Low-Temperature Adapted Nitrifying Microbial Communities of Finnish Wastewater Treatment Systems
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
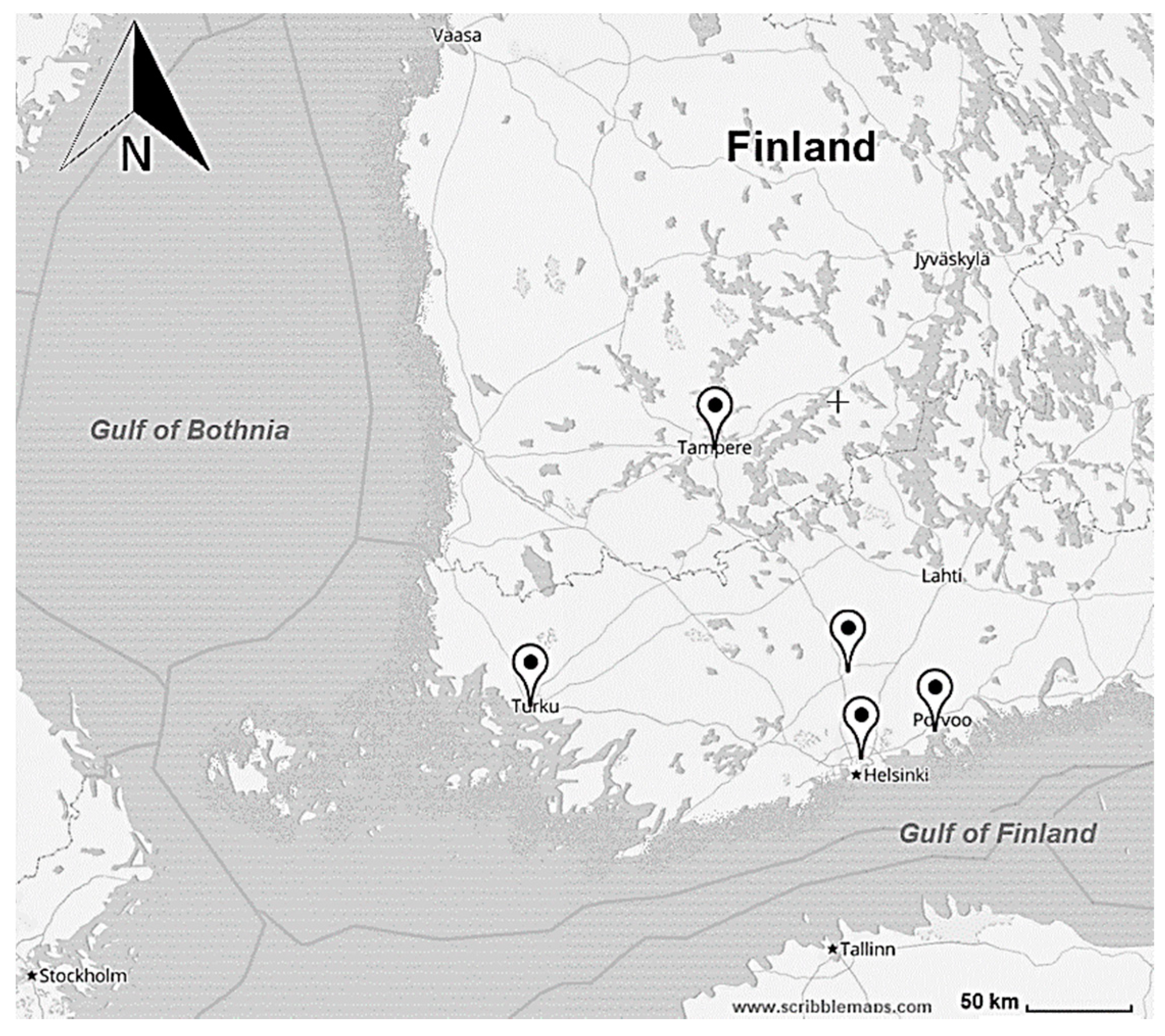
2.1.1. Full-Scale Study
2.1.2. Pilot Study
2.2. Samples Processing
3. Results and Discussion
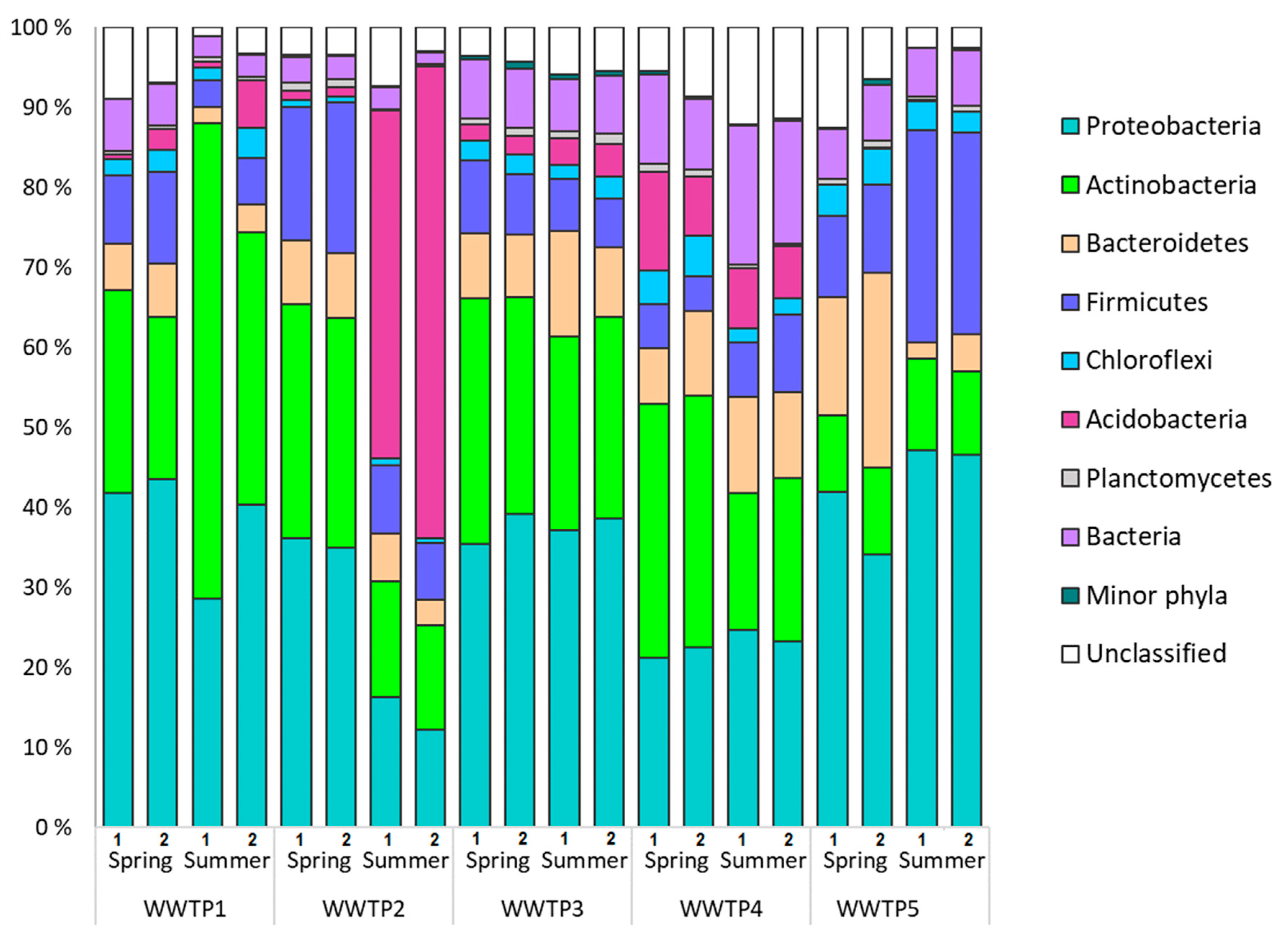
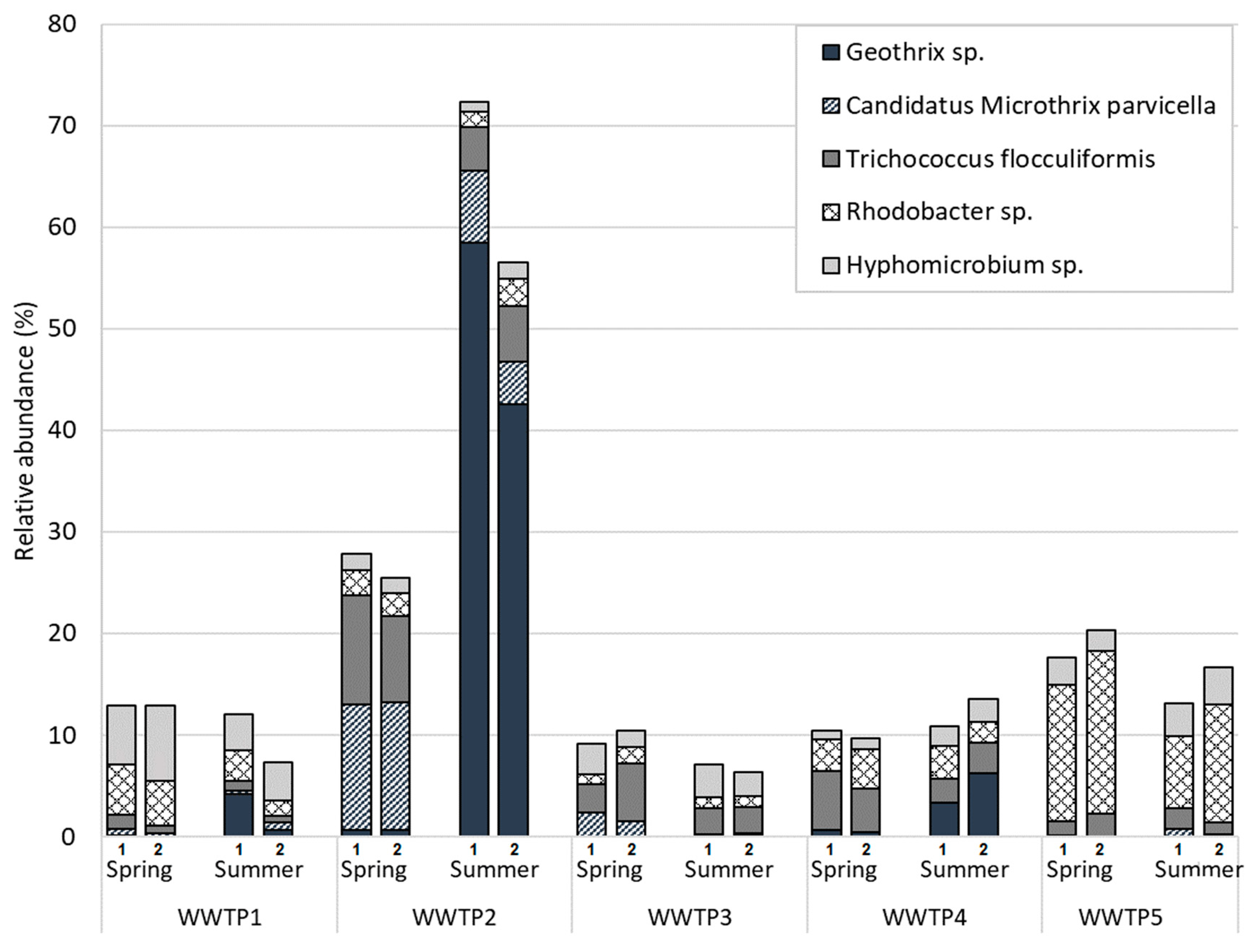
3.1. Microbial Communities of Finnish WWTPs
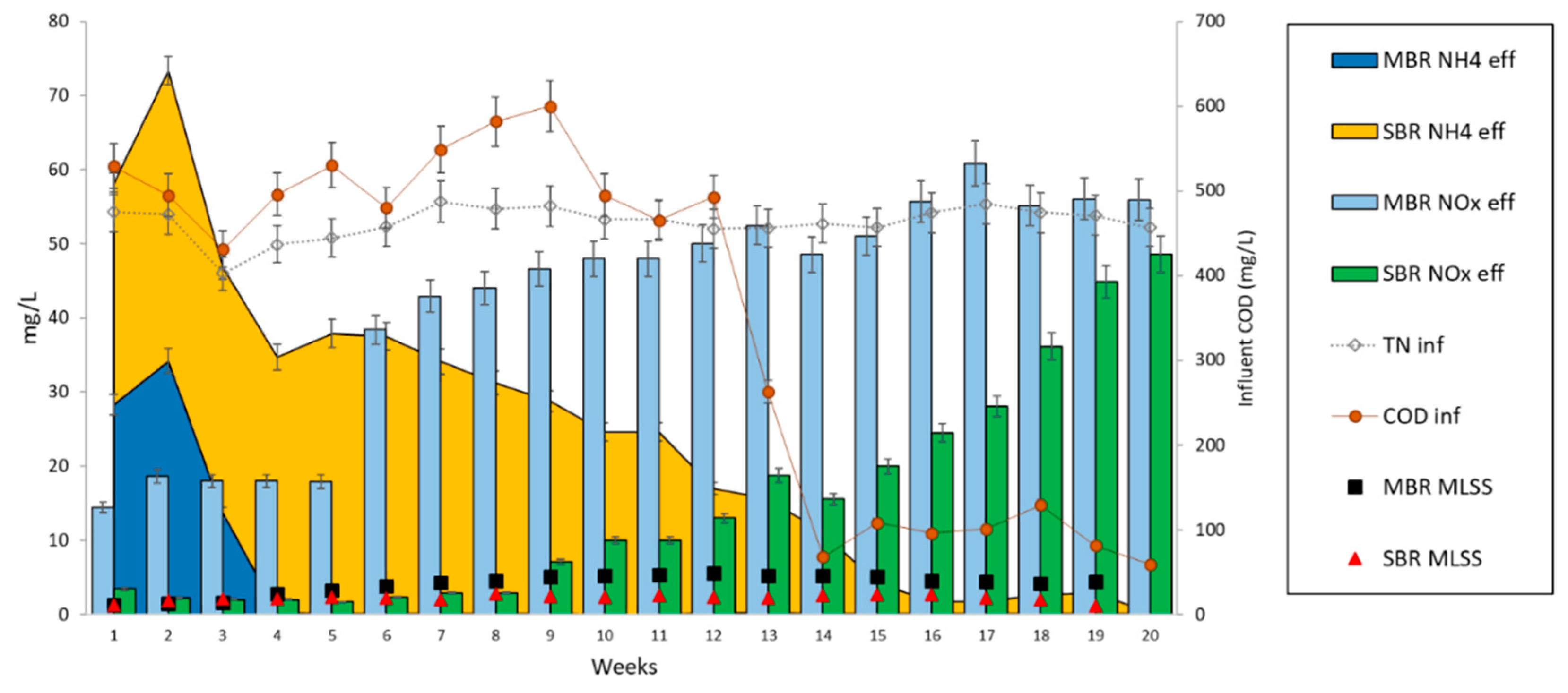
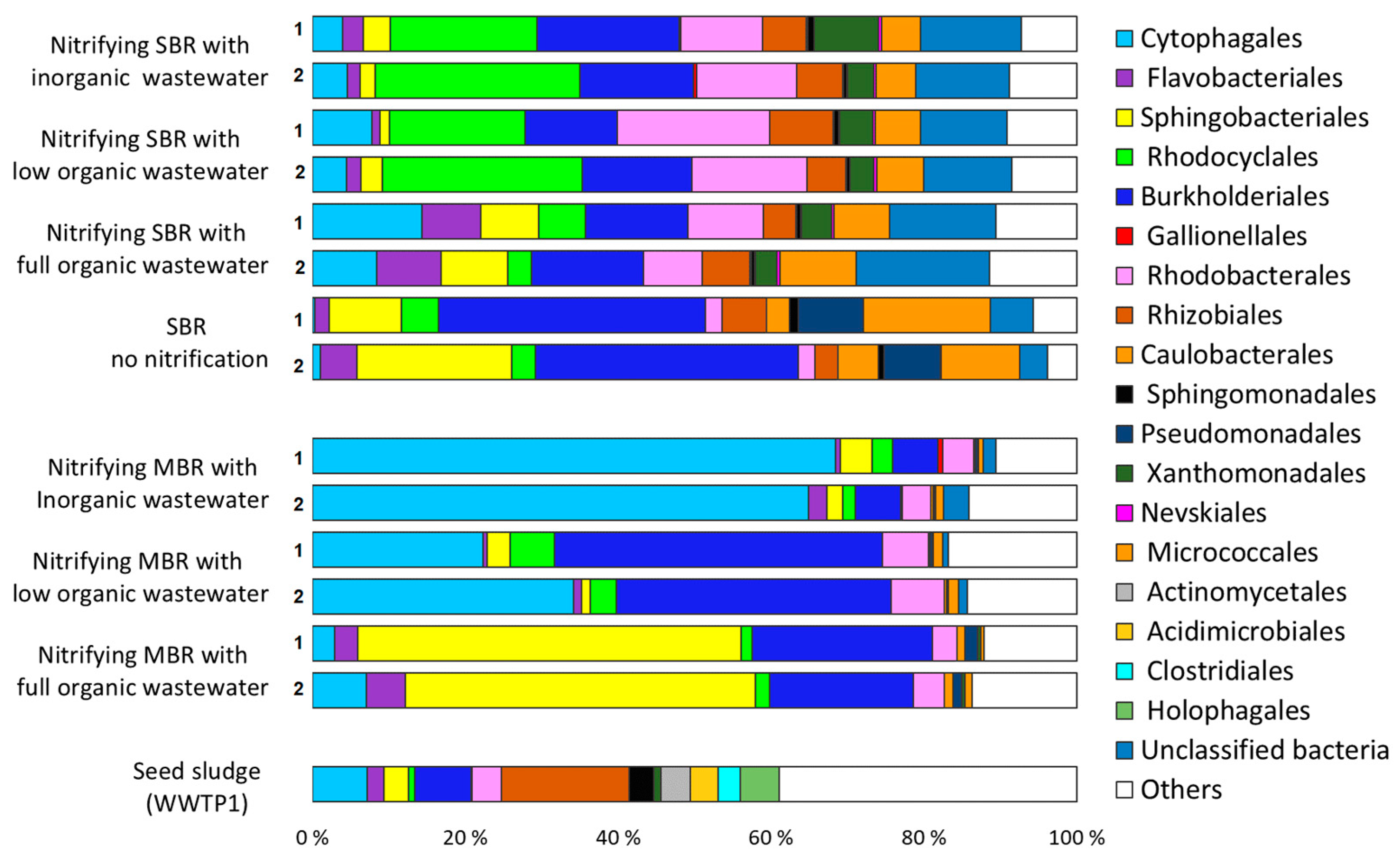
3.2. Acclimation of Activated Sludge to Laboratory Conditions
3.3. Effect of Organic Carbon Source on the Dynamics of Activated Sludge Microbial Community
3.3.1. Bacteria
3.3.2. Archaea
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaranowska, P.; Cydzik-Kwiatkowska, A.; Zielińska, M. Configuration of biological wastewater treatment line and influent composition as the main factors driving bacterial community structure of activated sludge. World J. Microbiol. Biotechnol. 2013, 29, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Awolusi, O.O.; Kumari, S.K.S.; Bux, F. Ecophysiology of nitrifying communities in membrane bioreactors. Int. J. Environ. Sci. Technol. 2015, 12, 747–762. [Google Scholar] [CrossRef][Green Version]
- Alawi, M.; Lipski, A.; Sanders, T.; Eva Maria, P.; Spieck, E. Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J. 2007, 1, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Mattila, K.; Tamminen, M.; Virta, M. Cold temperature decreases bacterial species richness in nitrogen-removing bioreactors treating inorganic mine waters. Biotechnol. Bioeng. 2011, 108, 2876–2883. [Google Scholar] [CrossRef] [PubMed]
- Kruglova, A.; Ahlgren, P.; Korhonen, N.; Rantanen, P.; Mikola, A.; Vahala, R. Biodegradation of ibuprofen, diclofenac and carbamazepine in nitrifying activated sludge under 12 °C temperature conditions. Sci. Total Environ. 2014, 499, 394–401. [Google Scholar] [CrossRef]
- Kruglova, A.; Kråkström, M.; Riska, M.; Mikola, A.; Rantanen, P.; Vahala, R.; Kronberg, L. Comparative study of emerging micropollutants removal by aerobic activated sludge of large laboratory-scale membrane bioreactors and sequencing batch reactors under low-temperature conditions. Bioresour. Technol. 2016, 214, 81–88. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Sihvonen, M.; Muñoz-Palazon, B.; Rodriguez-Sanchez, A.; Mikola, A.; Vahala, R. Microbial ecology of full-scale wastewater treatment systems in the Polar Arctic Circle: Archaea, Bacteria and Fungi. Sci. Rep. 2018, 8, 2208. [Google Scholar] [CrossRef]
- Urakawa, H.; Tajima, Y.; Numata, Y.; Tsuneda, S. Low Temperature Decreases the Phylogenetic Diversity of Ammonia-Oxidizing Archaea and Bacteria in Aquarium Biofiltration Systems. Appl. Environ. Microbiol. 2008, 74, 894–900. [Google Scholar] [CrossRef]
- Kruglova, A.; Gonzalez-Martinez, A.; Kråkström, M.; Mikola, A.; Vahala, R. Bacterial diversity and population shifts driven by spotlight wastewater micropollutants in low-temperature highly nitrifying activated sludge. Sci. Total Environ. 2017, 605, 291–299. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Siddiqui, K.S.; Andrews, D.; Sowers, K.R. Low-temperature extremophiles and their applications. Curr. Opin. Biotechnol. 2002, 13, 253–261. [Google Scholar] [CrossRef]
- Orcutt, B.N.; Lapham, L.L.; Delaney, J.; Sarode, N.; Marshall, K.S.; Whaley-Martin, K.J.; Slater, G.; Wheat, C.G.; Girguis, P.R. Microbial response to oil enrichment in Gulf of Mexico sediment measured using a novel long-term benthic lander system. Elem. Sci. Anth. 2017, 5, 18. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Garcia-Ruiz, M.J.; Rodriguez-Sanchez, A.; Osorio, F.; Gonzalez-Lopez, J. Archaeal and bacterial community dynamics and bioprocess performance of a bench-scale two-stage anaerobic digester. Appl. Microbiol. Biotechnol. 2016, 100, 6013–6033. [Google Scholar] [CrossRef] [PubMed]
- RTL Genomics 2016. Data Analysis Methodology. Version 1.8. 2016. Available online: http://www.researchandtesting.com/docs/Data_Analysis_Methodology.pdf (accessed on 17 November 2016).
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Caporaso, J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.; Fierer, N.; Peña, A.; Goodrich, J.; Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, A.T.; Saunders, A.M.; Larsen, P.; Albertsen Stevenson, M.; Nielsen, J.L.; Nielsen, P.H. The Microbial Database for Danish wastewater treatmentplants with nutrient removal (MiDas-DK)—A tool forunderstanding activated sludge population dynamicsand community stability. Water. Sci. Technol. 2013, 67, 2519–2526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Hu, M.; Xia, Y.; Wen, X.; Ding, K. Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl. Environ. Microbiol. 2012, 78, 7042–7047. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, M.; Ye, L. 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef]
- Fredriksson, N.J.; Hermansson, M.; Wilén, B.-M. Long-term dynamics of the bacterial community in a Swedish full-scale wastewater treatment plant. Environ. Technol. 2019, 40, 912–928. [Google Scholar] [CrossRef]
- Hu, M.; Wang, X.; Wen, X.; Xia, Y. Microbial community structures in different wastewater treatment plants as revealed by 454-pyrosequencing analysis. Bioresour. Technol. 2012, 117, 72–79. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Rodriguez-Sanchez, A.; Lotti, T.; Garcia-Ruiz, M.; Osorio, F.; Gonzalez-Lopez, J.; van Loosdrecht, M.C.M. Comparison of bacterial communities of conventional and a-stage activated sludge systems. Sci. Rep. 2016, 6, 18786. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Saunders, A.M.; Albertsen, M.; Nierychlo, M.; McIlroy, B.; Hansen, A.A.; Karst, S.M.; Nielsen, J.L.; Nielsen, P.H. MiDAS: The field guide to the microbes of activated sludge. Database 2015, 2015, bav062. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Feng, J.; Liu, J.; Fu, W.; Li, X.; Li, B. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res. 2019, 151, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Bi, X.; Xu, C. Ammonia-Oxidizing Archaea (AOA) Play with Ammonia-Oxidizing Bacteria (AOB) in Nitrogen Removal from Wastewater. Archaea 2018, 2018, 8429145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ye, L.; Tong, A.H.Y.; Shao, M.-F.; Lok, S. Ammonia-oxidizing archaea and ammonia-oxidizing bacteria in six full-scale wastewater treatment bioreactors. Appl. Microbiol. Biotechnol. 2011, 91, 1215–1225. [Google Scholar] [CrossRef] [PubMed]



| Sample | Name | WWTP1 | WWTP2 | WWTP3 | WWTP4 | WWTP5 | |
|---|---|---|---|---|---|---|---|
| Location | Helsinki | Porvoo | Hyvinkää | Turku | Tampere | ||
| Influent characteristics | T month.ave(°C) | Spring | 11.5 ± 1 | 6.9 ± 0.5 | 8.6 ± 0.5 | 11 ± 0.5 | 15 ± 0.5 |
| Summer | 17.1 ± 0.5 | 17 ± 0.5 | 14.5 ± 0.5 | 17.9 ± 0.5 | 20.5 ± 0.5 | ||
| Q ave (m3/day) | Spring | 280,000 | 16,000 | 112,000 | 180,000 | 85,000 | |
| Summer | 280,000 | 10,000 | 9500 | 60,000 | 75,000 | ||
| pH | Spring | 6.1 | 7.1 | 7.3 | 7.4 | 7.5 | |
| Summer | 6.2 | 7.1 | 7.4 | 7.3 | 7.4 | ||
| BOD7ATU (mg/L) | Spring | 350 | 370 | 190 | 220 | 100 | |
| Summer | 320 | 270 | 200 | 360 | 310 | ||
| CODCr (mg/L) | Spring | 570 | 780 | 450 | 550 | 810 | |
| Summer | 660 | 580 | 650 | 750 | 540 | ||
| Ntot (mg/L) | Spring | 51 | 46 | 48 | 43 | 61 | |
| Summer | 60 | 55 | 52 | 76 | 54 | ||
| NH4-N (mg/L) | Spring | 35 | 27 | 45 | 26 | 28 | |
| Summer | 35 | 44 | 47 | 57 | 37 | ||
| Ptot (mg/L) | Spring | 6.9 | 8.8 | 6.3 | 6 | 3.7 | |
| Summer | 8.9 | 6.6 | 7.4 | 8.3 | 8.5 | ||
| SS (mg/L) | Spring | 300 | 710 | 180 | 430 | 60 | |
| Summer | 380 | 260 | 270 | 240 | 570 | ||
| Operational parameters | T month.ave(°C) | Spring | 14 | 8 | 10 | 13 | 16 |
| Summer | 19 | 17 | 17 | 19 | 21 | ||
| SRT (day) | Spring | 12 | 14 | 14 | 16 | 6 | |
| Summer | 12 | 8 | 16 | 14 | 10 | ||
| DO (mg/L) | Spring | 1.9 ± 1.7 | 2.6 ± 0.1 | 1.7 ± 0.4 | 1.6 ± 1.1 | 3.1 ± 0.5 | |
| Summer | 1.9 ± 1.7 | 3.5 ± 1 | 1.9 ± 0.3 | 2.2 ± 1.3 | 2.1 ± 0.5 | ||
| MLSS (mg/L) | Spring | 3300 | 4800 | 6800 | 4600 | 4700 | |
| Summer | 2300 | 2800 | 4500 | 4100 | 5300 | ||
| WWTP performance | BOD7ATU, removal (%) | 97.9 | 98.8 | 98.8 | 98.7 | 98.5 | |
| CODCr, removal (%) | 92.5 | 95 | 95.8 | 94.1 | 94 | ||
| Ntot, removal (%) | 91.5 | 75.7 | 82.9 | 80.3 | 28.3 | ||
| Ptot, removal (%) | 96.5 | 97 | 97.7 | 97.6 | 97.4 | ||
| Nitrification efficiency (%) | 98.2 | 93.3 | 99.6 | 97 | 97.8 | ||
| SS, removal (%) | 98 | 99 | 98.8 | 99.1 | 98.4 | ||
| Laboratory Reactor | Type of Wastewater (Experimental Stage) | Sequencing Batch Reactors | Membrane Bioreactors | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |||
| Synthetic influent wastewater characteristics | T (°C) | 10 ± 1 | ||||
| V (L) | 12 15 | |||||
| Q (L/day) | 6 | 15 | ||||
| pH | 8 ± 0.4 | |||||
| BOD7 ATU (mg/L) | Municipal-like | 360 | ||||
| 50% lower organic carbon | 101 | |||||
| No organic carbon source | 22.5 | |||||
| CODCr (mg/L) | Municipal-like | 525 | ||||
| 50% lower organic carbon | 300 | |||||
| No organic carbon source | 85 | |||||
| Ntot (mg/L) | Municipal-like | 53 | ||||
| 50% lower organic carbon | 52 | |||||
| No organic carbon source | 53 | |||||
| NH4-N (mg/L) | Municipal-like | 15 | ||||
| 50% lower organic carbon | 34 | |||||
| No organic carbon source | 52 | |||||
| Ptot (mg/L) | Municipal-like | 12 | ||||
| 50% lower organic carbon | 10 | |||||
| No organic carbon source | 9 | |||||
| Operational | T (°C) | 10 ± 1 | ||||
| SRT (d) | 14 | 100 | ||||
| DO (mg/l) | 6 | 8 | ||||
| MLSS (mg/L) | Municipal-like | 2.3 | 2.3 | 5 | 4.8 | |
| 50% lower organic carbon | 2.2 | 2.4 | 5.3 | 4.9 | ||
| No organic carbon source | 1.1 | 1.2 | 4.5 | 4.4 | ||
| MF membrane | − | + | ||||
| Process performance | CODCr, removal (%) | Municipal-like | 86 | 87 | 87 | 90 |
| 50% lower organic carbon | 62 | 70 | 47 | 60 | ||
| No organic carbon source | 75 | 63 | 93 | 64 | ||
| NH4+, removal (%) | Municipal-like | 74 | 90 | 99 | 97 | |
| 50% lower organic carbon | 95 | 99 | 99 | 99 | ||
| No organic carbon source | 97 | 99 | 99 | 99 | ||
| SS, removal (%) | Municipal-like | 99.5 | 99.5 | 99.9 | 99.9 | |
| 50% lower organic carbon | 99.5 | 99 | 99.9 | 99.9 | ||
| No organic carbon source | 99.5 | 98.7 | 99.9 | 99.9 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruglova, A.; Kesulahti, J.; Minh Le, K.; Gonzalez-Martinez, A.; Mikola, A.; Vahala, R. Low-Temperature Adapted Nitrifying Microbial Communities of Finnish Wastewater Treatment Systems. Water 2020, 12, 2450. https://doi.org/10.3390/w12092450
Kruglova A, Kesulahti J, Minh Le K, Gonzalez-Martinez A, Mikola A, Vahala R. Low-Temperature Adapted Nitrifying Microbial Communities of Finnish Wastewater Treatment Systems. Water. 2020; 12(9):2450. https://doi.org/10.3390/w12092450
Chicago/Turabian StyleKruglova, Antonina, Jenni Kesulahti, Khoi Minh Le, Alejandro Gonzalez-Martinez, Anna Mikola, and Riku Vahala. 2020. "Low-Temperature Adapted Nitrifying Microbial Communities of Finnish Wastewater Treatment Systems" Water 12, no. 9: 2450. https://doi.org/10.3390/w12092450
APA StyleKruglova, A., Kesulahti, J., Minh Le, K., Gonzalez-Martinez, A., Mikola, A., & Vahala, R. (2020). Low-Temperature Adapted Nitrifying Microbial Communities of Finnish Wastewater Treatment Systems. Water, 12(9), 2450. https://doi.org/10.3390/w12092450







