Quintaneiro, C.; Golovko, O.; Žlábek, V.; Barata, C.; Soares, A.M.; Pestana, J.L.T. Life history and biochemical effects of chlorantraniliprole on Chironomus riparius. Sci. Total Environ. 2015, 508, 506–513. [Google Scholar] [CrossRef]
- Bird, R.; Draper, H. [35] Comparative studies on different methods of malonaldehyde determination. Methods Enzymol. 1984, 105, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Clairborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A.E., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Acetylcholinesterase Activity in Juveniles of Daphnia magna Straus. Bull. Environ. Contam. Toxicol. 1996, 57, 979–985. [Google Scholar] [CrossRef]
- Domingues, I.; Gravato, C. Oxidative Stress Assessment in Zebrafish Larvae. In Advanced Structural Safety Studies; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; Volume 1797, pp. 477–486. [Google Scholar]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Campos, D.; Gravato, C.; Fedorova, G.; Burkina, V.; Soares, A.M.V.M.; Pestana, J.L.T. Ecotoxicity of two organic UV-filters to the freshwater caddisfly Sericostoma vittatum. Environ. Pollut. 2017, 228, 370–377. [Google Scholar] [CrossRef]
- Rodrigues, A.D.C.; Gravato, C.; Silva, C.J.; Pires, S.F.S.; Costa, A.P.L.; Conceição, L.E.; Santos, P.; Costas, B.; Calheiros, J.; Castro-Cunha, M.; et al. Seasonal Temperature Fluctuations Differently Affect the Immune and Biochemical Parameters of Diploid and Triploid Oncorhynchus mykiss Cage-Cultured in Temperate Latitudes. Sustainability 2020, 12, 8785. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recover. 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rønnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Saele, Ø.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquac. 2013, 5, S59–S98. [Google Scholar] [CrossRef]
- Kim, S.W.; Chae, Y.; Kim, D.; An, Y.-J. Zebrafish can recognize microplastics as inedible materials: Quantitative evidence of ingestion behavior. Sci. Total Environ. 2019, 649, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, H.; Folguera, J.S.; Kortet, R.; Akkanen, J.; Vainikka, A.; Janhunen, M.; Kekäläinen, J. Do whitefish (Coregonus lavaretus) larvae show adaptive variation in the avoidance of microplastic ingestion? Environ. Pollut. 2020, 262, 114353. [Google Scholar] [CrossRef]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A.; et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Aldana, M.; Ojeda, F.P.; Duarte, C.; Galbán-Malagón, C.J. Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Zhou, A.; Ye, Q.; Feng, Y.; Wang, Z.; Wang, S.; Xu, G.; Zou, J. Species-specific effect of microplastics on fish embryos and observation of toxicity kinetics in larvae. J. Hazard. Mater. 2021, 403, 123948. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.A.; Forman, H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Sen, C.K. Nutritional biochemistry of cellular glutathione. J. Nutr. Biochem. 1997, 8, 660–672. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaei, S.; Kenari, A.A.; Hedayati, M.; Sadati, M.A.Y.; Metón, I. Effect of diet composition on growth performance, hepatic metabolism and antioxidant activities in Siberian sturgeon (Acipenser baerii, Brandt, 1869) submitted to starvation and refeeding. Fish Physiol. Biochem. 2016, 42, 1509–1520. [Google Scholar] [CrossRef]
- Oliveira, M.C.L.; Ribeiro, A.L.P.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Kentepozidou, E.; Feidantsis, K.; Roufidou, C.; Despoti, S.; Chatzifotis, S. Starvation and re-feeding affect Hsp expression, MAPK activation and antioxidant enzymes activity of European Sea Bass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 79–88. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 1–16. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 1022–1031. [Google Scholar] [CrossRef] [PubMed]

| [PE-MPs] mg/L | without Food | with Food |
|---|---|---|
| 0 | - | - |
| 0.1 | 1 ± 0.2 | 0.2 ± 0.3 |
| 1 | 1.2 ± 0.1 | 0.8 ± 0.2 |
| 10 | 1.3 ± 0.4 | 1.1 ± 0.2 |
| Sums of Squares | F-Value, df | p-Value | ||
|---|---|---|---|---|
| LPO | [PE-MPs] | 0.084 | F (3, 32) = 4.207 | 0.013 |
| p/a food | 0.005 | F (1, 32) = 0.7594 | 0.390 | |
| Interaction | 0.099 | F (3, 32) = 4.919 | 0.006 | |
| CAT | [PE-MPs] | 1.57 | F (3, 32) = 12.40 | <0.001 |
| p/a food | 0.45 | F (1, 32) = 10.61 | 0.003 | |
| Interaction | 0.03 | F (3, 32) = 0.2496 | 0.861 | |
| GST | [PE-MPs] | 62.81 | F (3, 32) = 2.952 | 0.047 |
| p/a food | 448.30 | F (1, 32) = 63.21 | <0.0001 | |
| Interaction | 4.524 | F (3, 32) = 0.2126 | 0.887 | |
| tGSH | [PE-MPs] | 2407 | F (3, 32) = 46.88 | <0.0001 |
| p/a food | 27.55 | F (1, 32) = 1.610 | 0.214 | |
| Interaction | 43.74 | F (3, 32) = 0.8519 | 0.476 | |
| ETS | [PE-MPs] | 2.68 | F (3, 32) = 7.756 | 0.0005 |
| p/a food | 4.47 | F (1, 32) = 38.76 | <0.0001 | |
| Interaction | 0.66 | F (3, 32) = 1.919 | 0.146 | |
| ChE | [PE-MPs] | 9037 | F (3, 32) = 25.16 | <0.0001 |
| p/a food | 862.5 | F (1, 32) = 7.204 | 0.0114 | |
| Interaction | 998.4 | F (3, 32) = 2.779 | 0.0570 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, D.; Rodrigues, A.C.M.; Rocha, R.J.M.; Martins, R.; Candeias-Mendes, A.; Castanho, S.; Soares, F.; Pousão-Ferreira, P.; Soares, A.M.V.M.; Gravato, C.; et al. Are Microplastics Impairing Marine Fish Larviculture?—Preliminary Results with Argyrosomus regius. Water 2021, 13, 104. https://doi.org/10.3390/w13010104
Campos D, Rodrigues ACM, Rocha RJM, Martins R, Candeias-Mendes A, Castanho S, Soares F, Pousão-Ferreira P, Soares AMVM, Gravato C, et al. Are Microplastics Impairing Marine Fish Larviculture?—Preliminary Results with Argyrosomus regius. Water. 2021; 13(1):104. https://doi.org/10.3390/w13010104
Chicago/Turabian StyleCampos, Diana, Andreia C. M. Rodrigues, Rui J. M. Rocha, Roberto Martins, Ana Candeias-Mendes, Sara Castanho, Florbela Soares, Pedro Pousão-Ferreira, Amadeu M. V. M. Soares, Carlos Gravato, and et al. 2021. "Are Microplastics Impairing Marine Fish Larviculture?—Preliminary Results with Argyrosomus regius" Water 13, no. 1: 104. https://doi.org/10.3390/w13010104
APA StyleCampos, D., Rodrigues, A. C. M., Rocha, R. J. M., Martins, R., Candeias-Mendes, A., Castanho, S., Soares, F., Pousão-Ferreira, P., Soares, A. M. V. M., Gravato, C., & Patrício Silva, A. L. (2021). Are Microplastics Impairing Marine Fish Larviculture?—Preliminary Results with Argyrosomus regius. Water, 13(1), 104. https://doi.org/10.3390/w13010104










