Dynamic Interception Effect of Internal and External Nitrogen and Phosphorus Migration of Ecological Ditches
Abstract
1. Introduction
2. Materials and Methods
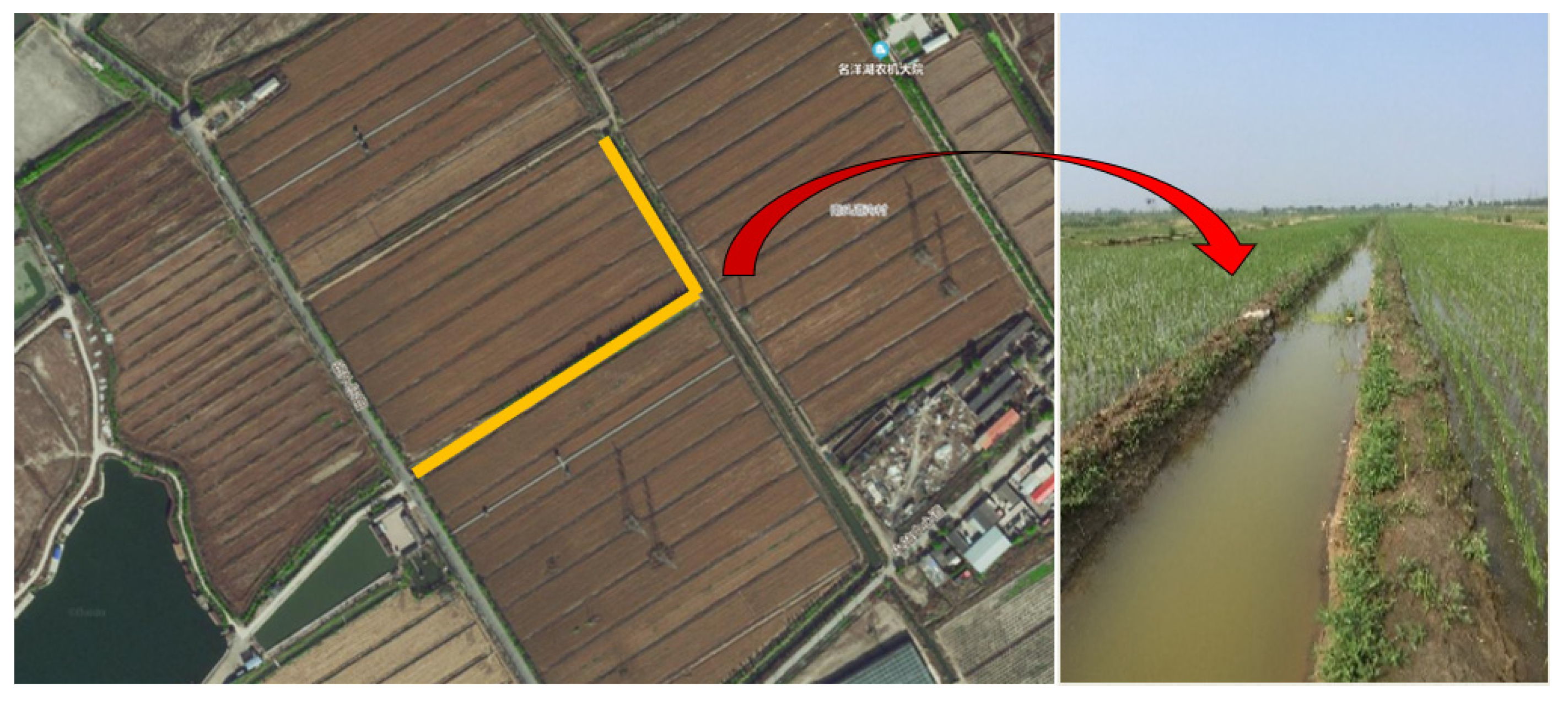
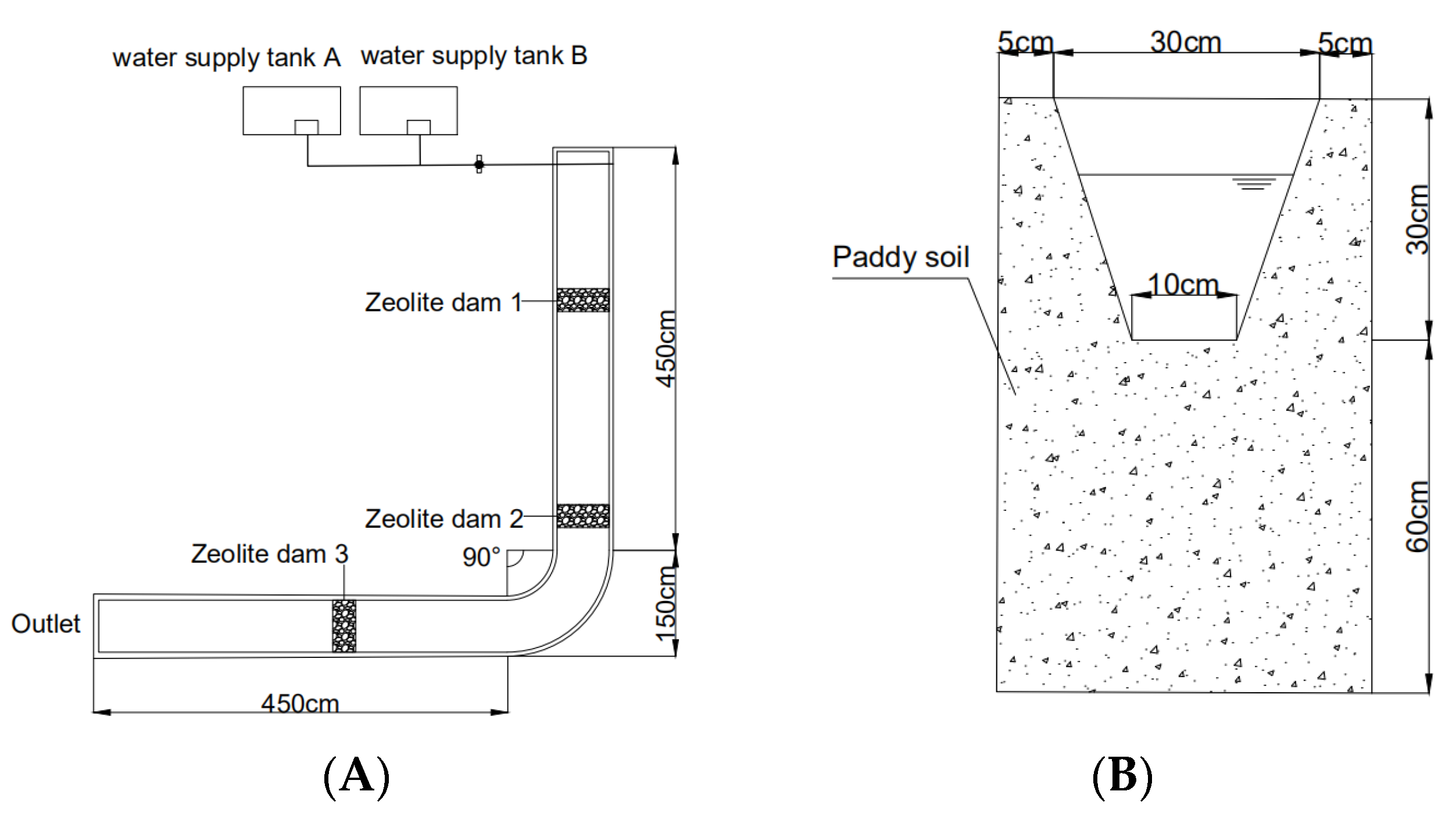
2.1. Ditch Model Construction
2.2. Experiment Design
2.2.1. Experimental Design of Blank Ditch
2.2.2. Experimental Design of Eco-Ditch
2.3. Sample Collection and Analysis
2.3.1. Collection and Analysis of the Monitoring Samples of N and P in Water and Sediment
2.3.2. Collection and Analysis of Zeolite Matrix Samples
2.3.3. Collection and Analysis of Microbiological Samples
3. Results
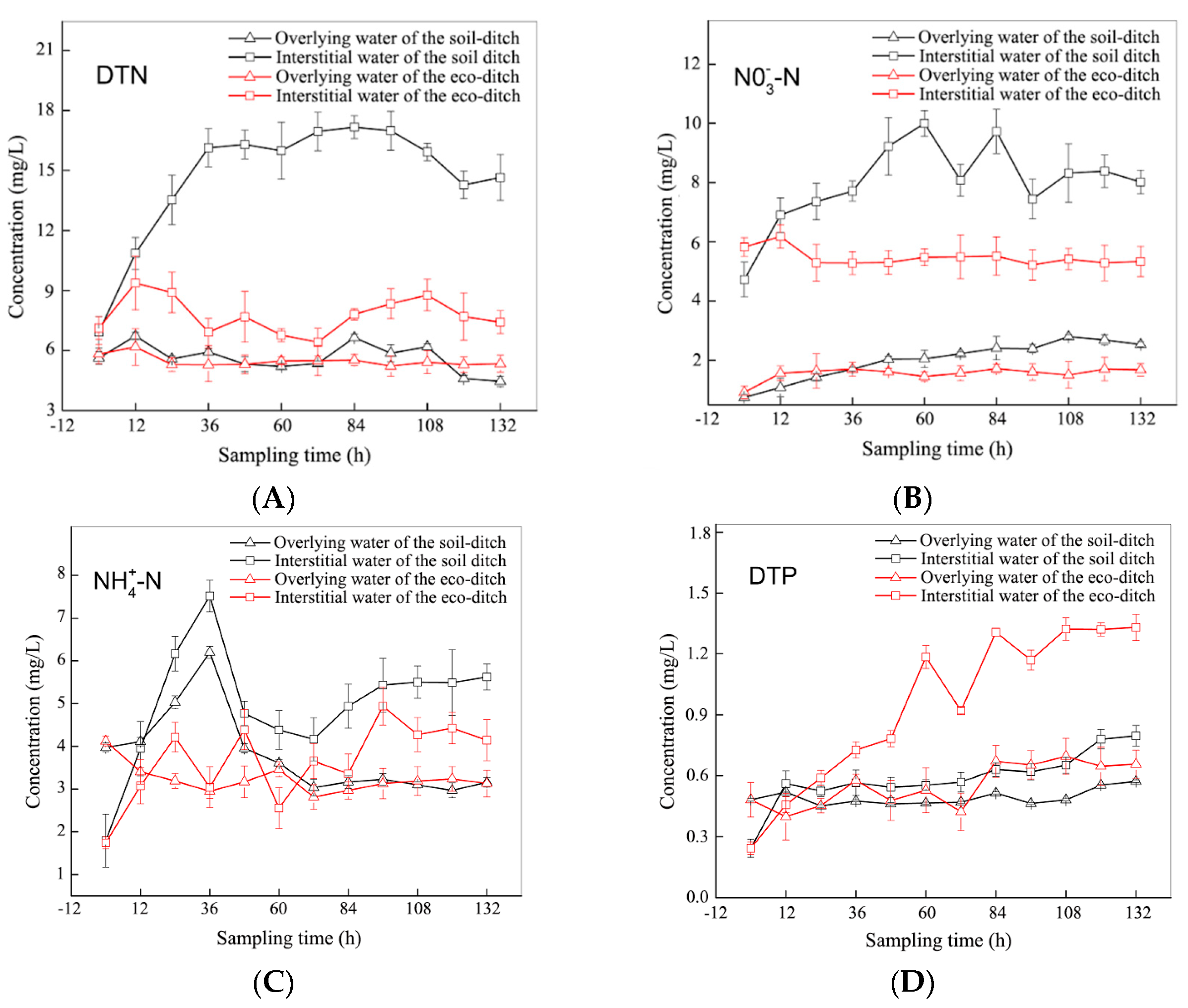
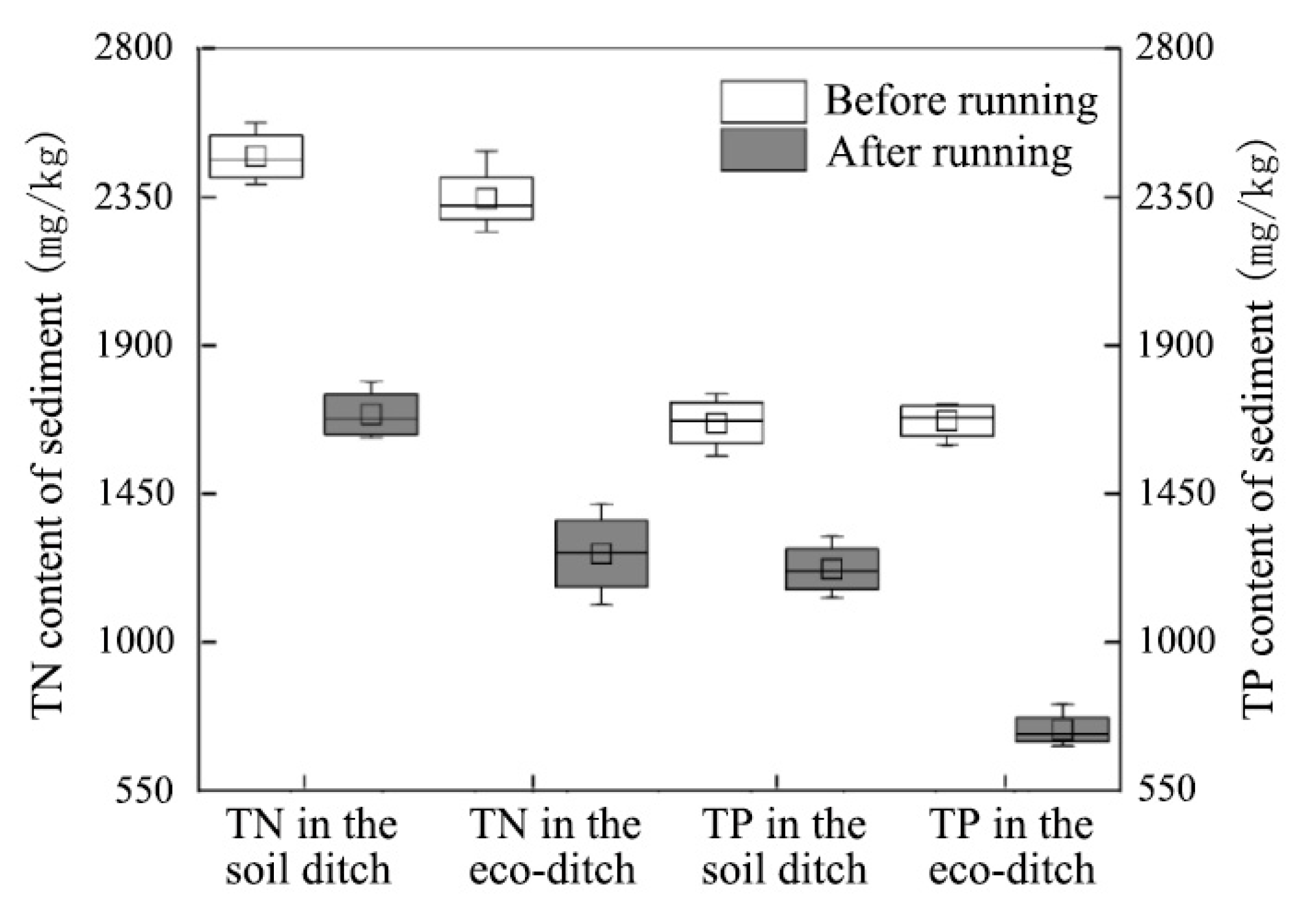
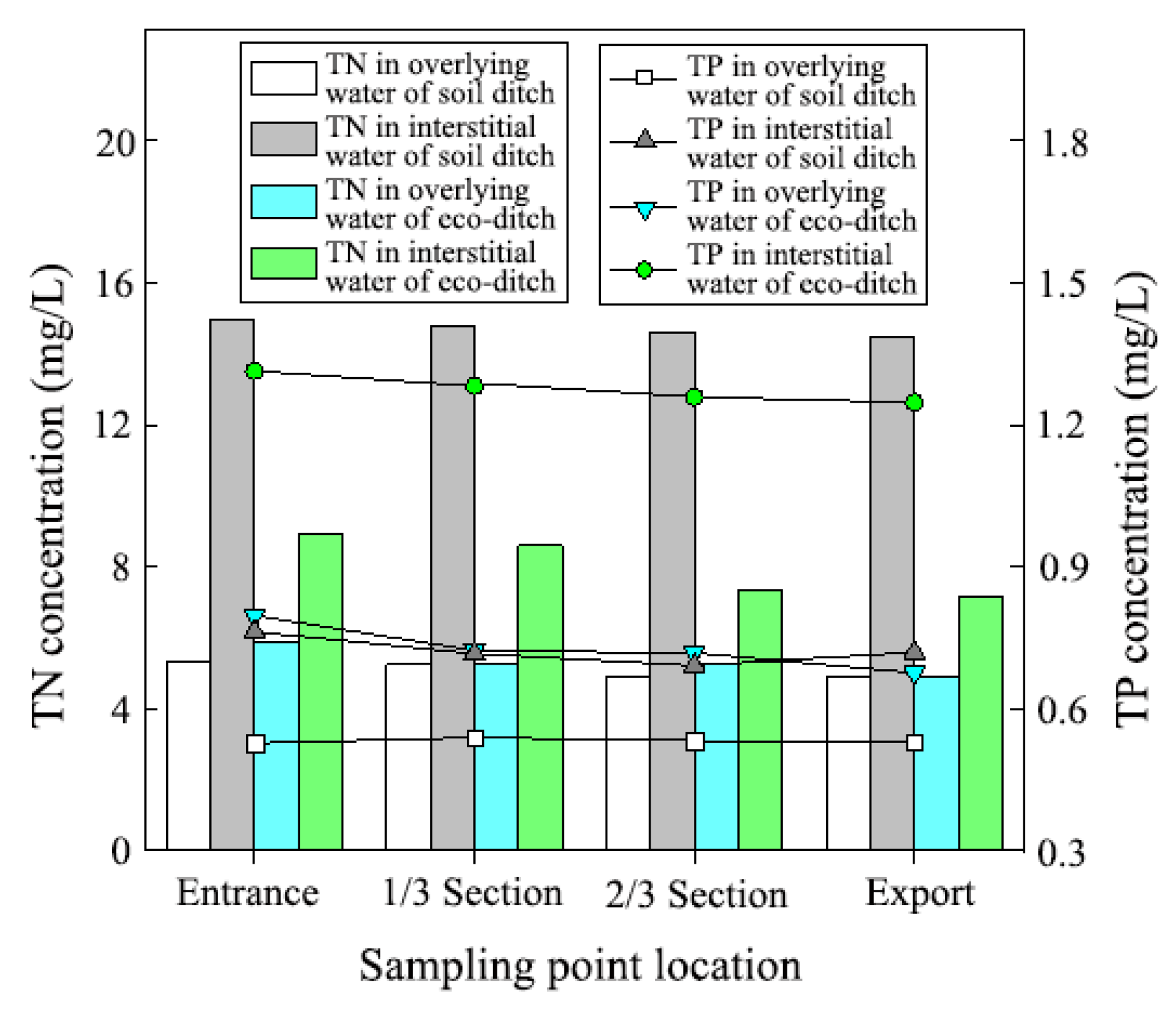
3.1. Distribution Law of N and P in Sediments, Interstitial Water, and Overlying Water in the Ecological and Soil Ditches
3.2. Analysis of the Surface Morphology of the Adsorption Matrix of the Matrix Dam of the Eco-Ditch
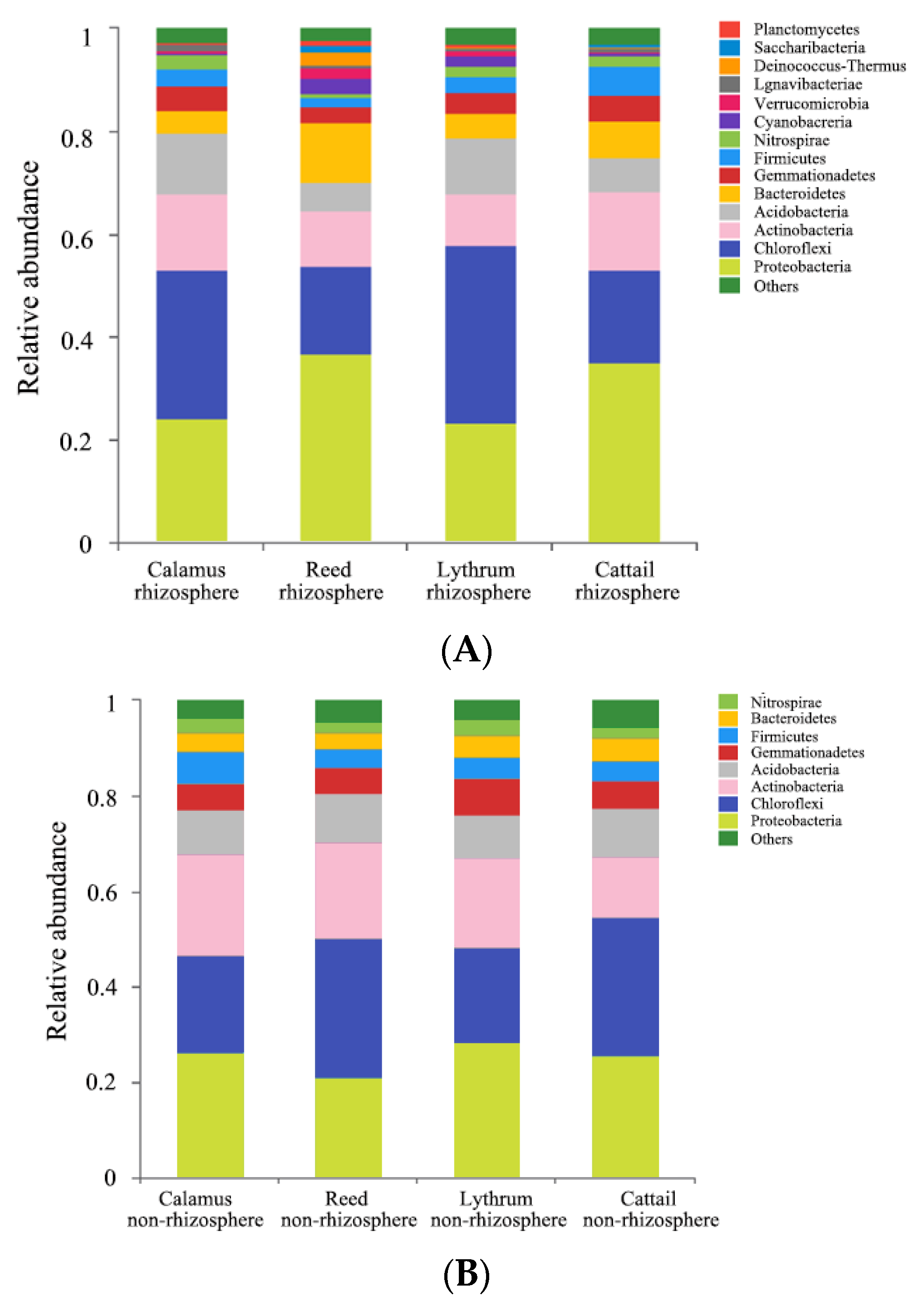
3.3. Analysis of the Characteristics of Microbial Communities in the Sediments of Ecological and Soil Ditches
4. Discussion
5. Conclusions
- (1)
- The concentration gradient is the main cause of pollutant transport and diffusion, and the eco-ditch weakens this concentration gradient diffusion via the comprehensive function of plants and abundant microbial activities. The functions of eco-ditch plants and the structure of the microorganism community effectively inhibits the endogenous release of sediment N, which mainly acts on the interstitial water. The total N mainly depends on the nitrate N output, and no obvious correlation exists between the nitrate N and dissolved total N in sediment, interstitial water, and overlying water. Compared with soil ditches, eco-ditches have a stronger reoxygenation capacity and a better pollutant interception effect. The attenuation in N and P in the interstitial water was slightly higher than that in the overlying water.
- (2)
- A large number of mycelia exist on the surface of the dam substrate of eco-ditches. The large surface area and clearance also provide a good attachment framework for microorganisms; the low-water substrate dam extends the residence time of the water flow, which is beneficial for the eco-ditch’s adsorbing and purification of pollutants in water bodies.
- (3)
- The sediments of ecological and soil ditches are dominated by Proteobacteria, Chloroflexi, Actinomycetes, and Acidobacteria, a species composition resembling that of some rivers, lakes, and wetland sediments. This indicated that the farmland drainage ditches can be characterized as linear wetlands. The microbial diversity and abundance in the eco-ditch were higher than in the soil ditch. Proteobacteria, which play a central role in the degradation of pollutants such as biological N and P, were found in the eco-ditch with a relative abundance in sediment (30.41%) higher than that in the soil ditch (25.33%). The growth of aquatic plants in the eco-ditch changed the microenvironment of the sediment, which was beneficial for the purification effect of microorganisms on N and P pollutants in water.
Author Contributions
Funding
Conflicts of Interest
References
- Dal Ferro, N.; Ibrahim, H.M.S.; Borin, M. Newly-established free water-surface constructed wetland to treat agricultural waters in the low-lying venetian plain: Performance on nitrogen and phosphorus removal. Sci. Total Environ. 2018, 639, 852–859. [Google Scholar] [CrossRef]
- Chen, C.; Yu, Z.B.; Xiang, L.; He, J.J.; Fu, X.L. Effects of rainfall intensity and amount on the transport of total nitrogen and phosphorus in a small agricultural watershed. Appl. Mech. Mater. 2012, 212–213, 268–271. [Google Scholar] [CrossRef]
- Han, J.G.; Li, Z.B.; Li, P. Nitrogen and phosphorous concentrations in runoff from a purple soil in an agricultural watershed. Agric. Water Manag. 2010, 97, 757–762. [Google Scholar] [CrossRef]
- Hu, Q.H.; Zhu, Y.M.; Song, J.; Li, Z.Y.; Wen, J.J. Effects of Ph and Eh on release of nitrogen and phosphorus from sediments of West Lake. J. Zhejiang Univ. Sci. 2003, 4, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Xue, P.Y.; Geng, L.P.; Chen, M.M.; Sun, H.X.; Wang, D.W.; Feng, Y.J.; Wang, D.M. Distribution characteristics of nitrogen and phosphorus in sediments-submerged macrophytes-water systems of Baiyangdian Lake. J. Soil Water Conserv. 2017, 31, 304–309. [Google Scholar]
- Norris, B.; Laws, E.A. Nutrients and phytoplankton in a shallow, hypereutrophic urban lake: Prospects for restoration. Water 2017, 9, 431. [Google Scholar] [CrossRef]
- Li, S.M.; Wang, X.L.; Tu, J.M.; Qiao, B.; Li, J.S. Nitrogen removal in an ecological ditch based on an orthogonal test. Water Air Soil Pollut. 2016, 227, 396. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Zhu, B. Effectiveness of vegetated drainage ditches for domestic sewage effluent mitigation. Bull. Environ. Contam. Toxicol. 2017, 98, 682–689. [Google Scholar] [CrossRef]
- Taylor, J.M.; Moore, M.T.; Scott, J.T. Contrasting nutrient mitigation and denitrification potential of agricultural drainage environments with different emergent aquatic macrophytes. J. Environ. Qual. 2015, 44, 1304–1314. [Google Scholar] [CrossRef]
- Lavrnic, S.; Braschi, I.; Anconelli, S.; Blasioli, S.; Solimando, D.; Mannini, P. Long-term monitoring of a surface flow constructed wetland treating agricultural drainage water in northern italy. Water 2018, 10, 644. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Zhu, B.; Muyembe, D.K.; Dzakpasu, M. Growth characteristics and nutrient removal capability of eco-ditch plants in mesocosm sediment receiving primary domestic wastewater. Environ. Sci. Pollut. Res. 2017, 24, 23926–23938. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Peng, S.; Luo, Y. A paddy eco-ditch and wetland system to reduce non-point source pollution from rice-based production system while maintaining water use efficiency. Environ. Sci. Pollut. Res. 2015, 22, 4406–4417. [Google Scholar] [CrossRef] [PubMed]
- Olli, G.; Amélie, D.; Destouni, G. Field study of phosphorous transport and retention in drainage reaches. J. Hydrol. 2009, 365, 46–55. [Google Scholar] [CrossRef]
- Zhou, L.; Bai, C.R.; Cai, J. Bio-cord plays a similar role as submerged macrophytes in harboring bacterial assemblages in an eco-ditch. Environ. Sci. Pollut. Res. 2018, 25, 26550–26561. [Google Scholar] [CrossRef]
- Wang, X.L.; Qiao, B.; Li, S.M.; Li, J.S. Using natural Chinese zeolite to remove ammonium from rainfall runoff following urea fertilization of a paddy rice field. Environ. Sci. Pollut. Res. 2016, 23, 5342–5351. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, J.S.; Li, S.M.; Zheng, X.T. A study on removing nitrogen from paddy field rainfall runoff by an ecological ditch–zeolite barrier system. Environ. Sci. Pollut. Res. 2017, 24, 27090–27103. [Google Scholar] [CrossRef]
- Bangalee, M.Z.I.; Miau, J.J.; Lin, S.Y.; Yang, J.H. Flow visualization, PIV measurement and CFD calculation for fluid-driven natural cross-ventilation in a scale model. Energy Build. 2013, 66, 306–314. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, C.; Zhao, Y.; Liu, Z.; Guan, B. Effects of diversity, coverage and biomass of submerged macrophytes on nutrient concentrations, water clarity and phytoplankton biomass in two restored shallow lakes. Water 2020, 12, 1425. [Google Scholar] [CrossRef]
- Tian, W.H.; Wen, X.H.; Cui, Y.P.; Q, Y. Influence of filter particle size on nitrification performance of aerated biological filter. CHN. Water Wastewater 2003, 19, 48–50. [Google Scholar]
- Yu, J.; Zhong, J.; Chen, Q.; Huang, W.; Fan, C. An investigation of the effects of capping on internal phosphorus release from sediments under rooted macrophytes (Phragmites Australis) revegetation. Environ. Sci. Pollut. Res. 2018, 25, 24682–24694. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, H.M.; Zhang, D. Removal of ammonium from greywater using natural zeolite. Desalination 2011, 277, 15–23. [Google Scholar] [CrossRef]
- Davis, M.E.; Lobo, R.F. Zeolite and molecular sieve synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988; pp. 81–99. ISBN 978-94-015-7360-3. [Google Scholar]
- Ayi, Q.L.; Zeng, B.; Liu, J.H.; Li, S.Q.; van Bodegom, P.M.; Cornelissen, J.H.C. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 2016, 118, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ansola, G.; Arroyo, P.; Miera, L.E.S.D. Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci. Total Environ. 2014, 473, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, A.N.; Krogstad, T.; Kleinman, P.J.A.; Haggard, B.; Shigaki, F.; Saporito, L.S. Managing natural processes in drainage ditches for nonpoint source phosphorus control. J. Soil Water Conserv. 2007, 62, 197–206. [Google Scholar] [CrossRef]
- Li, P.; Bi, X.J.; Wang, J.; Ru, S.G. Microbial diversity in activated sludges of conventional and reversed A~2/O processes. China Environ. Sci. 2017, 37, 1137–1145. [Google Scholar]
- Ciccazzo, S.; Esposito, A.; Rolli, E.; Zerbe, S.; Daffonchio, D.; Brusetti, L. Safe-site effects on rhizosphere bacterial communities in a high-altitude alpine environment. Biomed Res. Int. 2014, 2014, 480170. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Y.; Otte, M.L.; Liu, Y.; Qin, L.; Tian, X.; Lu, X.G.; Jiang, M.; Zou, Y.C. Performance of iron plaque of wetland plants for regulating iron, manganese, and phosphorus from agricultural drainage water. Water 2018, 10, 42. [Google Scholar] [CrossRef]
- Taylor, J.; Moore, M.; Speir, S.L.; Testa, S. Vegetated ditch habitats provide net nitrogen sink and phosphorus storage capacity in agricultural drainage networks despite senescent plant leaching. Water 2020, 12, 875. [Google Scholar] [CrossRef]






| Ditch Type | Interstitial Water | Overlying Water | ||||||
|---|---|---|---|---|---|---|---|---|
| NH4+-N | NO3−-N | TN | TP | NH4+-N | NO3−-N | TN | TP | |
| (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | |
| Ecological Ditch | 3.65 (0.88) a | 5.47 (0.26) a | 7.77 (0.88) a | 0.95 (0.37) a | 3.23 (0.32) a | 1.56 (0.21) a | 5.47 (0.26) a | 0.56 (0.11) a |
| Soil Ditch | 4.98 (1.33) b | 8.00 (1.34) b | 14.64 (2.90) b | 0.59 (0.13) b | 3.80 (0.93) a | 2.01 (0.62) a | 5.63 (0.68) a | 0.49 (0.04) a |
| Sampling Location | Interstitial Water in Ecological Ditch | Interstitial Water in Soil Ditch | Overlying Water in Ecological Ditch | Overlying Water in Soil Ditch | ||||
|---|---|---|---|---|---|---|---|---|
| TN | TP | TN | TP | TN | TP | TN | TP | |
| (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | |
| Entrance | 8.90 (0.02) a | 1.38 (0.00) a | 16.10 (0.10) a | 0.68 (0.02) a | 5.89 (0.14) a | 0.76 (0.01) a | 5.31 (1.10) a | 0.53 (0.01) a |
| 1/3 Section | 8.61 (0.14) b | 1.37 (0.00) ab | 15.62 (0.28) b | 0.73 (0.00) ab | 5.27 (0.09) b | 0.72 (0.01) b | 5.25 (1.08) a | 0.54 (0.01) a |
| 2/3 Section | 7.32 (0.02) c | 1.36 (0.00) b | 14.12 (0.09) c | 0.80 (0.00) b | 5.29 (0.01) b | 0.59 (0.00) c | 4.90 (0.34) a | 0.53 (0.01) a |
| Export | 7.16 (0.05) c | 1.32 (0.01) c | 14.11 (0.08) c | 0.76 (0.00) ab | 4.90 (0.15) c | 0.52 (0.00) d | 4.92 (0.88) a | 0.53 (0.01) a |
| Day | Overlying Water in Ecological Ditch | Overlying Water in Soil Ditch | ||
|---|---|---|---|---|
| DO | pH | DO | pH | |
| 1 | 6.41 (0.17) a | 8.00 (0.01) a | 6.22 (0.17) a | 8.24 (0.01) a |
| 2 | 5.56 (0.14) b | 7.99 (0.01) a | 5.39 (0.22) b | 8.16 (0.04) b |
| 3 | 5.57 (0.53) b | 7.99 (0.00) a | 4.81 (0.03) c | 8.13 (0.03) b |
| 4 | 5.66 (0.41) ab | 7.98 (0.00) a | 4.73 (0.04) c | 8.12 (0.00) bc |
| 5 | 5.51 (0.47) b | 7.99 (0.00 a | 4.43 (0.13) d | 8.11 (0.01) bc |
| 6 | 4.29 (0.15) c | 7.98 (0.01) a | 4.03 (0.09) e | 8.07 (0.03) c |
| Sample | Shannon Index | Chao1 Index | Coverage Index |
|---|---|---|---|
| Yellow calamus rhizosphere | 6.70221 | 2137.14286 | 0.98013 |
| Yellow calamus non-rhizosphere | 6.63140 | 1997.88703 | 0.98195 |
| Cattail rhizosphere | 6.73678 | 2188.34 | 0.98190 |
| Cattail non-rhizosphere | 6.72451 | 2088.84333 | 0.98099 |
| Reed rhizosphere | 6.34987 | 2169.68817 | 0.97526 |
| Reed non-rhizosphere | 6.63545 | 1977.63265 | 0.98281 |
| Lythrum rhizosphere | 6.78560 | 2131.41791 | 0.98153 |
| Lythrum non-rhizosphere | 6.73279 | 2063.13433 | 0.98158 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, S.; Wang, X.; An, Y.; Wang, R. Dynamic Interception Effect of Internal and External Nitrogen and Phosphorus Migration of Ecological Ditches. Water 2020, 12, 2553. https://doi.org/10.3390/w12092553
Liu Y, Li S, Wang X, An Y, Wang R. Dynamic Interception Effect of Internal and External Nitrogen and Phosphorus Migration of Ecological Ditches. Water. 2020; 12(9):2553. https://doi.org/10.3390/w12092553
Chicago/Turabian StyleLiu, Yuyang, Songmin Li, Xiaoling Wang, Yuchen An, and Ruonan Wang. 2020. "Dynamic Interception Effect of Internal and External Nitrogen and Phosphorus Migration of Ecological Ditches" Water 12, no. 9: 2553. https://doi.org/10.3390/w12092553
APA StyleLiu, Y., Li, S., Wang, X., An, Y., & Wang, R. (2020). Dynamic Interception Effect of Internal and External Nitrogen and Phosphorus Migration of Ecological Ditches. Water, 12(9), 2553. https://doi.org/10.3390/w12092553




