Characteristics of the Biochemical Composition and Bioavailability of Phytoplankton-Derived Particulate Organic Matter in the Chukchi Sea, Arctic
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling and Measurements of the Environmental Variables
2.2. Biochemical Composition and Enzyme-Hydrolysable Experiments Related to the POM
2.3. Statistical Analysis
3. Results
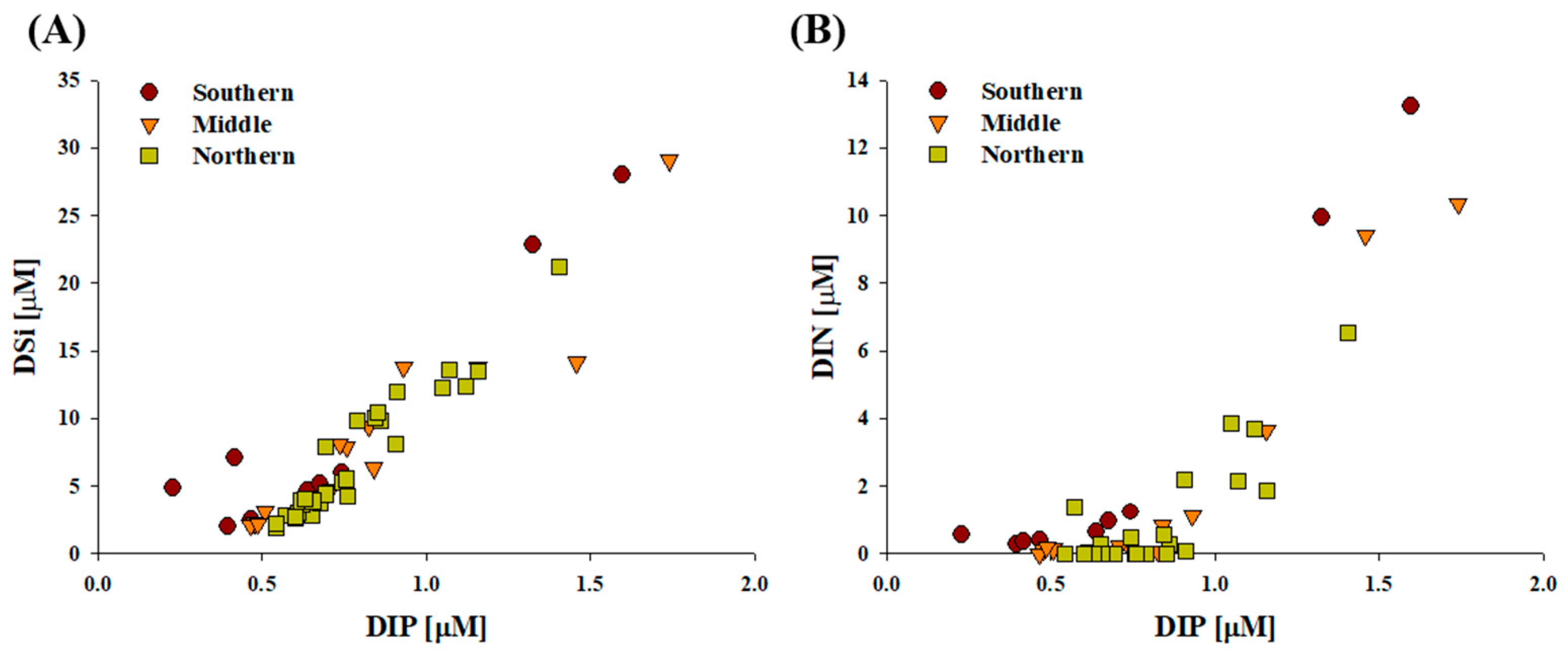
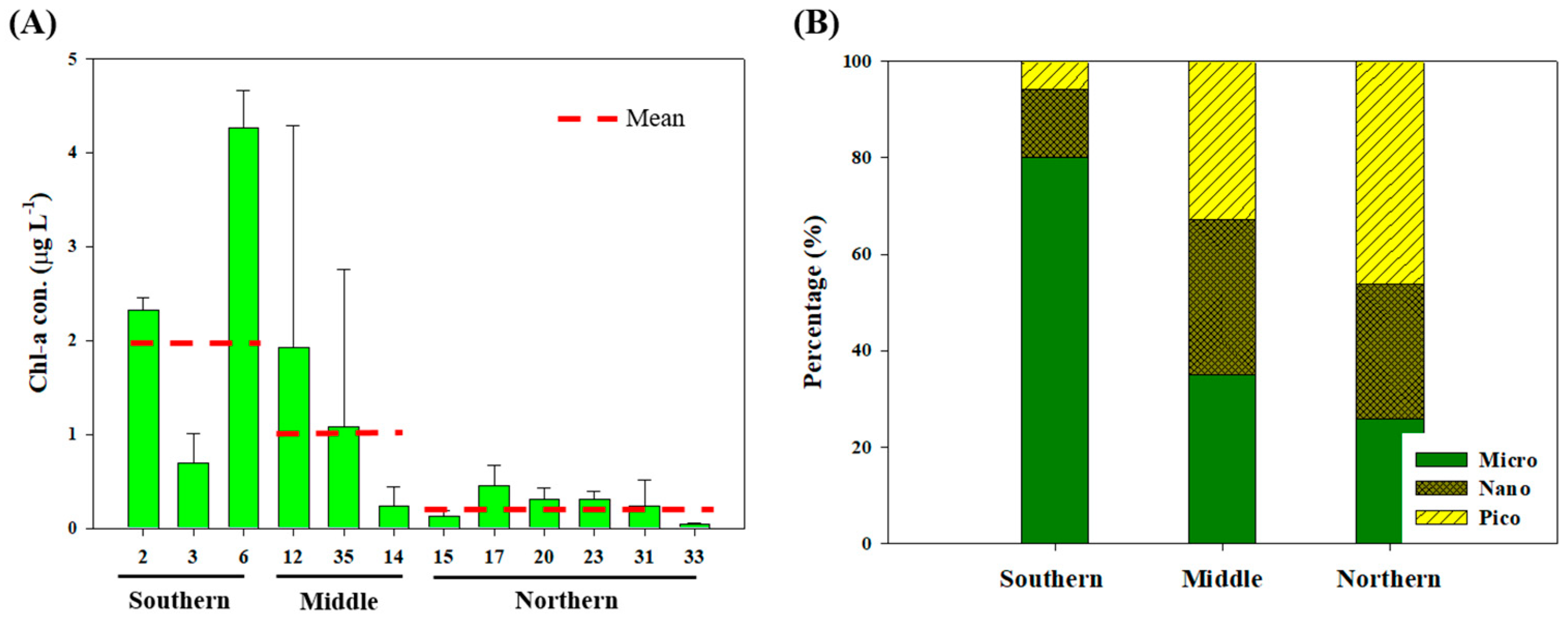
3.1. Physicochemical and Biological Characteristics During the Sampling Period
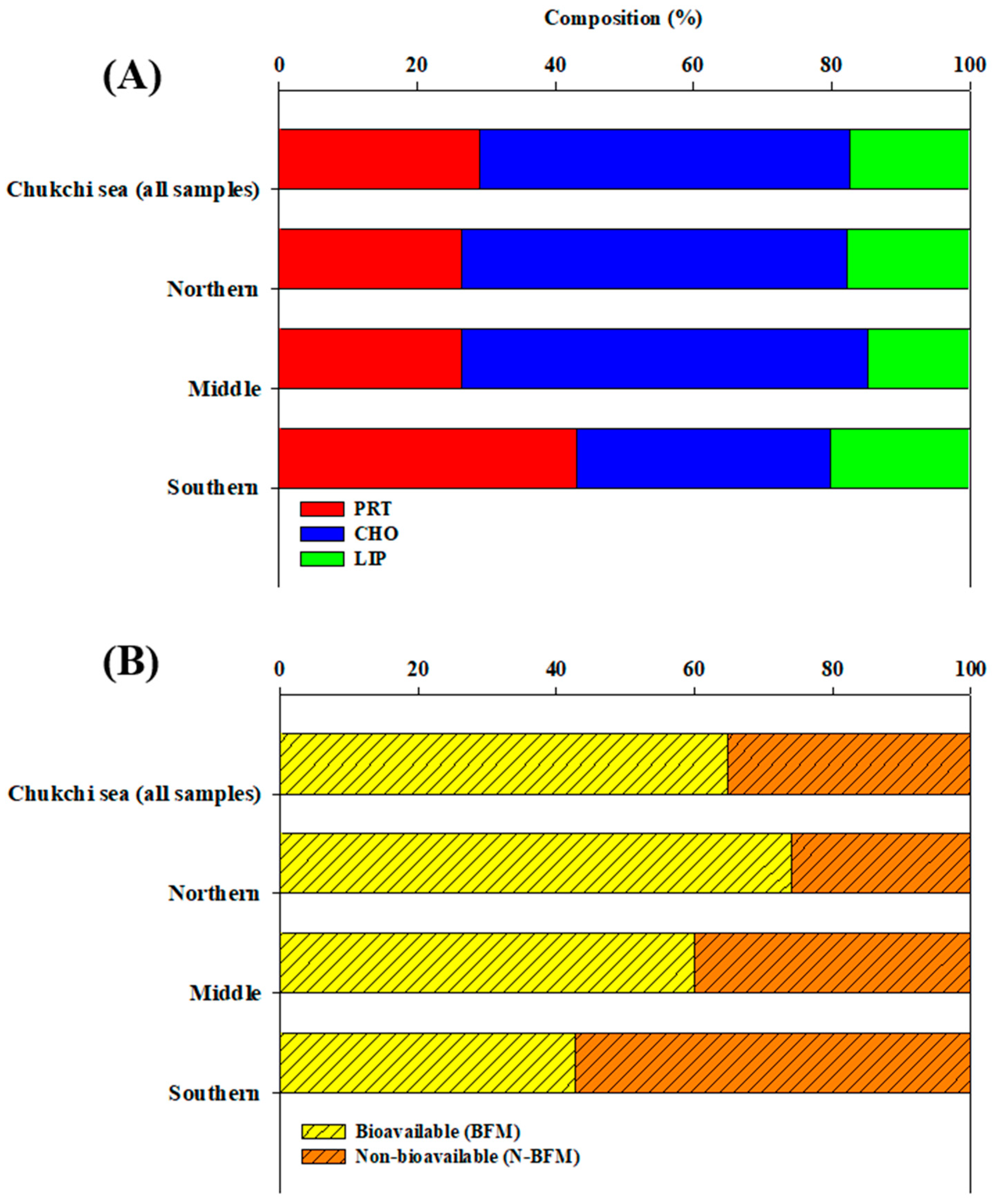
3.2. Biochemical Composition (PRT, LIP, and CHO) of POM
3.3. Hydrolysable Compounds of POM
3.4. Multivariate Statistical Analysis
4. Discussion
4.1. Origin and Quantity of POM
4.2. Biochemical Composition in Relation to Environmental Parameters
4.3. Bioavailability of POM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Søreide, J.E.; Leu, E.; Berge, J.; Graeve, M.; Falk-Petersen, S. Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob. Chang. Boil. 2010, 16. [Google Scholar] [CrossRef]
- Engel, A.; Bracher, A.; Dinter, T.; Endres, S.; Grosse, J.; Metfies, K.; Peeken, I.; Piontek, J.; Salter, I.; Nöthig, E.-M. Inter-annual variability of organic carbon concentration in the eastern Fram Strait during summer (2009–2017). Front. Mar. Sci. 2019, 6, 1–17. [Google Scholar] [CrossRef]
- Wassmann, P.; Duarte, C.M.; Agustí, S.; Sejr, M.K. Footprints of climate change in the Arctic marine ecosystem. Glob. Chang. Boil. 2011, 17, 1235–1249. [Google Scholar] [CrossRef]
- Bhavya, P.S.; Kim, B.K.; Jo, N.; Kim, K.; Kang, J.J.; Lee, J.H.; Lee, D.; Lee, J.H.; Joo, H.; Ahn, S.H.; et al. A review on the macromolecular compositions of phytoplankton and the implications for aquatic biogeochemistry. Ocean Sci. J. 2018, 54, 1–14. [Google Scholar] [CrossRef]
- Kreeger, D.; Goulden, C.; Kilham, S.; Lynn, S.; Datta, S.; Interlandi, S. Seasonal changes in the biochemistry of lake seston. Freshw. Boil. 1997, 38, 539–554. [Google Scholar] [CrossRef]
- Fichez, R. Composition and fate of organic matter in submarine cave sediments; implications for the biogeochemical cycle of organic carbon. Oceanol. Acta 1991, 14, 369–377. [Google Scholar]
- Danovaro, R.; Fabiano, M.; Croce, N.D. Labile organic matter and microbial biomasses in deep-sea sediments (Eastern Mediterranean Sea). Deep. Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 953–965. [Google Scholar] [CrossRef]
- Fabiano, M.; Danovaro, R.; Crisafi, E.; La Ferla, R.; Povero, P.; Acosta-Pomar, L.; Ferla, R. Particulate matter composition and bacterial distribution in Terra Nova Bay (Antarctica) during summer 1989–1990. Polar Boil. 1995, 15, 393–400. [Google Scholar] [CrossRef]
- Parsons, T.R.; Stephens, K.; Strickland, J.D.H. On the chemical composition of eleven species of marine phytoplankters. J. Fish. Res. Board Can. 1961, 18, 1001–1016. [Google Scholar] [CrossRef]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Boil. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Fabiano, M.; Povero, P.; Danovaro, R. Distribution and composition of particulate organic matter in the Ross Sea (Antarctica). Polar Boil. 1993, 13, 525–533. [Google Scholar] [CrossRef]
- Danovaro, R.; Dell’Anno, A.; Pusceddu, A.; Marrale, D.; Croce, N.D.; Fabiano, M.; Tselepides, A. Biochemical composition of pico-, nano- and micro-particulate organic matter and bacterioplankton biomass in the oligotrophic Cretan Sea (NE Mediterranean). Prog. Oceanogr. 2000, 46, 279–310. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, S.; Ha, S.-Y.; Jung, J.; Kim, T.-W.; Yang, E.J.; Jo, N.; Lim, Y.J.; Park, J.; Lee, S.H. Vertical distributions of macromolecular composition of particulate organic matter in the water column of the amundsen sea polynya during the summer in 2014. J. Geophys. Res. Oceans 2018, 123, 1393–1405. [Google Scholar] [CrossRef]
- Hecky, R.E.; Kilham, P. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 1988, 33, 796–822. [Google Scholar] [CrossRef]
- Kilham, S.; Kreeger, D.; Goulden, C.; Lynn, S. Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshw. Boil. 1997, 38, 591–596. [Google Scholar] [CrossRef]
- Lee, S.H.; Whitledge, T.E.; Kang, S.-H. Carbon uptake rates of sea ice algae and phytoplankton under different light intensities in a landfast sea ice zone, Barrow, Alaska. Arctic 2008, 61, 281–291. [Google Scholar] [CrossRef][Green Version]
- Smith, A.E.; Morris, I. Pathways of carbon assimilation in phytoplankton from the Antarctic Ocean1. Limnol. Oceanogr. 1980, 25, 865–872. [Google Scholar] [CrossRef]
- Takagi, M.; Watanabe, K.; Yamaberi, K.; Yoshida, T. Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Appl. Microbiol. Biotechnol. 2000, 54, 112–117. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.E.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Winberg, G.G. Symbols, Units and Conversion Factors in Study of Fresh Waters Productivity; International Biological Programme: London, UK, 1971; p. 23. [Google Scholar]
- Dell’Anno, A.; Fabiano, M.; Mei, M.; Danovaro, R. Enzymatically hydrolysed protein and carbohydrate pools in deep-sea sediments: Estimates of the potentially bioavailable fraction and methodological considerations. Mar. Ecol. Prog. Ser. 2000, 196, 15–23. [Google Scholar] [CrossRef][Green Version]
- Pusceddu, A.; Dell’Anno, A.; Danovaro, R.; Manini, E.; Sarà, G.; Fabiano, M. Enzymatically hydrolyzable protein and carbohydrate sedimentary pools as indicators of the trophic state of detritus sink systems: A case study in a Mediterranean coastal lagoon. Estuaries 2003, 26, 641–650. [Google Scholar] [CrossRef]
- Misic, C.; Harriague, A.C.; Mangoni, O.; Aulicino, G.; Castagno, P.; Cotroneo, Y. Effects of physical constraints on the lability of POM during summer in the Ross Sea. J. Mar. Syst. 2017, 166, 132–143. [Google Scholar] [CrossRef]
- Woodgate, R.A.; Aagaard, K.; Weingartner, T.J. Monthly temperature, salinity, and transport variability of the Bering Strait through inflow. Geophys. Res. Lett. 2005, 32, L04601. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, X. Summer changes in water mass characteristics and vertical thermohaline structure in the Eastern Chukchi Sea, 1974–2017. Water 2020, 12, 1434. [Google Scholar] [CrossRef]
- Korsak, M.N. Primary production of organic matter. In Results of the Third Joint US-USSR Bering and Chukchi Seas Expedition (BERPAC): Summer 1988; Nagel, P.A., Ed.; US Fish and Wildlife Service: Washington, DC, USA, 1992; pp. 215–218. [Google Scholar]
- Grebmeier, J.; Cooper, L.; Feder, H.M.; Sirenko, B.I. Ecosystem dynamics of the Pacific-influenced Northern Bering and Chukchi Seas in the Amerasian Arctic. Prog. Oceanogr. 2006, 71, 331–361. [Google Scholar] [CrossRef]
- Grebmeier, J.M. Shifting patterns of life in the Pacific arctic and Sub-Arctic seas. Annu. Rev. Mar. Sci. 2012, 4, 63–78. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.H.; Yun, M.S.; Joo, H.; Song, H.J.; Yang, E.J.; Chung, K.H.; Kang, S.-H.; Lee, S.H. High lipid composition of particulate organic matter in the northern Chukchi Sea, 2011. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 120, 72–81. [Google Scholar] [CrossRef]
- Yun, M.S.; Lee, D.B.; Kim, B.K.; Kang, J.J.; Lee, J.H.; Yang, E.J.; Park, W.G.; Chung, K.H.; Lee, S.H. Comparison of phytoplankton macromolecular compositions and zooplankton proximate compositions in the northern Chukchi Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 120, 82–90. [Google Scholar] [CrossRef]
- Yun, M.S.; Joo, H.T.; Park, J.W.; Kang, J.J.; Kang, S.-H.; Lee, S.H. Lipid-rich and protein-poor carbon allocation patterns of phytoplankton in the northern Chukchi Sea, 2011. Cont. Shelf Res. 2018, 158, 26–32. [Google Scholar] [CrossRef]
- Ahn, S.H.; Whitledge, T.E.; Stockwell, D.A.; Lee, J.H.; Lee, H.W.; Lee, S.H. The biochemical composition of phytoplankton in the Laptev and East Siberian seas during the summer of 2013. Polar Boil. 2018, 42, 133–148. [Google Scholar] [CrossRef]
- Marra, J.F.; Lance, V.P.; Vaillancourt, R.D.; Hargreaves, B.R. Resolving the ocean’s euphotic zone. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2014, 83, 45–50. [Google Scholar] [CrossRef]
- Catalano, G.; Povero, P.; Fabiano, M.; Benedetti, F.; Goffart, A. Nutrient utilisation and particulate organic matter changes during summer in the upper mixed layer (Ross Sea, Antarctica). Deep. Sea Res. Part I Oceanogr. Res. Pap. 1997, 44, 97–112. [Google Scholar] [CrossRef]
- Rivaro, P.F.; Abelmoschi, M.L.; Grotti, M.; Ianni, C.; Magi, E.; Margiotta, F.; Massolo, S.; Saggiomo, V. Combined effects of hydrographic structure and iron and copper availability on the phytoplankton growth in Terra Nova Bay Polynya (Ross Sea, Antarctica). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2012, 62, 97–110. [Google Scholar] [CrossRef]
- Bates, N.R.; Garley, R.; Frey, K.E.; Shake, K.L.; Mathis, J.T. Sea-ice melt CO2–carbonate chemistry in the western Arctic Ocean: Meltwater contributions to air–sea CO2 gas exchange, mixed-layer properties and rates of net community production under sea ice. Biogeosciences 2014, 11, 6769–6789. [Google Scholar] [CrossRef]
- Fetterer, F.; Knowles, K.; Meier, W.N.; Savoie, M.; Windnagel, A.K. Updated Daily. Sea Ice Index, Version 3. [Indicate Subset Used]; National Snow and Ice Data Center (NSIDC): Boulder, CO, USA, 2017. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- Coplen, T.B.; Brand, W.A.; Gehre, M.; Gröning, M.; Meijer, H.A.J.; TOMAN, B.; Verkouteren, R.M. New guidelines for δ13C measurements. Anal. Chem. 2006, 78, 2439–2441. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar]
- Dall, W.; Moriaty, J.W. The biology of crustacea. In Functional Aspects of Nutrition and Digestion; Mantel, L.H., Ed.; Academic Press: New York, NY, USA, 1983; pp. 215–261. [Google Scholar]
- Gordon, D.C., Jr. A microscopic study of organic particles in the North Atlantic Ocean. Deep. Sea Res. Oceanogr. Abstr. 1970, 17, 175–185. [Google Scholar] [CrossRef]
- Mayer, L.M.; Linda, S.L.; Sawyer, T.; Plante, C.J.; Jumars, P.A.; Self, R.L.; Schick, L.L. Bioavailable amino acids in sediments: A biomimetic, kinetics based approach. Limnol. Oceanogr. 1995, 40, 511–520. [Google Scholar] [CrossRef]
- Fabiano, M.; Pusceddu, A. Total and hydrolizable particulate organic matter (carbohydrates, proteins and lipids) at a coastal station in Terra Nova Bay (Ross Sea, Antarctica). Polar Boil. 1998, 19, 125–132. [Google Scholar] [CrossRef]
- Pusceddu, A.; Bianchelli, S.; Danovaro, R. Quantity and biochemical composition of particulate organic matter in a highly trawled area (Thermaikos Gulf, Eastern Mediterranean Sea). Adv. Oceanogr. Limnol. 2015, 6, 21–32. [Google Scholar] [CrossRef]
- Gong, D.; Pickart, R.S. Summertime circulation in the eastern Chukchi Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 118, 18–31. [Google Scholar] [CrossRef]
- Stroh, J.N.; Panteleev, G.; Kirillov, S.; Makhotin, M.; Shakhova, N. Sea-surface temperature and salinity product comparison against external in situ data in the Arctic Ocean. J. Geophys. Res. Ocean. 2015, 120, 7223–7236. [Google Scholar] [CrossRef]
- Volkman, J.K.; Tanoue, E. Chemical and biological studies of particulate organic matter in the ocean. J. Oceanogr. 2003, 25, 265–279. [Google Scholar] [CrossRef]
- Yun, M.S.; Chung, K.H.; Zimmermann, S.; Zhao, J.; Joo, H.M.; Lee, S.H. Phytoplankton productivity and its response to higher light levels in the Canada Basin. Polar Boil. 2012, 35, 257–268. [Google Scholar] [CrossRef]
- Lee, S.H.; Whitledge, T.E.; Kang, S.-H. Recent carbon and nitrogen uptake rates of phytoplankton in Bering Strait and the Chukchi Sea. Cont. Shelf Res. 2007, 27, 2231–2249. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Kim, B.K.; Saitoh, S.-I.; Kang, C.-K.; Kang, S.-H.; Whitledge, T. Latitudinal carbon productivity in the Bering and Chukchi Seas during the summer in 2007. Cont. Shelf Res. 2013, 59, 28–36. [Google Scholar] [CrossRef]
- Yun, M.S.; Whitledge, T.E.; Stockwell, D.; Son, S.H.; Lee, J.H.; Lee, D.B.; Lee, S.H.; Park, J.W.; Park, J. Primary production in the Chukchi Sea with potential effects of freshwater content. Biogeosciences 2016, 13, 737–749. [Google Scholar] [CrossRef]
- Nishino, S.; Kikuchi, T.; Fujiwara, A.; Hirawake, T.; Aoyama, M. Water mass characteristics and their temporal changes in a biological hotspot in the southern Chukchi Sea. Biogeosciences 2016, 13, 2563–2578. [Google Scholar] [CrossRef]
- Zeeman, S.I. The importance of primary production and CO2. In Results of the Third Joint US-USSR Bering and Chukchi Seas Expedition (BERPAC), Summer 1988; U.S. Fish and Wildlife Service: Washington, DC, USA, 1992; pp. 39–49. [Google Scholar]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A. The Influence of Organisms on the Composition of Sea-Water in the Sea; Hill, M.N., Ed.; Springer: New York, NY, USA, 1963; pp. 26–77. [Google Scholar]
- Fogg, G.E.; Thake, B. Cultures of limited volume. In Algal Cultures and Phytoplankton Ecology; University of Wisconsin Press: Maddison, WI, USA, 1987; pp. 12–42. [Google Scholar]
- Hu, Q. Environmental effects on cell composition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Ed.; Oxford University Press: Oxford, UK, 2004; pp. 114–122. [Google Scholar]
- Jin, H.; Zhuang, Y.; Li, H.; Chen, J.; Gao, S.; Ji, Z.; Zhang, Y. Response of phytoplankton community to different water types in the western Arctic Ocean surface water based on pigment analysis in summer 2008. Acta Oceanol. Sin. 2017, 36, 109–121. [Google Scholar] [CrossRef]
- Li, W.K.W.; McLaughlin, F.A.; Lovejoy, C.; Carmack, E.C. Smallest algae thrive as the arctic ocean freshens. Science 2009, 326, 539. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Hirawake, T.; Suzuki, K.; Imai, I.; Saitoh, S.-I. Timing of sea ice retreat can alter phytoplankton community structure in the western Arctic Ocean. Biogeosciences 2014, 11, 1705–1716. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Mantoura, R.F.C.; Bjørnland, T. Data for the identification of 47 key phytoplankton pigments. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods. Monographs on Oceanographic Methodology; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO: Paris, France, 1997; pp. 449–559. [Google Scholar]
- Haug, A.; Myklestad, S.; Sakshaug, E. Studies on the phytoplankton ecology of the Trondheimsfjord. I. The chemical composition of phytoplankton populations. J. Exp. Mar. Boil. Ecol. 1973, 11, 15–26. [Google Scholar] [CrossRef]
- Shifrin, N.S.; Chisholm, S.W. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles1. J. Phycol. 2008, 17, 374–384. [Google Scholar] [CrossRef]
- Jo, N.; Kang, J.J.; Park, W.G.; Lee, B.R.; Yun, M.S.; Lee, J.H.; Kim, S.M.; Lee, D.; Joo, H.; Lee, J.H.; et al. Seasonal variation in the biochemical compositions of phytoplankton and zooplankton communities in the southwestern East/Japan Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 82–90. [Google Scholar] [CrossRef]
- Yun, M.S.; Joo, H.M.; Kang, J.J.; Park, J.W.; Lee, J.H.; Kang, S.-H.; Sun, J.; Lee, S.H. Potential implications of changing Photosynthetic end-products of Phytoplankton caused by sea ice conditions in the Northern Chukchi Sea. Front. Microbiol. 2019, 10, 2274. [Google Scholar] [CrossRef]
- Handa, N.; Tominaga, H. A detailed analysis of carbohydrates in marine particulate matter. Mar. Boil. 1969, 2, 228–235. [Google Scholar] [CrossRef]
- Dawson, R.; Liebezeit, G. Biochemical compounds in the pelagic and sedimentary environment of Antarctic waters. Publs. Cent. Natn. Exploit. Océans (Sér. Act. Colloques) 1982, 14, 67–86. [Google Scholar]
- Christian, J.R.; Karl, D.M. Bacterial ectoenzymes in marine waters: Activity ratios and temperature responses in three oceanographic provinces. Limnol. Oceanogr. 1995, 40, 1042–1049. [Google Scholar] [CrossRef]
- Liebezeit, G. Particulate carbohydrates in relation to phytoplankton in the euphotic zone of the Bransfield Strait. Polar Boil. 1984, 2, 225–228. [Google Scholar] [CrossRef]
- Cowie, G.L.; Hedges, J.I. Digestion and alteration of the biochemical constituents of a diatom (Thalassiosira weisflogii) ingested by an herbivorous zooplankton (Calanus pacificus). Limnol. Oceanogr. 1996, 41, 581–594. [Google Scholar] [CrossRef]
- DeNicola, D.M. A review of diatoms found in highly acidic environments. Hydrobiology 2000, 433, 111–122. [Google Scholar] [CrossRef]
- Hamm, C.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef]
- Vargas, C.A.; Escribano, R.; Poulet, S. Phytoplankton food quality determines time windows for successful zooplankton reproductive pulses. Ecology 2006, 87, 2992–2999. [Google Scholar] [CrossRef]
- Friedrichs, L.; Hornig, M.; Schulze, L.; Bertram, A.; Jansen, S.; Hamm, C. Size and biomechanic properties of diatom frustules influence food uptake by copepods. Mar. Ecol. Prog. Ser. 2013, 481, 41–51. [Google Scholar] [CrossRef][Green Version]
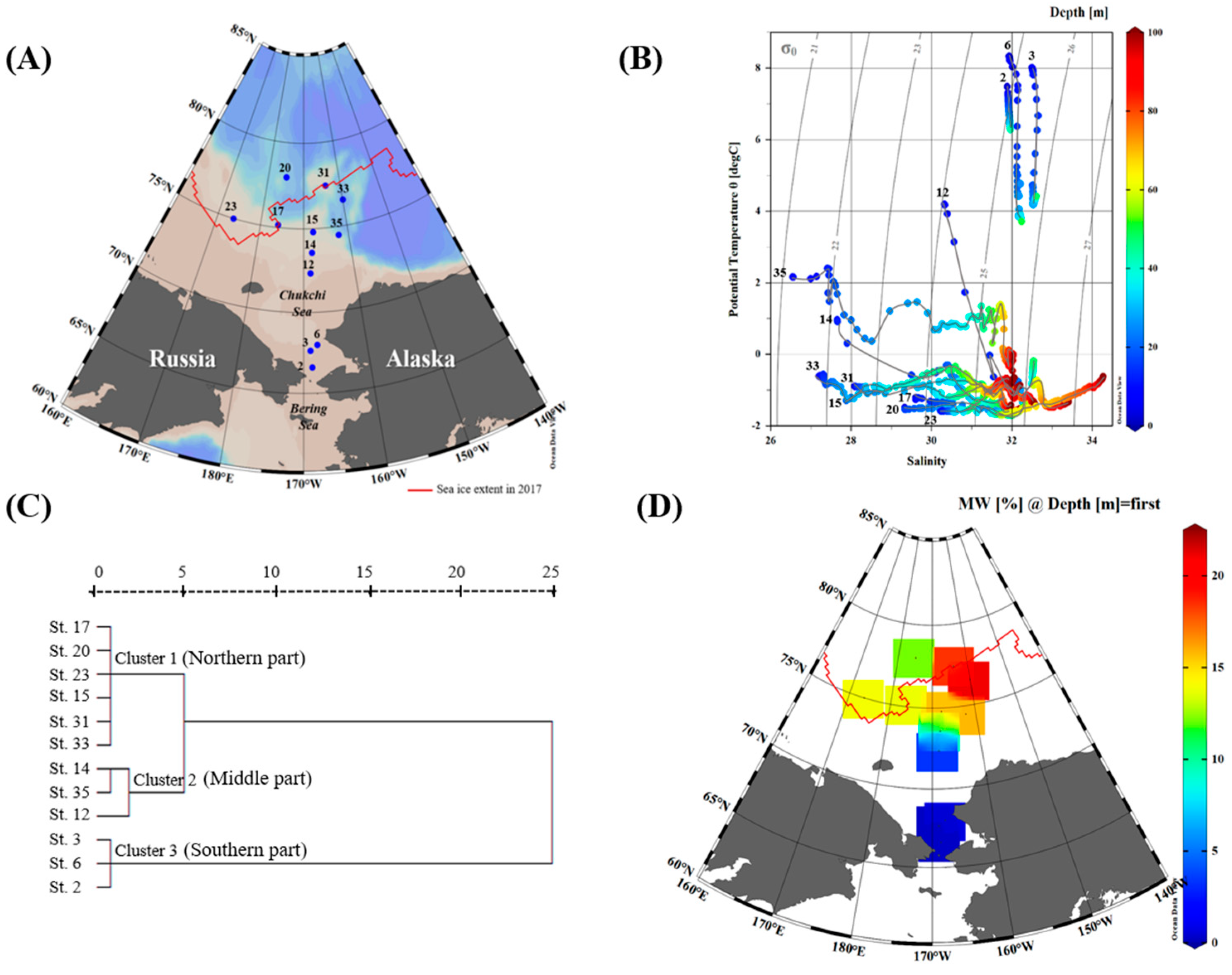


| Station | Date (dd-mm-yyyy) | Latitude (°N) | Longitude (°W) | Bottom Depth (m) | Euphotic Depth (m) | Sampling Depth (m) | Group |
|---|---|---|---|---|---|---|---|
| 2 | 07-08-2017 | 66.6298 | 168.6874 | 43 | 20 | 0, 10, 20 | Southern part |
| 3 | 07-08-2017 | 67.6699 | 168.9601 | 48 | 31 | 0, 10, 18, 30 | Southern part |
| 6 | 07-08-2017 | 68.0130 | 167.8668 | 50 | 17 | 0, 10 | Southern part |
| 12 | 09-08-2017 | 72.3601 | 168.6668 | 48 | 35 | 0, 10, 15, 20, 30 | Middle part |
| 14 | 09-08-2017 | 73.5803 | 168.2824 | 119 | 44 | 0, 10, 20, 30, 44 | Middle part |
| 35 | 24-08-2017 | 74.5003 | 162.2487 | 1536 | 43 | 0, 10, 20, 30, 40 | Middle part |
| 15 | 10-08-2017 | 74.7987 | 167.8904 | 192 | 54 | 0, 10, 15, 20, 30, 44 | Northern part |
| 17 | 11-08-2017 | 75.1509 | 176.0166 | 319 | 33 | 0, 10, 13, 18, 30 | Northern part |
| 20 | 12-08-2017 | 77.9999 | 174.9342 | 1672 | 54 | 0, 10, 14, 22, 24, 30 | Northern part |
| 23 | 18-08-2017 | 75.0008 | 173.6090 | 135 | 39 | 0, 10, 20, 25, 34 | Northern part |
| 31 | 22-08-2017 | 77.4722 | 164.1178 | 267 | 40 | 0, 10, 15, 20, 30, 40 | Northern part |
| 33 | 23-08-2017 | 76.5254 | 159.9693 | 2102 | 73 | 0, 10, 20, 30 | Northern part |
| Component | Principal Component | |
|---|---|---|
| PC1 | PC2 | |
| Eigenvalue | 7.8 | 3.3 |
| Percentage | 60.2 | 25.0 |
| Cumulative percent | 60.2 | 85.2 |
| Eigenvector | ||
| Variables | Eigenvectors | |
| Salinity | 0.8882 | 0.450 |
| Temperature | 0.931 | −0.138 |
| Density | 0.785 | 0.579 |
| DIP | −0.242 | 0.920 |
| DSi | 0.159 | 0.936 |
| DIN | 0.287 | 0.805 |
| MW | −0.907 | −0.303 |
| Microphytoplankton (%) | 0.891 | 0.389 |
| Nanophytoplankton (%) | −0.753 | −0.351 |
| Picophytoplankton (%) | −0.880 | −0.373 |
| LIP (%) | 0.240 | 0.673 |
| PRT (%) | 0.824 | −0.463 |
| CHO (%) | −0.919 | 0.103 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.K.; Jung, J.; Lee, Y.; Cho, K.-H.; Gal, J.-K.; Kang, S.-H.; Ha, S.-Y. Characteristics of the Biochemical Composition and Bioavailability of Phytoplankton-Derived Particulate Organic Matter in the Chukchi Sea, Arctic. Water 2020, 12, 2355. https://doi.org/10.3390/w12092355
Kim BK, Jung J, Lee Y, Cho K-H, Gal J-K, Kang S-H, Ha S-Y. Characteristics of the Biochemical Composition and Bioavailability of Phytoplankton-Derived Particulate Organic Matter in the Chukchi Sea, Arctic. Water. 2020; 12(9):2355. https://doi.org/10.3390/w12092355
Chicago/Turabian StyleKim, Bo Kyung, Jinyoung Jung, Youngju Lee, Kyoung-Ho Cho, Jong-Ku Gal, Sung-Ho Kang, and Sun-Yong Ha. 2020. "Characteristics of the Biochemical Composition and Bioavailability of Phytoplankton-Derived Particulate Organic Matter in the Chukchi Sea, Arctic" Water 12, no. 9: 2355. https://doi.org/10.3390/w12092355
APA StyleKim, B. K., Jung, J., Lee, Y., Cho, K.-H., Gal, J.-K., Kang, S.-H., & Ha, S.-Y. (2020). Characteristics of the Biochemical Composition and Bioavailability of Phytoplankton-Derived Particulate Organic Matter in the Chukchi Sea, Arctic. Water, 12(9), 2355. https://doi.org/10.3390/w12092355





