Optimization-Based Water-Salt Dynamic Threshold Analysis of Cotton Root Zone in Arid Areas
Abstract
1. Introduction
2. Materials and Methods
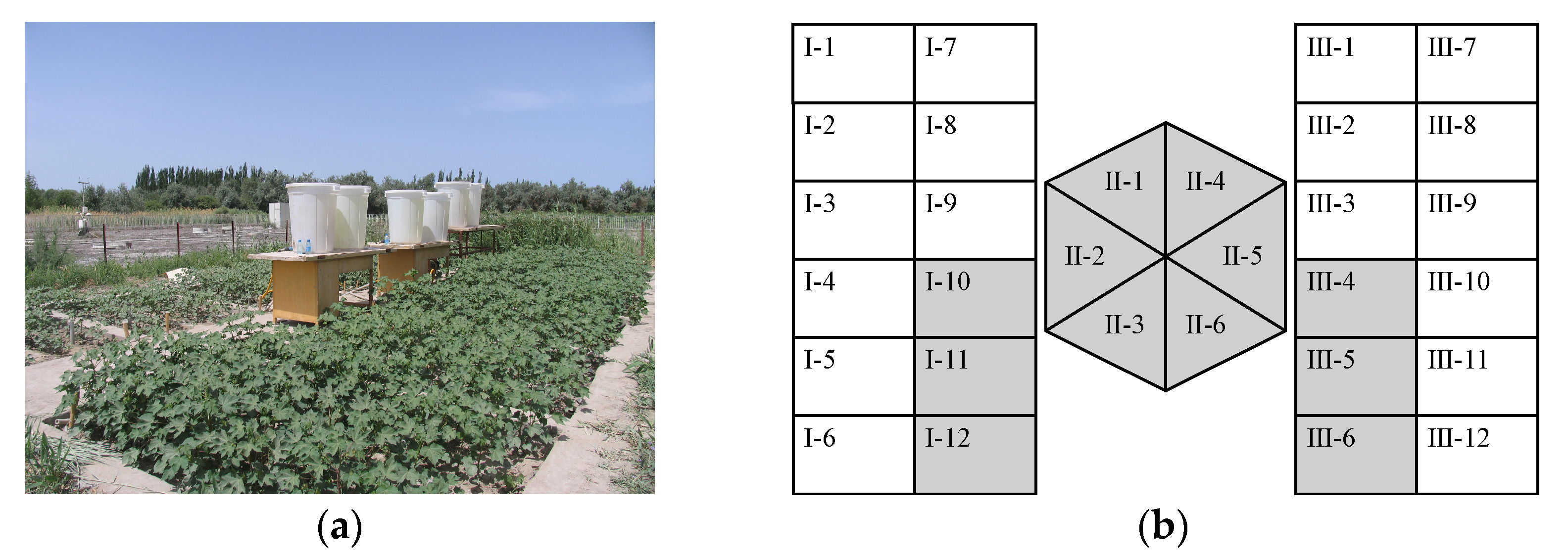
2.1. Field Experiments
2.1.1. Experiment One
Experimental Site and Design
Observation and Measurements
2.1.2. Experiment Two
2.2. Model of Response to Crop Water and Salinity
2.2.1. Response of Yield to Water and Salt
2.2.2. Response of Evapotranspiration to Water and Salt
2.3. Optimization Model of Water and Salinity Threshold
2.3.1. Optimization Model of Soil Water and Salt Thresholds in the Root Zone
- (1)
- water balance constraint:Wi+1 = Wi + Pi + Ii − ETai + Qei − QDi − Ri,
- (2)
- salt balance constraint:Sai+1 = Sai + SIi + SPi + Sei − SDi − SCi
- (3)
- irrigation quantity constraints:
- (4)
- boundary condition constraints:
- (5)
- non-negative constraintswhere: Wi and Wi+1 are soil moisture content (mm) at the beginning and end of growth stage i; ETasi, Ii, Pi, Qei, QDi, Ri, and θi are evapotranspiration (mm), irrigation (mm), precipitation (mm), capillary water (mm), drainage (mm), runoff (mm), and soil moisture content (cm3 cm−3) at growth stage i; Sai and Sai+1 are salt content of the root zone soil at the beginning and end of growth stage i (kg m−2); SIi, SPi, Sei, and SDi are salt content of irrigation water, precipitation, capillary water, and deep drainage at growth stage i (kg m−2), and SCi is plant salt absorption (kg m−2); M is the irrigation quota (mm); θwp is the wilt coefficient and θfc is the field capacity (cm3 cm−3); Si and Si,max are root soil salt content and maximum salt content (g kg−1) at the beginning of growth stage i; Sr is the maximum allowable salt content (g kg−1) at the end of the growth stage; W0 is initial water content (mm) and S0 is initial salt content (g kg−1).
2.3.2. Root Zone Water Balance Module
2.3.3. Root Zone Salt Content Module
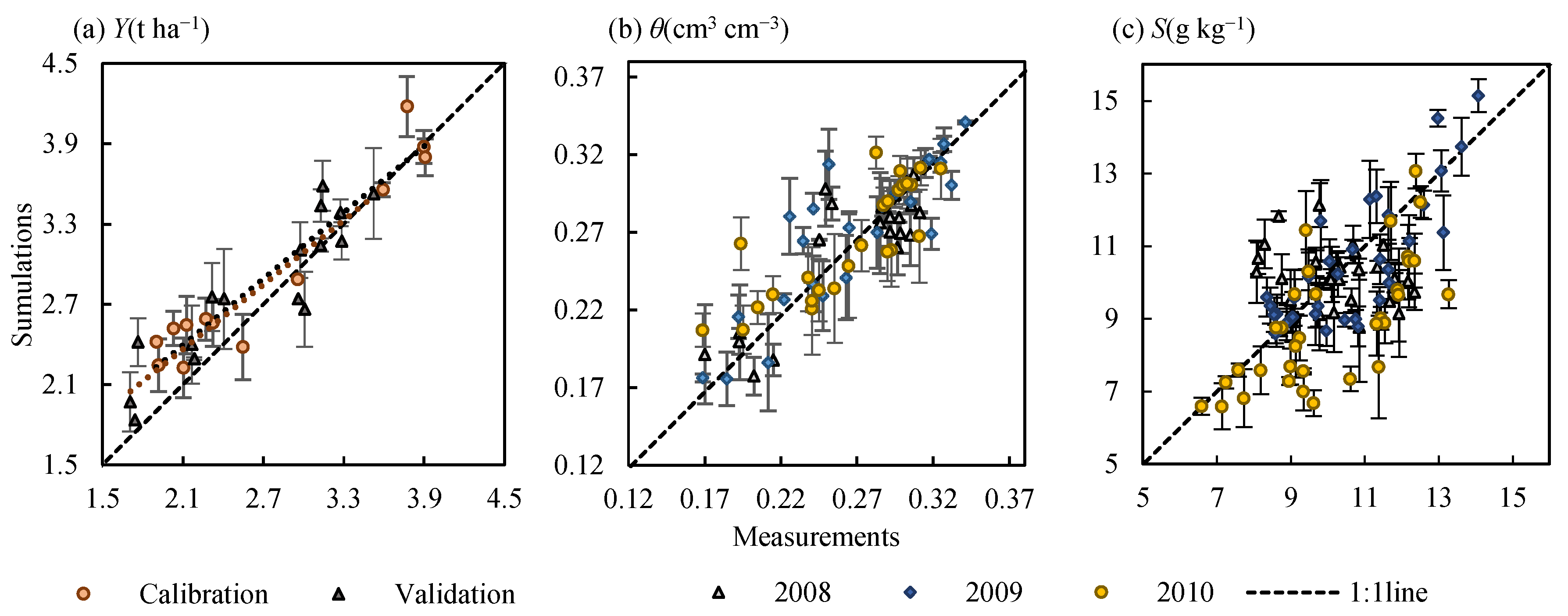
2.3.4. Model Evaluation
3. Results and Discussion Generate
3.1. Analysis of Model Parameters
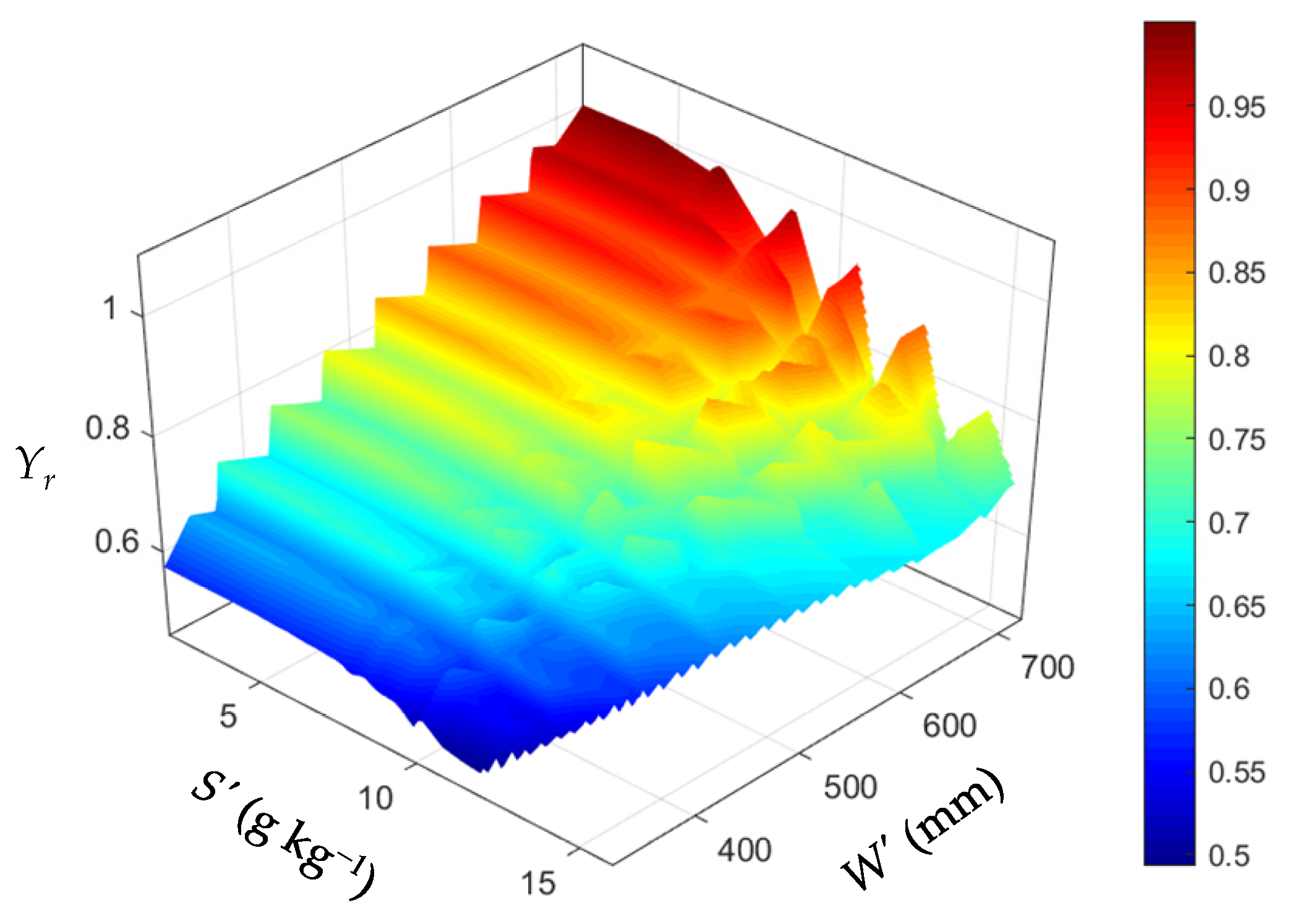
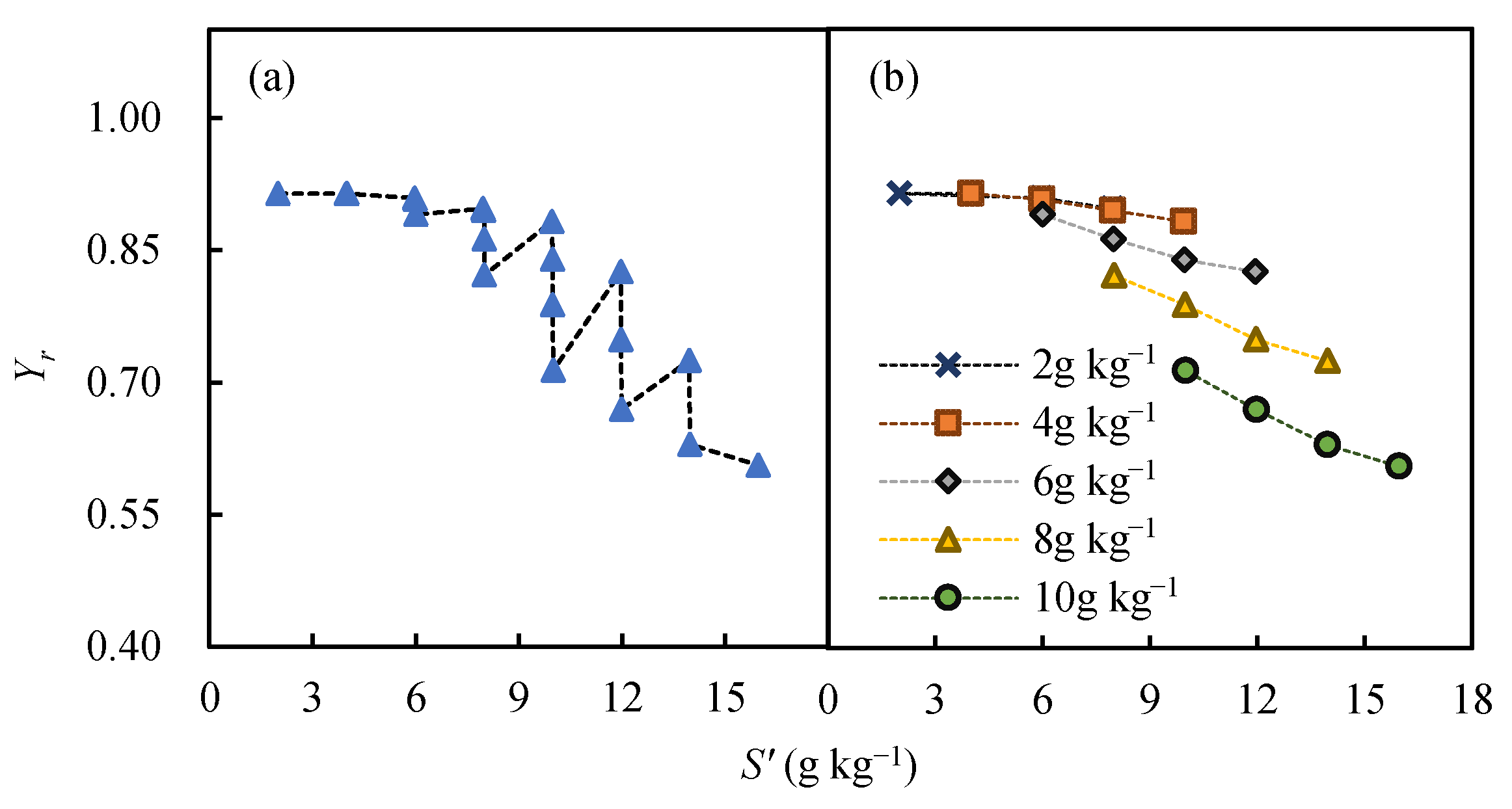
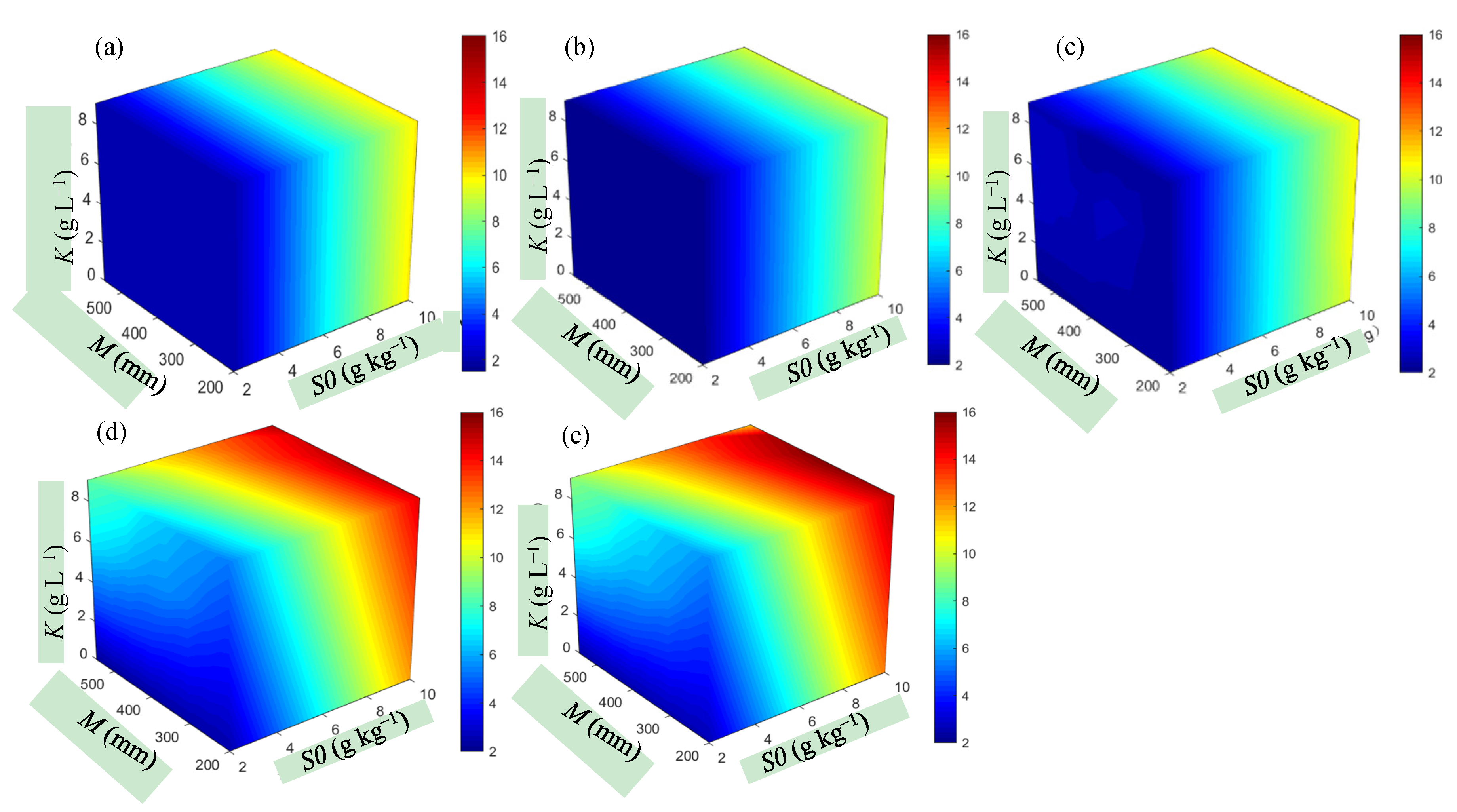
3.2. Response of Yield to Soil Water and Salinity under Different Scenarios
3.2.1. Effect of Available Water on Yield
3.2.2. Effect of Soil Salinity on Yield
3.2.3. Soil Water and Salt Content under Different Yield Reduction Levels
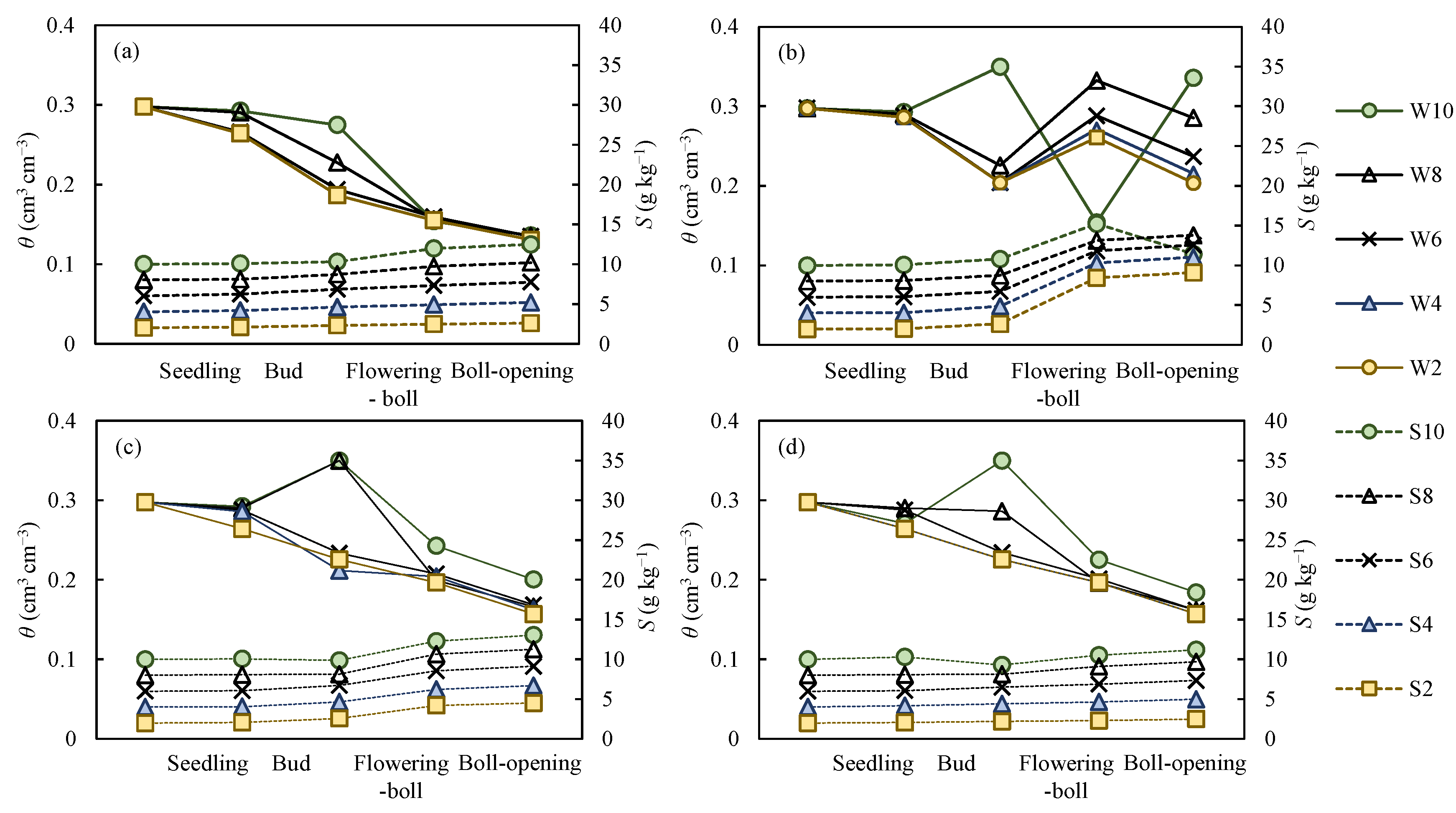
3.3. Dynamic Thresholds of Water and Salinity in the Cotton Root Zone During the Growth Period
3.3.1. Threshold Values of Soil Water and Salinity in the Root Zone during the Growth Period
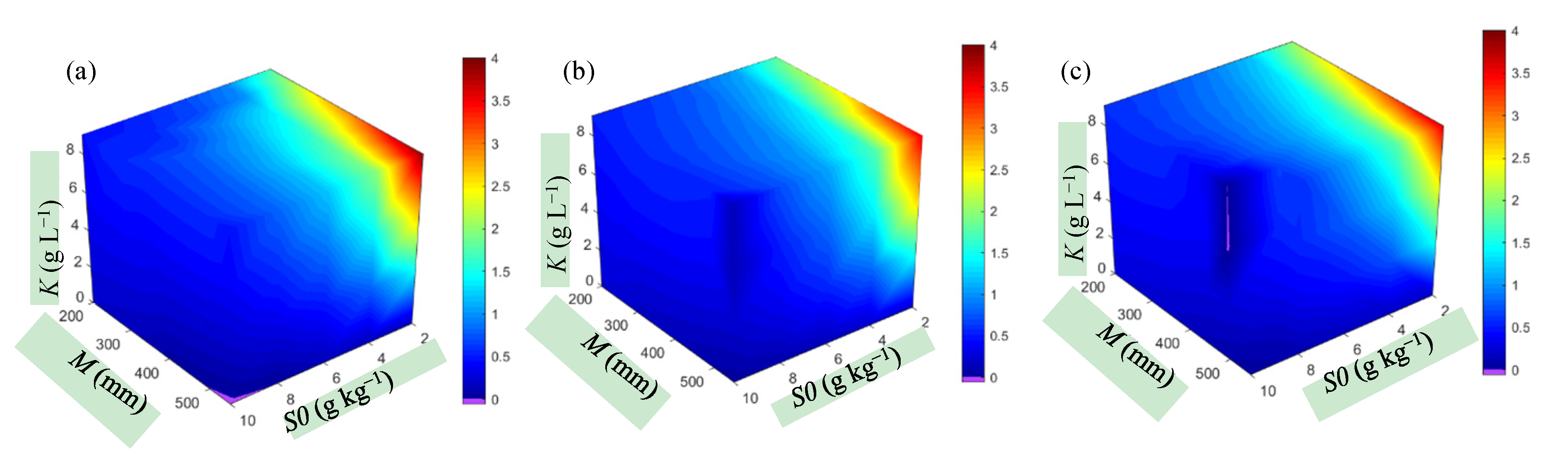
3.3.2. Soil Salt Accumulation over the Entire Growth Period
4. Conclusions
- cotton plants differ in sensitivity to soil moisture content and salinity at different growth stages. In descending order of sensitivity, the stages for soil water sensitivity are ordered: flowering–boll > bud > boll-opening > seedling; and the stages for sensitivity to salinity are ordered: flowering–boll > seedling > bud > boll-opening. The flowering–boll stage is the crucial period for cotton yield; therefore, particular attention should be given to the control of soil water and salinity during that period;
- cotton yield is significantly affected by irrigation quota M, initial soil moisture content W0, initial soil salt content S0, and irrigation water saltinity K. To ensure that the relative yield of cotton is above 0.85, the available water W’ (the sum of W0 and M) must meet the requirement W’ > 535 mm (with W0 ≥ 0.85θfc and M ≥ 400 mm), and total soil salt S’ should meet the requirement S’ < 9 g kg−1 (with S0 < 8 g kg−1 and K < 6 g L−1) in southern Xinjiang;
- the threshold levels of water content and salt in the root zone under different scenarios vary considerably. This result indicates that the change in threshold levels depended on the initial boundary conditions and other factors. The Akesu Irrigation District in southern Xinjiang, where soil salt content is relatively low, can be represented reasonably well by the scenario W0 = 0.85θfc, S0 = 4 g kg−1, M = 400 mm, K = 0 g L−1. In this scenario, when no actions were taken to remove salt during the growth period, the threshold levels of soil water at different growth stages (seedling, bud, flowering–boll and boll-opening) were respectively 0.75–0.85θfc, 0.65–0.75θfc, 0.56–0.65θfc, and 0.45–0.56θfc, and the threshold levels of salt were, respectively, 4–4.16, 4.16–4.39, 4.39–4.64, and 4.64–4.97 g kg−1. In most cases, due to salt accumulation over the entire growth period, it is necessary to reduce the salt content of the root zone to ensure sustainable agriculture.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| Parameters and Variables | Meaning and Description |
| i | The ith growth stage |
| n | The number of growth stages |
| λi | The water deficit sensitivity index of yield at growth stage i |
| θpi | The critical water content (cm3 cm−3) at growth stage i |
| ρ, σ | Salt stress sensitivity index of ET and yield |
| Ya, Ym | The actual crop yield and the potential crop yield (kg ha−1) |
| ETai, ETmi | Actual and maximum evapotranspiration (mm) of growth stage i |
| SY,max,SY,min | The critical and maximum soil salinity which affect the yield at different growth stages (g kg−1) |
| Set,max,Set,min | The critical and maximum soil salinity which affect ET at different growth stages (g kg−1) |
| Ks | The stress factor |
| Ksw | The water stress factor |
| Kss | The salt stress factor |
| Parameters and Variables | Meaning and Description |
| Ya, Ym | The actual crop yield and the potential crop yield (kg ha−1) |
| gi | The response factors of yield to water and salinity at growth stage i. |
| Wi,Wi+1 | The soil moisture content (mm) at the beginning and end of growth stage i |
| ETsi, Ii, Pi, Qei, QDi, Ri, θi | The evapotranspiration (mm), irrigation (mm), precipitation (mm), capillary water (mm), drainage (mm), runoff (mm), and soil moisture content (cm3 cm−3) at growth stage i |
| Sai,Sai+1 | The salt content of the root zone soil at the beginning and end of growth stage i (kg m−2) |
| SIi, SPi, Sei, Sdi,Sci | The salt content of irrigation water, precipitation, capillary water, deep drainage and absorbed by the plants at growth stage i (kg m−2) |
| θwp, θfc | Wilt coefficient and field capacity (cm3 cm−3) |
| Si, Si,max | The root soil actual and maximum salt content (g kg−1) at the beginning of growth stage i |
| Sr | The maximum allowable salt content (g kg−1) at the end of the growth stage |
| M | The irrigation quota (mm) |
| W0, S0 | Initial water content (mm) and initial salt content (g kg−1) |
| Parameters and Variables | Meaning and Description |
| a, b | The exchange coefficients of capillary rise and drainage |
| Hi | The layer depth in the root zone (m) |
| f | The leaching coefficient |
| β | The capillary rise coefficient |
| Ki, CPi, Cei, CDi | The salt concentration of irrigation water, precipitation, capillary rise and deep drainage in growth stage i (kg m−3) |
| Ci | The salt concentration of the root soil at the beginning of growth stage i (kg m−3) |
| Cwi | The average salt concentration of irrigation water and precipitation (kg m−3) |
References
- Asfaw, E.; Suryabhagavan, K.V.; Argaw, M. Soil salinity modeling and mapping using remote sensing and GIS: The case of Wonji sugar cane irrigation farm, Ethiopia. J. Saudi Soc. Agric. Sci. 2018, 17, 250–258. [Google Scholar] [CrossRef]
- Ma, C.J.; Naidu, R.; Liu, F.G.; Lin, C.H.; Ming, H. Influence of hybrid giant Napier grass on salt and nutrient distributions with depth in a saline soil. Biodegradation 2012, 23, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.X.; Tian, C.Y.; Li, C.J. Soil salinity dynamics under drip irrigation and mulch film and their effects on cotton root length. Commun. Soil Sci. Plan. 2013, 44, 1489–1502. [Google Scholar] [CrossRef]
- Dixit, P.N.; Chen, D. Modification of a spatially referenced crop model to simulate the effect of spatial pattern of subsoil salinity. Comput. Electron. Agric. 2010, 74, 313–320. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J. Vegetative bioremediation of calcareous sodic soils: History, mechanisms, and evaluation. Irrig. Sci. 2002, 21, 91–101. [Google Scholar]
- Letey, J.; Hoffman, G.J.; Hopmans, J.W.; Grattan, S.R.; Suarez, D.; Corwin, D.L.; Oster, J.D.; Wu, L.; Amrhein, C. Evaluation of soil salinity leaching requirement guidelines. Agric. Water Manag. 2011, 98, 502–506. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. A simplified regional-scale electromagnetic induction-Salinity calibration model using ANOCOVA modeling techniques. Geoderma 2014, 230, 288–295. [Google Scholar] [CrossRef]
- Mao, W.; Yang, J.Z.; Zhu, Y.; Ye, M.; Wu, J.W. Loosely coupled SaltMod for simulating groundwater and salt dynamics under well-canal conjunctive irrigation in semi-arid areas. Agric. Water Manag. 2017, 192, 209–220. [Google Scholar] [CrossRef]
- Campbell, B.T.; Saha, S.; Percy, R.; Frelichowski, J.; Jenkins, J.N.; Park, W.; Mayee, C.D.; Gotmare, V.; Dessauw, D.; Giband, M. Status of the global cotton germplasm resources. Crop Sci. 2010, 50, 1161–1179. [Google Scholar] [CrossRef]
- Zhang, H.; Khalid, H.; Li, W.; He, Y.; Liu, G.; Chen, C. Employing response surface methodology (RSM) to improve methane production from cotton stalk. Environ. Sci. Pollut. Res. 2018, 25, 7618–7624. [Google Scholar] [CrossRef]
- Yang, Z.N.; Tang, J.J.; Yu, X.L. Xinjiang Cotton industry present situation and countermeasure research. Res. Agric. Mod. 2013, 34, 298–302. (In Chinese) [Google Scholar]
- Wang, Z.M.; Jin, M.G.; Šimůnek, J.; van Genuchten, M.T. Evaluation of mulched drip irrigation for cotton in arid Northwest China. Irrig. Sci. 2014, 32, 15–27. [Google Scholar] [CrossRef]
- Penna, J.C.V.; Verhalen, L.M.; Kirkham, M.B.; McNew, R.W. Screening cotton genotypes for seedling drought tolerance. Genet. Mol. Biol. 1998, 21, 545–549. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance-current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Pace, P.F.; Cralle, H.T.; El-Halawany, S.H.; Cothren, J.T.; Senseman, S.A. Drought-induced changes in shoot and root growth of young cotton plants. J. Cotton Sci. 1999, 3, 183–187. [Google Scholar]
- Turner, N.C.; Hearn, A.B.; Begg, J.E.; Constable, G.A. Cotton (Gossypium-hirsutum-l): Physiological and morphological responses to water deficits and their relationship to yield. Field Crop. Res. 1986, 14, 153–170. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Van Genuchten, M.T.; Hoffman, G.J. Analysis of crop production. Soil. Salin. Irrig. 1984, 51, 258–271. [Google Scholar]
- Jensen, M.E. Water Consumption by Agricultural Plants (Chapter 1); Academic Press: New York, NY, USA, 1968. [Google Scholar]
- Chen, J.L.; Kang, S.Z.; Du, T.S.; Guo, P.; Qiu, R.J.; Chen, R.Q.; Gu, F. Modeling relations of tomato yield and fruit quality with water deficit at different growth stages under greenhouse condition. Agric. Water Manag. 2014, 146, 131–148. [Google Scholar] [CrossRef]
- Wang, J.T.; Guo, S.S.; Kang, S.Z.; Wang, Y.F.; Du, T.S.; Tong, L. Joint optimization of irrigation and planting pattern to guarantee seed quality, maximize yield, and save water in hybrid maize seed production. Eur. J. Agron. 2020, 113, 125970. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Xu, C.; Wu, J.W.; Huang, J.S. Sunflower seed yield estimation under the interaction of soil salinity and nitrogen application. Field Crop. Res. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Russo, D.; Bakker, D. Crop-water production functions for sweet corn and cotton irrigated with saline waters. Soil Sci. Soc. Am. J. 1987, 51, 1554–1562. [Google Scholar] [CrossRef]
- Letey, J.; Dinar, A. Simulated crop-water production functions for several crops when irrigated with saline waters. Hilgardia 1986, 54, 1–32. [Google Scholar] [CrossRef]
- Shani, U.; Ben-Gal, A.; Tripler, E.; Dudley, L.M. Plant response to the soil environment: An analytical model integrating yield, water, soil type, and salinity. Water Resour. Res. 2007, 43, W08418. [Google Scholar] [CrossRef]
- Skaggs, T.H.; Anderson, R.G.; Corwin, D.L.; Suarez, D.L. Analytical steady-state solutions for water-limited cropping systems using saline irrigation water. Water Resour. Res. 2014, 50, 9656–9674. [Google Scholar] [CrossRef]
- Jalali, V.; Asadi Kapourchal, S.; Homaee, M. Evaluating performance of macroscopic water uptake models at productive growth stages of durum wheat under saline conditions. Agric. Water Manag. 2017, 180, 13–21. [Google Scholar] [CrossRef]
- Wang, R.S.; Kang, Y.H.; Wan, S.Q.; Hu, W.; Liu, S.P.; Liu, S.H. Salt distribution and the growth of cotton under different drip irrigation regimes in a saline area. Agric. Water Manag. 2011, 100, 58–69. [Google Scholar] [CrossRef]
- Wang, R.S.; Wan, S.Q.; Sun, J.X.; Xiao, H.J. Soil salinity, sodicity and cotton yield parameters under different drip irrigation regimes during saline wasteland reclamation. Agric. Water Manag. 2018, 209, 20–31. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liao, R.K.; Lin, H.; Jiang, G.J.; He, X.L.; Wu, W.Y.; Lili, Z.Z. Effects of drip irrigation levels on soil water, salinity and wheat growth in North China. Int. J. Agric. Biol. Eng. 2018, 11, 146–156. [Google Scholar] [CrossRef]
- Kumar, P.; Sarangi, A.; Singh, D.K.; Parihar, S.S. Evaluation of AquaCrop model in predicting wheat yield and water productivity under irrigated saline regimes. Irrig. Drain. 2014, 63, 474–487. [Google Scholar] [CrossRef]
- Supit, I.; Hooijer, A.A.; van Diepen, C.A. System Description of the WOFOST 6.0 Crop Simulation Model Implemented in CGMS, Vol. 1: Theory and Algorithms; Joint Research Centre, Commission of the European Communities: Luxembourg, 1994; p. 146. [Google Scholar]
- Van Dam, J.C.; Huygen, J.; Wesseling, J.G.; Feddes, R.A.; Kabat, P.; Van Walsum, P.; Groenendijk, P.; van Diepen, C.A. Theory of SWAP Version 2.0; Simulation of Water Flow, Solute Transport and Plant Growth in the Soil-Water-Atmosphere-Plant Environment; DLO Winand Staring Centre: Wageningen, The Netherlands, 1997. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. FAO Rome 1998, 300, D5109. [Google Scholar]
- Guang, M.L.; Jing, S.Y.; Rong, J.Y. Chemical factors to electrical conductivity of soil extract and their intensity. Acta Pedol. Sinica 2005, 2, 247–252. (In Chinese) [Google Scholar]
- Sun, G.F.; Zhu, Y.; Ye, M.; Yang, J.Z.; Qu, Z.Y.; Mao, W.; Wu, J.W. Development and application of long-term root zone salt balance model for predicting soil salinity in arid shallow water table area. Agric. Water Manag. 2019, 213, 486–498. [Google Scholar] [CrossRef]
- Wang, F.; Sun, J.; Liu, Z.; Ning, H.; Qiang, X.; Shen, X. Effect of different irrigation scheduling on salt distribution and leaching in cotton field. Trans. Chin. Soc. Agric. Mach. 2013, 44, 120–127. (In Chinese) [Google Scholar]
- Zhang, Z.Y.; Guo, X.P. Dynamic response model of crop to water-salinity. J. Hydraul. Eng. 1998, 12, 66–70. (In Chinese) [Google Scholar]
- Hu, M. Mechanisms and Simulation for Two Representative Desert Shrubs Response to Water and Salt Stress in Arid Region. Ph.D. Thesis, China Agricultural University, Beijing, China, November 2010. (In Chinese). [Google Scholar]
- Zhu, Z.D. Principles and Applications of Drainage, II. Theory of Field Drainage and Runoff; Agriculture Press: Beijing, China, 1981. (In Chinese) [Google Scholar]
- Luo, H.H.; Zhang, Y.L.; Zhang, W.F. Effects of water stress and rewatering on photosynthesis, root activity, and yield of cotton with drip irrigation under mulch. Photosynthetica 2016, 54, 65–73. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer Science & Business Media: Berlin, Germany, 2003. [Google Scholar]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Zhu, J. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crop. Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Zonta, J.H.; Brandao, Z.N.; Rodrigues, J.I.D.S.; Sofiatti, V. Cotton response to water deficits at different growth stages. Rev. Caatinga 2017, 30, 980–990. [Google Scholar] [CrossRef]
- Longenecker, D.E. The influence of high sodium in soils upon fruiting and shedding, boll characteristics, fiber properties, and yields of two cotton species. Soil Sci. 1974, 118, 387–396. [Google Scholar] [CrossRef]
- Fowler, J.L. Salinity and Fruiting; The Cotton Foundation: Memphis, TN, USA, 1986; pp. 107–111. [Google Scholar]
- Feng, D.; Zhang, J.P.; Sun, J.S.; Zheng, C.L.; Cao, C.Y.; Li, K.J.; Liu, Z.G. Cotton salt tolerance appraisal indices and eigenvalue under border irrigation with saline water. Trans. Chin. Soc. Agric. Eng. 2012, 28, 52–57. (In Chinese) [Google Scholar]
- Feng, D.; Zhang, J.P.; Sun, C.T.; Dang, H.K.; Liu, H.; Ning, H.F.; Sun, J.S.; Li, K.J. Responses of cotton growth and water physiological indices to salt stress at different growing stages. Chin. J. Ecol. 2014, 33, 1195–1199. (In Chinese) [Google Scholar]
- Pearson, K.E.; Bauder, J.W.; Warrance, N.J. The Basics of Salinity and Sodicity Effects on Soil Physical Properties; Montana State University: Bozeman, MO, USA, 2006; pp. 1–11. [Google Scholar]
- Yang, M.; Wang, G.; Lazin, R.; Shen, X.; Anagnostou, E. Impact of planting time soil moisture on cereal crop yield in the Upper Blue Nile Basin: A novel insight towards agricultural water management. Agric. Water Manag. 2020, 243, 106430. [Google Scholar] [CrossRef]
- Ali, M.H.; Hoque, M.R.; Hassan, A.A.; Khair, A. Effects of deficit irrigation on yield, water productivity, and economic returns of wheat. Agric. Water Manag. 2007, 92, 151–161. [Google Scholar] [CrossRef]
- Wang, Q.; Huo, Z.; Zhang, L.; Wang, J.; Zhao, Y. Impact of saline water irrigation on water use efficiency and soil salt accumulation for spring maize in arid regions of China. Agric. Water Manag. 2016, 163, 125–138. [Google Scholar] [CrossRef]
- Stewart, J.I.; Hagan, R.M. Functions to predict effects of crop water deficits. J. Irrig. Drain. Div. 1973, 99, 421–439. [Google Scholar]
- García Vila, M.; Fereres, E.; Mateos, L.; Orgaz, F.; Steduto, P. Deficit Irrigation Optimization of Cotton with AquaCrop. Agron. J. 2009, 101, 477–487. [Google Scholar] [CrossRef]
- Tan, S.; Wang, Q.J.; Zhang, J.H.; Chen, Y.; Shan, Y.Y.; Xu, D. Performance of AquaCrop model for cotton growth simulation under film-mulched drip irrigation in southern Xinjiang, China. Agric. Water Manag. 2018, 196, 99–113. [Google Scholar] [CrossRef]
- Patell, R.M.; Prasher, S.O.; Donnelly, D.; Bonnell, R.B. Effect of initial soil salinity and subirrigation water salinity on potato tuber yield and size. Agric. Water Manag. 2001, 46, 231–239. [Google Scholar] [CrossRef]
- Selim, T.; Berndtsson, R.; Persson, M.; Somaida, M.; El-Kiki, M.; Hamed, Y.; Mirdan, A.; Zhou, Q.Y. Influence of geometric design of alternate partial root-zone subsurface drip irrigation (APRSDI) with brackish water on soil moisture and salinity distribution. Agric. Water Manag. 2012, 103, 182–190. [Google Scholar] [CrossRef]
- Zhao, X.N.; Othmanli, H.; Schiller, T.; Zhao, C.; Sheng, Y.; Zia, S.; Müller, J.; Stahr, K. Water use efficiency in saline soils under cotton cultivation in the Tarim River Basin. Water 2015, 7, 3103–3122. [Google Scholar] [CrossRef]
- Chen, J.L. Modeling Fruit Growth and Sugar Accumulation and Optimizing Irrigation Schedule to Improve Water Use Efficiency and Fruit Quality of Tomato. Ph.D. Thesis, China Agricultural University, Beijing, China, November 2016. (In Chinese). [Google Scholar]
- Chen, W.P.; Hou, Z.N.; Wu, L.S.; Liang, Y.C.; Wei, C.Z. Evaluating salinity distribution in soil irrigated with saline water in arid regions of northwest China. Agric. Water Manag. 2010, 97, 2001–2008. [Google Scholar] [CrossRef]
- Chen, W.L.; Jin, M.G.; Ferré, T.P.A.; Liu, Y.F.; Xian, Y.; Shan, T.R.; Ping, X. Spatial distribution of soil moisture, soil salinity, and root density beneath a cotton field under mulched drip irrigation with brackish and fresh water. Field Crop. Res. 2018, 215, 207–221. [Google Scholar] [CrossRef]
- Zhao, B. Study on Control Indicator of Automatic Irrigation about Cotton under Film Drip Irrigation. Master’s Thesis, Shihezi University, Shihezi, China, January 2017. (In Chinese). [Google Scholar]
- Wang, Z.H.; Zhou, B.; Pei, L.; Zhang, J.Z.; He, X.L.; Lin, H. Controlling threshold in soil salinity when planting spring wheat and sequential cropping silage corn in Northern Xinjiang using drip irrigation. Int. J. Agric. Biol. Eng. 2018, 11, 108–114. [Google Scholar] [CrossRef]
- Mmolawa, K.; Or, D. Root zone solute dynamics under drip irrigation: A review. Plant Soil 2000, 222, 163–190. [Google Scholar] [CrossRef]
- Manjunatha, M.V.; Oosterbaan, R.J.; Gupta, S.K.; Rajkumar, H.; Jansen, H. Performance of subsurface drains for reclaiming waterlogged saline lands under rolling topography in Tungabhadra irrigation project in India. Agric. Water Manag. 2004, 69, 69–82. [Google Scholar] [CrossRef]




| Module | Parameter | Seedling Stage | Bud Stage | Flowering–Boll Stage | Boll-Opening Stage |
|---|---|---|---|---|---|
| Modified Jensen model | λ | −0.150 | 0.202 | 0.411 | 0.166 |
| σ | 0.121 | 0.051 | 0.29 | −0.683 | |
| SY,min (g kg−1) | 6.64 | 7.37 | 8.14 | 8.53 | |
| SY,max (g kg−1) | 21.01 | 22.19 | 24.69 | 27.87 | |
| Water balance module | ρ | 0.7 | 0.98 | 0.81 | 0.78 |
| Set,min (g kg−1) | 4.51 | 5.48 | 5.80 | 5.88 | |
| Set,max (g kg−1) | 25.0 | 17.9 | 28.0 | 25.0 | |
| θp (cm3 cm−3) | 0.245 | ||||
| a | 0.561 | ||||
| b | −0.635 | ||||
| Salt balance module | f | 0.2 | |||
| β | 1.4 | ||||
| Variable | Year | R2 | RMSE | nRMSE (%) |
|---|---|---|---|---|
| Yield | Calibration | 0.827 | 0.708 | 24.4 |
| (t ha−1) | Validation | 0.914 | 0.577 | 20.4 |
| Soil water content (cm3 cm−3) | 2008 | 0.753 | 0.040 | 15.0 |
| 2009 | 0.747 | 0.049 | 18.1 | |
| 2010 | 0.681 | 0.036 | 14.9 | |
| Soil salt content (g kg−1) | 2008 | 0.012 | 1.649 | 16.2 |
| 2009 | 0.657 | 1.690 | 16.0 | |
| 2010 | 0.485 | 1.843 | 18.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Kang, S.; Li, X.; Guo, P.; Hu, S. Optimization-Based Water-Salt Dynamic Threshold Analysis of Cotton Root Zone in Arid Areas. Water 2020, 12, 2449. https://doi.org/10.3390/w12092449
Wu H, Kang S, Li X, Guo P, Hu S. Optimization-Based Water-Salt Dynamic Threshold Analysis of Cotton Root Zone in Arid Areas. Water. 2020; 12(9):2449. https://doi.org/10.3390/w12092449
Chicago/Turabian StyleWu, Hui, Shaozhong Kang, Xiaojuan Li, Ping Guo, and Shunjun Hu. 2020. "Optimization-Based Water-Salt Dynamic Threshold Analysis of Cotton Root Zone in Arid Areas" Water 12, no. 9: 2449. https://doi.org/10.3390/w12092449
APA StyleWu, H., Kang, S., Li, X., Guo, P., & Hu, S. (2020). Optimization-Based Water-Salt Dynamic Threshold Analysis of Cotton Root Zone in Arid Areas. Water, 12(9), 2449. https://doi.org/10.3390/w12092449




