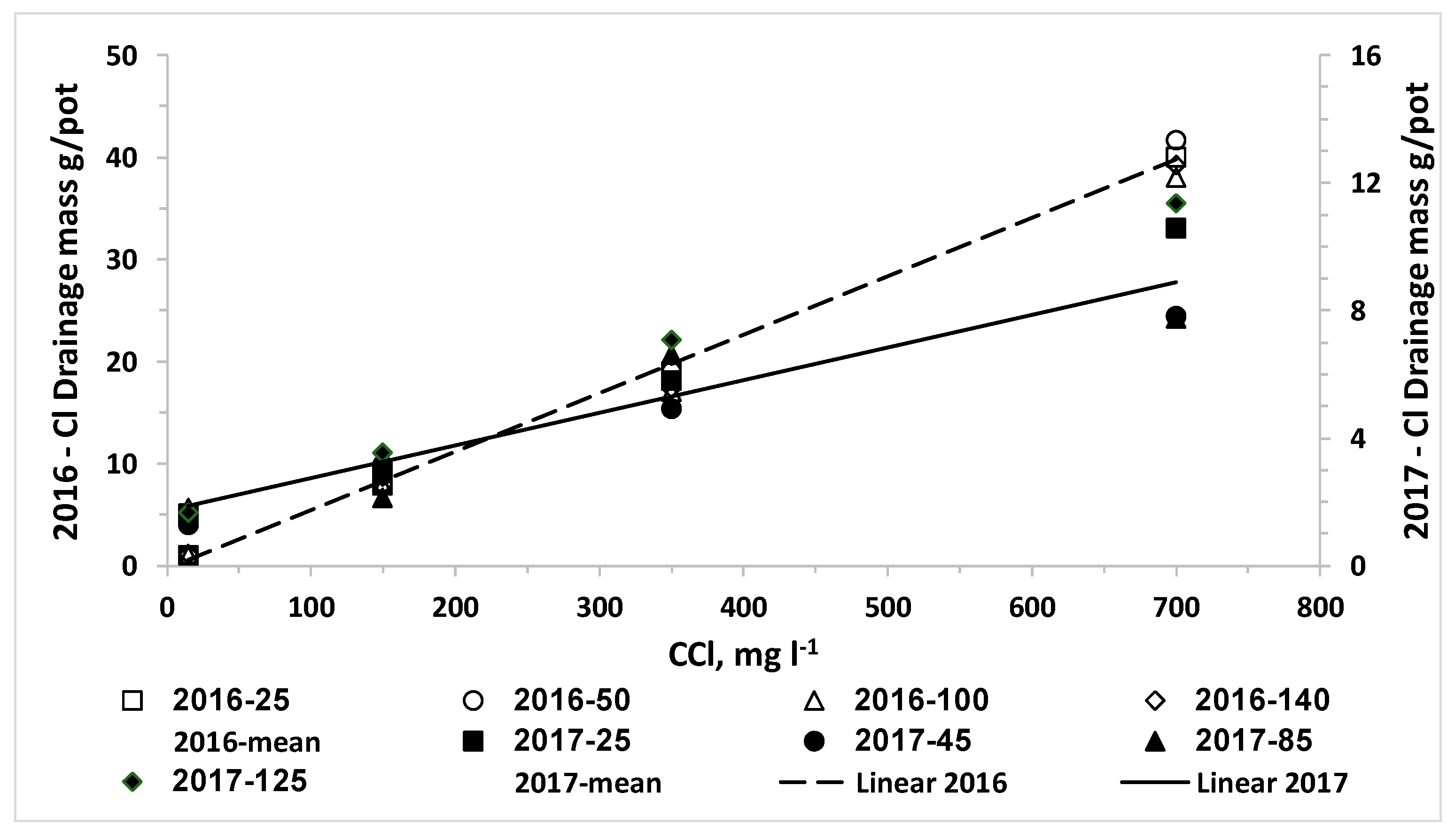1. Introduction
The growing demand for fresh water led to an increase in the production of desalinated water, mainly in arid and semiarid regions [
1,
2]. An annual production of 585 × 10
6 m
3/year in Israel was reported [
3], approximately 40% of the total fresh water consumption [
4]. The use of desalinated seawater for irrigation in Israel was estimated at 200 × 10
6 m
3/year [
5], which is ~40% of the national freshwater irrigation consumption.
In intensive agriculture, the majority of crops grown are defined as glycophyte plants, meaning high sensitivity to salinity above a threshold value, which is specific for each crop [
6,
7,
8,
9]. Salinity may interfere with mineral nutrition acquisition by plants in two ways [
10,
11]: (i) the total ionic strength of the soil solution, regardless of its composition, can reduce nutrient uptake and translocation, and (ii) uptake competition with specific ions such as sodium and chloride can reduce nutrient uptake. These interactions may lead to Na-induced Ca and/or K deficiencies [
12] and Cl
− induced inhibition of NO
3− uptake [
13,
14]. Antagonism between Cl
− and NO
3− uptake by plants was demonstrated in numerous publications [
13]. This antagonism was found in various plants, including substrate-grown crops such as melon and lettuce [
15], tomato and melon [
16,
17,
18], and rose [
14]. Direct competition between NO
3− and Cl
− on uptake by plants was reported in several publications [
19,
20,
21]. Therefore, there is a possibility that yield reduction due to increased salinity may be partially due to induced deficiency of N by the increased external Cl concentration. Addition of nitrate to the irrigation water reduced chloride accumulation in avocado plant and alleviated its adverse effects [
22], whereas another publication suggested that a reduction in water uptake led to the reduced nitrate uptake [
23]. Under conditions of salinity, nitrogen concentration in plant leaves decreased due to increasing chloride concentration in pepper [
24], tomato [
16], lettuce, and Chinese cabbage [
25].
Due to the above findings, increasing the NO
3− supply to sensitive crops irrigated with water containing high chloride concentrations was recommended in several publications [
13,
16,
22]. Consequently, the shift from irrigation with conventional water to using desalinated water calls for adjustment in the amount of additional minerals. Adjustment in the amount of minerals needed for plant growth requires understanding the effects of the quality of water supplied for irrigation on plants response to nutrients.
Irrigation with desalinated water was found to increase maximum yields of bell peppers by 50% and allowed a reduction in irrigation water application rate by half compared to irrigation with local brackish groundwater (electrical conductivity (EC = 3.2 dS·m
−1) [
26]. It was shown that the higher water dose required with saline water than with desalinated water was due to the required excess water for leaching out soluble salts from the root zone [
26]. This leaching application results in high volumes of drainage water that are often enriched with salts and also in other nutrients [
27] including nitrate [
28]. The reduction in the required leaching fraction with the reduction in water salinity was shown to reduce N leaching and enhance the efficiency of N fertilization [
29]. A simulation study of water and salts transport in soil of irrigated orchard in Mediterranean region showed that a shift from natural water to desalinated seawater reduced downward leaching of contaminants to the groundwater [
5].
Contamination of groundwater by nitrate is a major problem worldwide [
30,
31,
32]. In Israel, it has led to disqualification of a greater number of drinking water wells than any other environmental contaminant in the beginning of the 21st century [
33]. Nitrate is highly soluble and in most soils it is very mobile within the soil–water solution. Consequently, when nitrogen fertilizer inputs exceed the amount of nitrogen needed by the plant, the excess nitrate is then easily leached by irrigation water and rainwater to deeper soil layers, finally reaching groundwater [
34,
35].
We hypothesized that optimal nitrogen concentration for the highest yield will be lower and the total uptake of water and nitrogen will increase with decrease in the chloride concentration of the irrigating solution; consequently, chloride and nitrate downward leaching below the root zone will decrease with reducing salinity of the irrigation water. Optimization of N application in combination with irrigation with desalinated water will also lead to reduction of N and Cl fluxes below the root zone and protect underground water sources from N and Cl contamination.
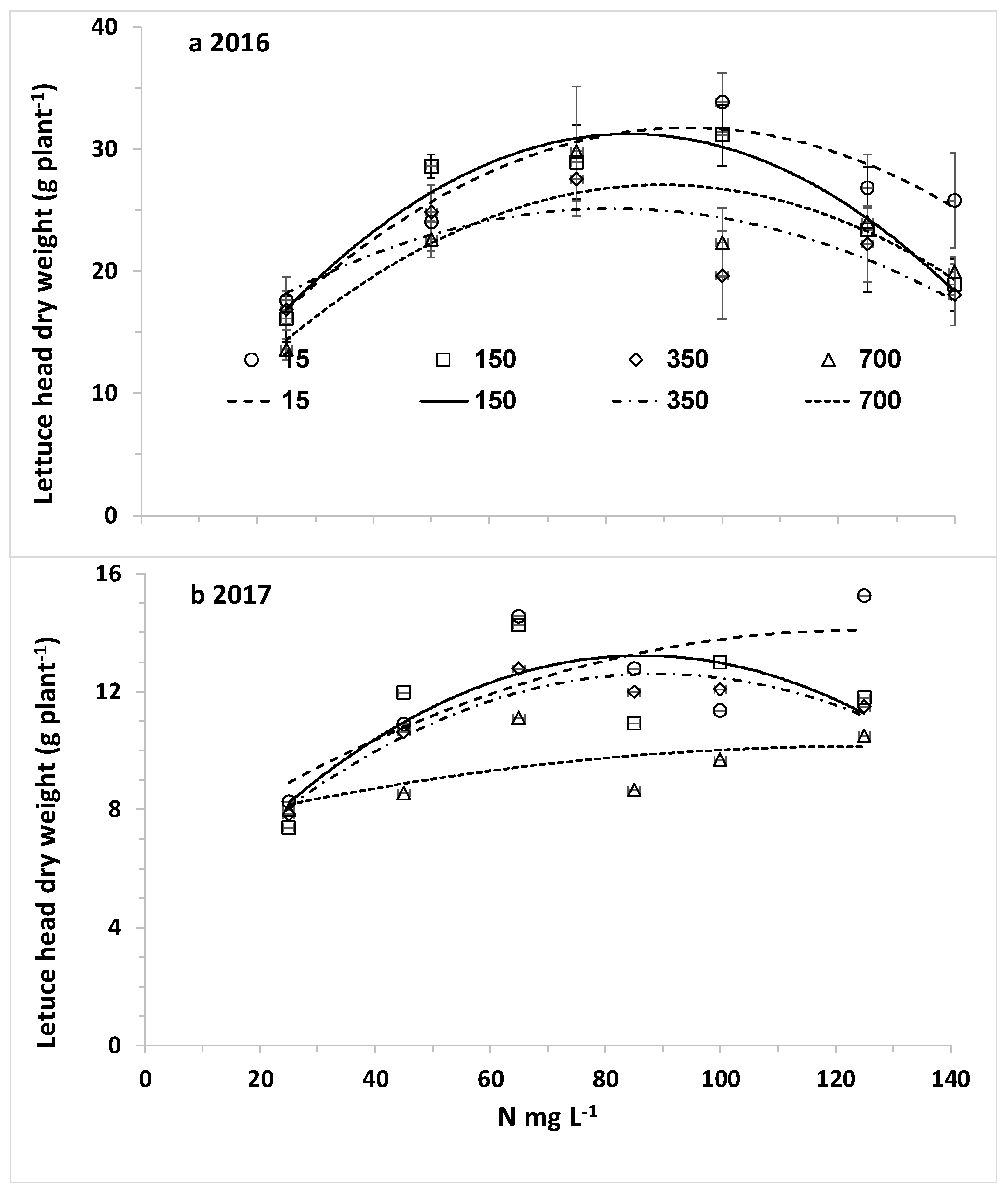
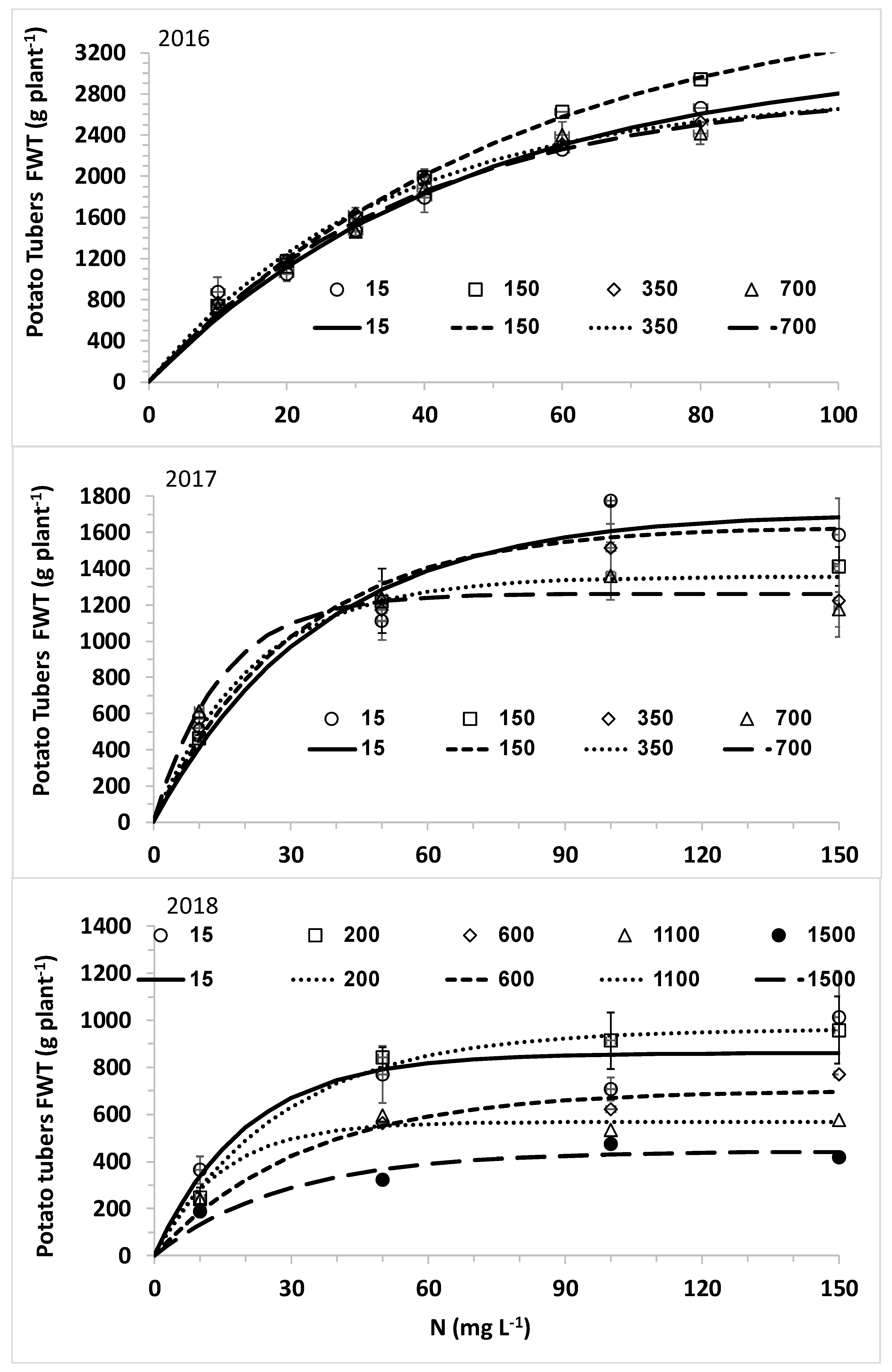
The overall aim of this research was to optimize nitrogen fertilization of plants under desalinated water and a wide range of chloride concentrations for high yield while minimizing downward leaching of nitrate and chloride. The specific objectives were (1) to determine the response curves of lettuce biomass and potato tubers to CN (N concentration in the irrigating solution) at different CCl (Cl concentration in the irrigating solution); (2) to explore the effects of CN at different CCl values on N and Cl concentrations in the leaves of lettuce and potato; and (3) to determine the effects of N and Cl concentrations in the irrigating water on the water leaching fraction (LF) and the downward leaching of nitrogen and chloride.
4. Discussion
The main hypothesis of this research was that the optimal nitrogen concentration for the highest yield will be lower with decreasing chloride concentration of the irrigating solution. Therefore, we hypothesized that lower nitrogen concentrations will be required with desalinated water for achieving maximum yield. However, the opposite results were obtained in the current experiment with two crops: lettuce and potato. Using the best fit response curves of lettuce heads (quadratic equation) and potato tubers (Mitcherlich model) we found that higher values of C
N were required for obtaining the maximal yield with desalinated or moderate salinity (low chloride concentrations) than for irrigation with high salinity water (high chloride concentrations). However, one should note that the maximum yields under desalinated or moderate salinity were bigger than under high salinity water. Extending the scope of the research from the question of the opportunity to reduce nitrogen fertilization with the use of desalinated water to the wider question of the possible interaction of salinity with fertilization, several published studies showed interactions of nitrogen with chloride in avocado [
22], tomato [
16,
17,
18,
19], and melon [
17], whereas other studies found no interaction in maize [
37], pepper [
38], and various horticultural crops [
39].
The hypothesis of the possible interaction of C
N with C
Cl leading to the opportunity to reduce the recommended C
N with desalinated water is based on findings on competition between the ions chloride and nitrate in uptake by plants [
14,
16,
17,
18]. Our assumptions were (i) the uptake and the concentration of chloride in organs of plants will be reduced by elevating C
N and (ii) the uptake of and the concentration of nitrogen in organs of plants irrigated with desalinated water will be higher than in plants irrigated with higher chloride concentrations when the same C
N is applied. As expected, we also found that the concentration of chloride in plant organs of lettuce and potato decreased with elevating the C
N (
Table 6 and
Table 7). However, no effect of C
Cl on the concentrations of reduced nitrogen in plant organs was found in lettuce and potato (
Table 6 and
Table 7), in agreement with published findings for pepper [
38]. On the other hand, nitrate concentration in lettuce and potato leaves decreased with increasing C
Cl, but this reduction had negligible impact on the total content of nitrogen in plants organs, because the reduced nitrogen is the main component of nitrogen in plant organs.
The response of plants biomass production to nitrogen is dependent of environmental conditions including salinity. Following Liebig’s law of the minimum, when water of low salinity, like desalinated water, is used, the potential for high biomass production is elevated and the demand for nitrogen is higher. Therefore, the optimal C
N for fertigation with desalinated water or another water source with low salinity and chloride concentration is higher or the same than the concentration recommended with other fresh water with relative low salinity. When the irrigating water containing high chloride concentration the salinity leads to reduction in the potential biomass and the demand for nitrogen, consequently in most investigations there was no positive effect of elevating C
N with saline water irrigation. The negative effect of high salinity on plant biomass is caused by two main factors: (i) osmotic effect on water uptake and (ii) specific toxic effects of ions. Elevating C
N as well as other nutrients is useless as a mitigating tool against the negative effect of the osmotic pressure; moreover, the elevated concentrations of nutrients contribute to higher osmotic pressure. Consequently, in many studies no positive effect of elevating nutrients concentrations above the recommended levels with fresh water were observed [
18,
37,
38]. The few cases where positive effects of elevating nutrients concentrations applied with saline water above the optimal concentration for plants irrigated with low salinity water were probably obtained with plants that are highly sensitive to specific toxic effects of some ions, especially Cl and Na [
13,
22,
40]. In the current research with both lettuce and potato, which are defined as moderately salt-sensitive [
6,
7], the major impact of the high chloride treatments was probably the total salinity affecting the osmotic pressure and the required energy for water uptake. In such crops the optimal required nitrogen is not higher in high salinity and chloride solutions.
Several reviews concluded that the results reported in the literature on the interaction between salinity and nutrients were contradictory or indicated no interactive effects [
10,
15,
37,
39,
41]. Grattan and Grieve (1999) [
39] concluded that “Despite a large number of studies that demonstrate that salinity reduces nutrient uptake and accumulation or affects nutrient partitioning within the plant, little evidence exists that adding nutrients at levels above what is considered optimal in non-saline environments, improves crop yield.” Recently, it was reported that nitrogen doses beyond the recommended values exacerbated the negative effects of salinity on growth and photosynthetic rates, in maize and cotton plants growing under moderate to high salinity conditions [
42]. They found that the negative effect of high salinity with high dose of nitrogen was stronger in maize which is less tolerant to salinity.
However, part of this conflict can be removed by using the Bernstein definitions [
43] of three different types of idealized salinity/nutrition interactions: (a) increased salt tolerance at suboptimal nutritional levels, (b) independent effects of salinity and nutrition at optimal and suboptimal nutritional levels, and (c) decreased salt tolerance at suboptimal nutritional levels. This method requires several salinity levels at each fertilization level. In the current research just in the third potato experiment there were more than four concentrations of C
Cl (salinity level) and in that experiment we found that the slope of reduction in yield as a function of C
Cl was not affected by the C
N (type b case in Bernstein model).
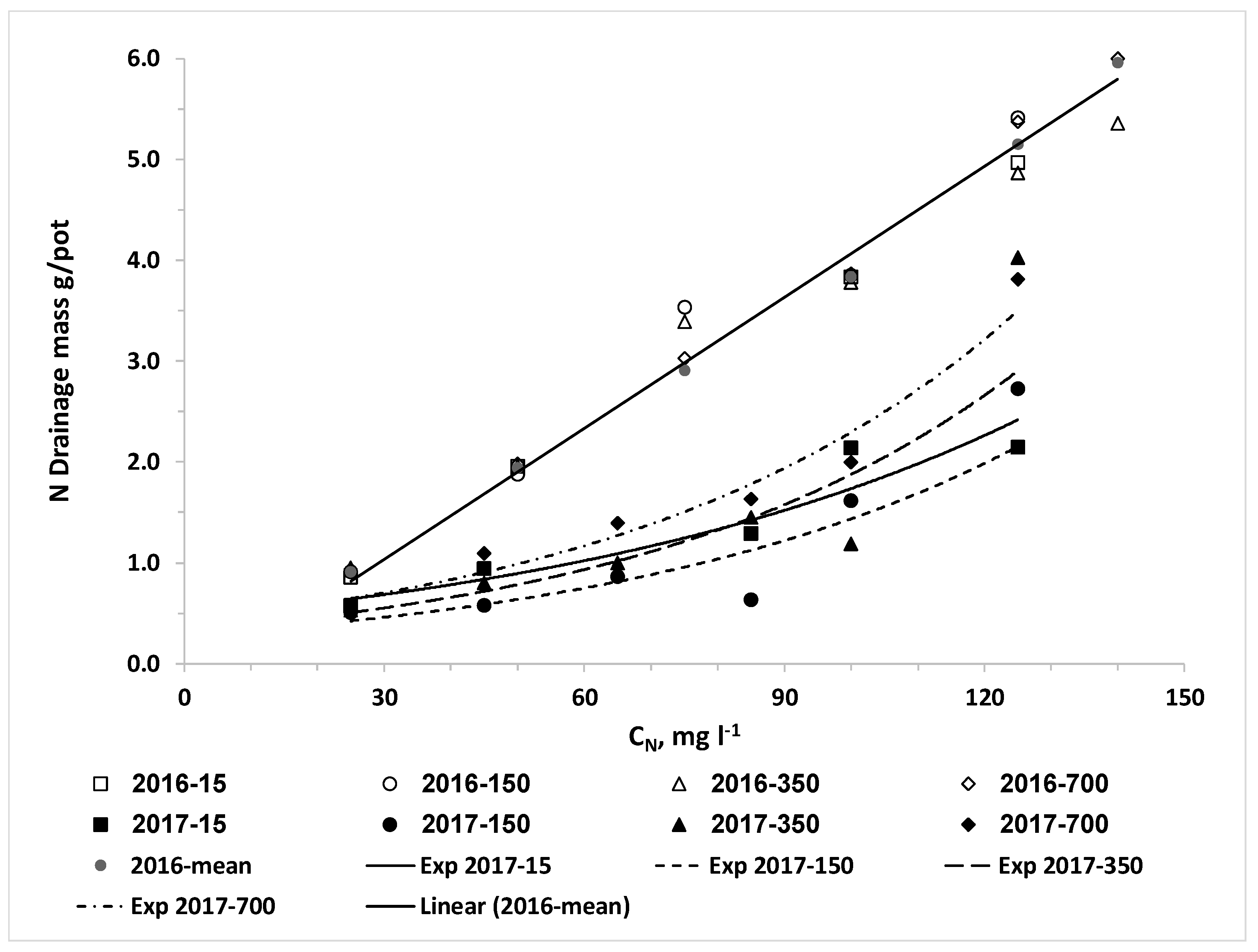
We also set the hypothesis that the uptake of water and nitrogen of plants irrigated with desalinated water will be higher than that of plants irrigated with water containing higher chloride concentrations; consequently, the LF and the downward leaching of chloride and nitrate below the root zone will be reduced by irrigation with desalinated water. The results of the current research approved this hypothesis for both crops when the irrigation volume was the same for all combinations of CN and CCl. In reality, the optimal management of irrigation with water sources with different salinity levels should be adjusted to minimize the salinity in and below the root zone. Therefore, the required volume and LF of irrigating water decrease as the salinity decreases and thus the lowest water volume is required when plants are irrigated with desalinated water. Consequently, the efficiency of the applied nitrogen is higher and the total amount of applied nitrogen with desalinated water might be lower despite the higher CN of the fertigation. We expected that the use of desalinated water will enhance plant biomass and water uptake and reduce the LF over water of moderate salinity. However, in most of the lettuce and potato experiments in the current study there was no advantage to desalinated water over the treatment of low CCl in the range of 100 to 200 mg L−1. It should be noted that the response of the plants is to the effective salinity, in the root zone, rather than the salinity of the irrigated water. The LF in irrigation of commercial fields and in most experiments is much lower than the LF values in the current experiments. Therefore, the effective salinity or Cl concentration in the soil solution in commercial fields is several times higher than that of the irrigation water, while in the current research the Cl concentration of the drainage was similar or just two times higher than CCl. Therefore, we suggest that in real life the enhancement of biomass production and reduction in LF and downward leaching of nitrate and chloride by a shift from moderate salinity to desalinated water will be bigger than in the current study.
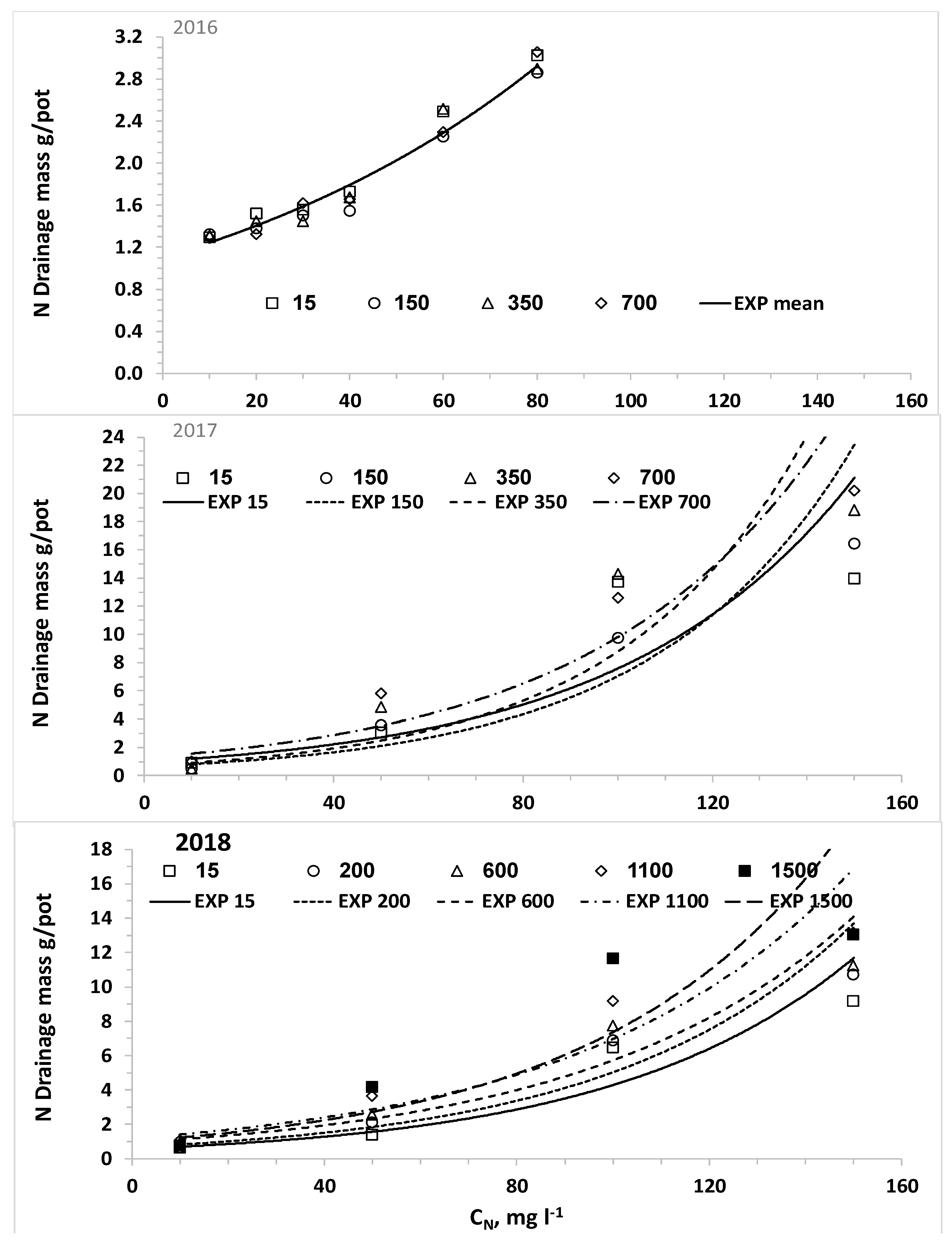
The nonlinear increase of C
NL and M
NL as a function of C
N with steep increase above a threshold that was obtained in all potato experiments is in agreement with our previous study [
44]. This result is typical of the reduced efficiency of N uptake with increased C
N, which is quantitatively described by nutrient uptake models like the Michalis–Menten equation [
14]. In part of the experiments, elevating C
CL level increased significantly M
NL as a result of the smaller biomass production and higher LF. Although the LF values in the current experiments were much higher than those in commercial fields, the general trend of higher leaching fraction with higher salinity is also practiced in commercial fields to prevent salts accumulation in the root-zone [
27,
28,
35,
41]. Thus, reduced M
NL and higher efficiency of C
N fertigation is expected with desalinated water, although irrigation with desalinated water did not enhance reduced nitrogen concentration in lettuce and potato organs. In contrast to potato, in lettuce no such clear threshold value of C
N was obtained. The main reason for this difference is that in lettuce the uptake of nitrogen in low C
N values is less efficient than in potato and there was no change in the N uptake efficiency as a function of C
N.The linear increase of C
ClL and M
ClL as function of C
Cl that was obtained for both crops in all experiments was expected due to the very low uptake of chloride [
13], which does not change considerably the concentration of chloride in the drainage from the irrigation water. Although C
N had significant effect on Cl concentration in plant organs, this effect is small relative to the total amount of Cl applied even in the desalinated water, and therefore it had no significant effect on C
ClL. Thus, the significant effect of C
N on M
Cl is due its’ effect on LF discussed above.