Removal of Cadmium from Contaminated Water Using Coated Chicken Bones with Double-Layer Hydroxide (Mg/Fe-LDH)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coated Chicken Bones
3. Adsorption Experiment
4. Results and Discussion
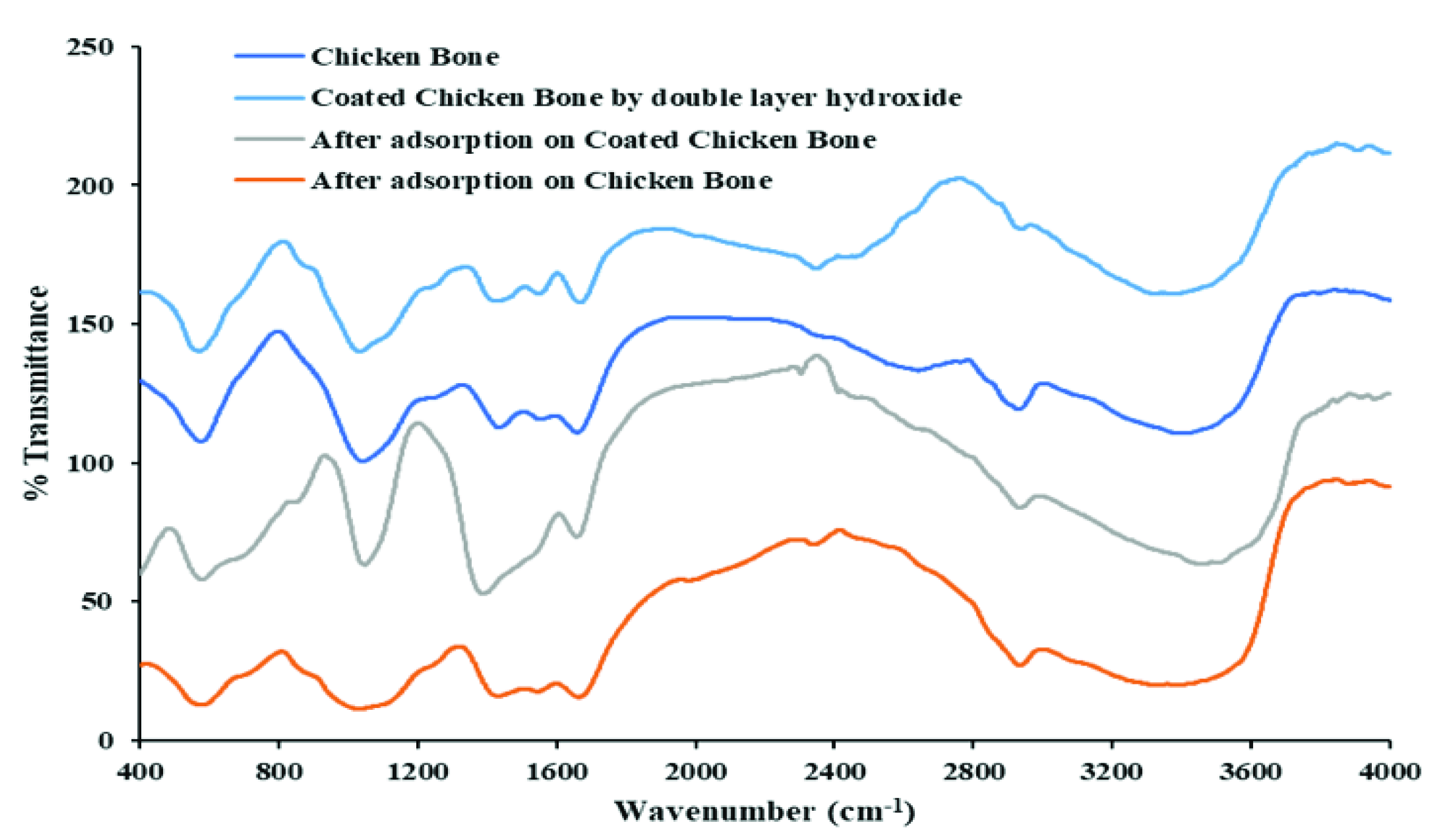
4.1. Characterization of the Coated Chicken Bone Particles Using FTIR
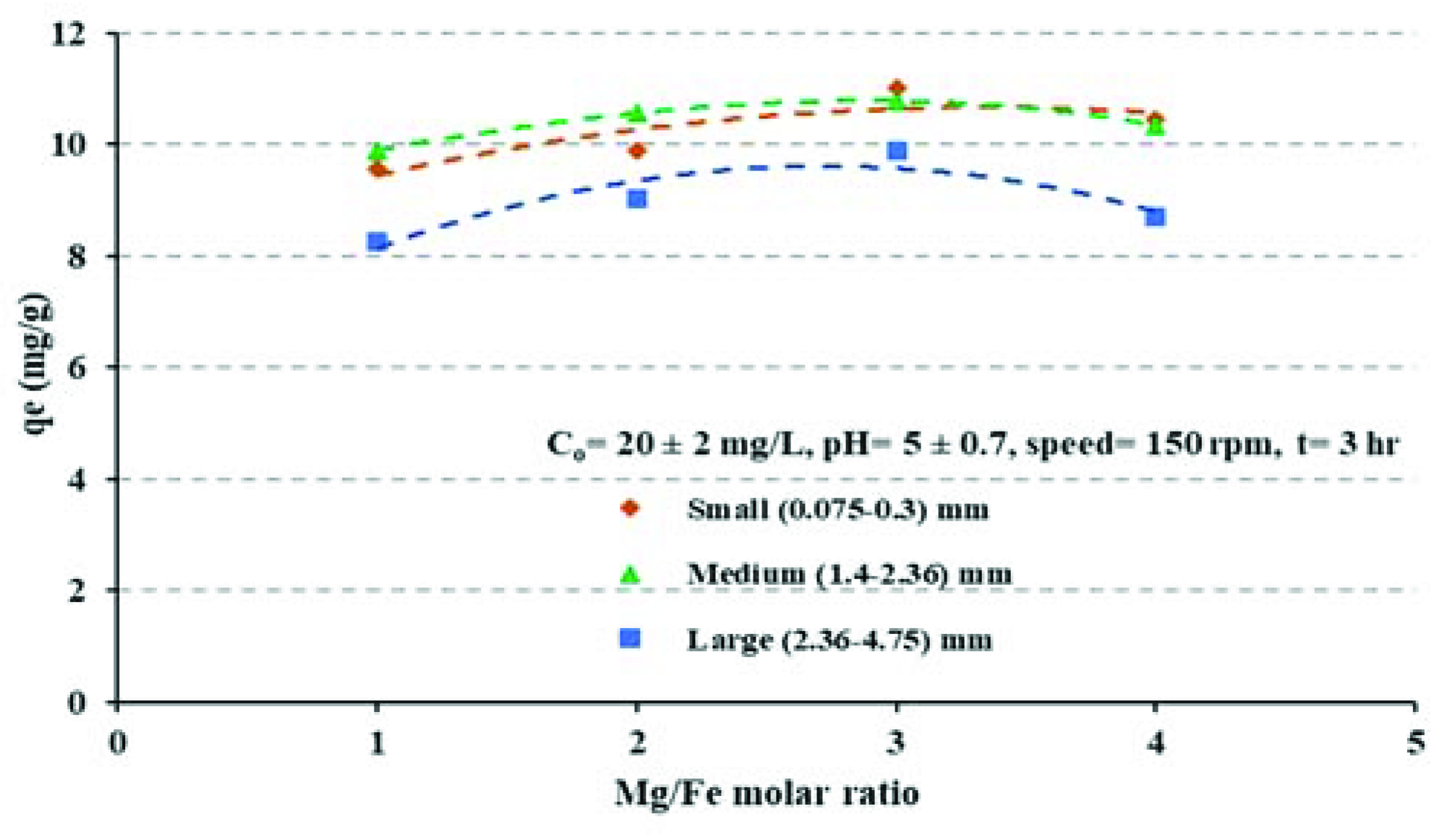
4.2. Effects of Mg/Fe Molar Ratio
4.3. Batch Experiments
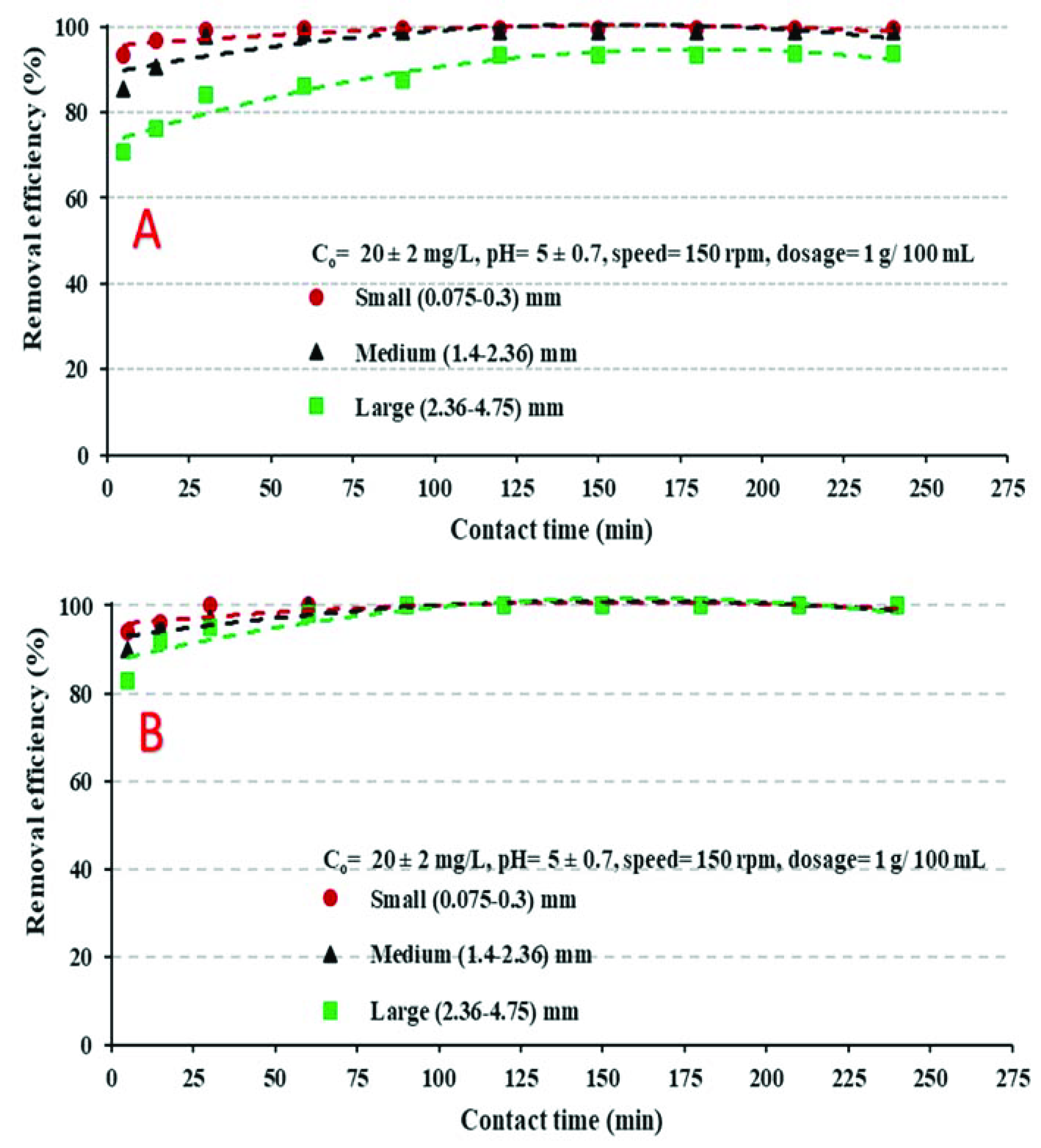
4.3.1. Influence of Contact Time
4.3.2. Influence of Agitation Speed
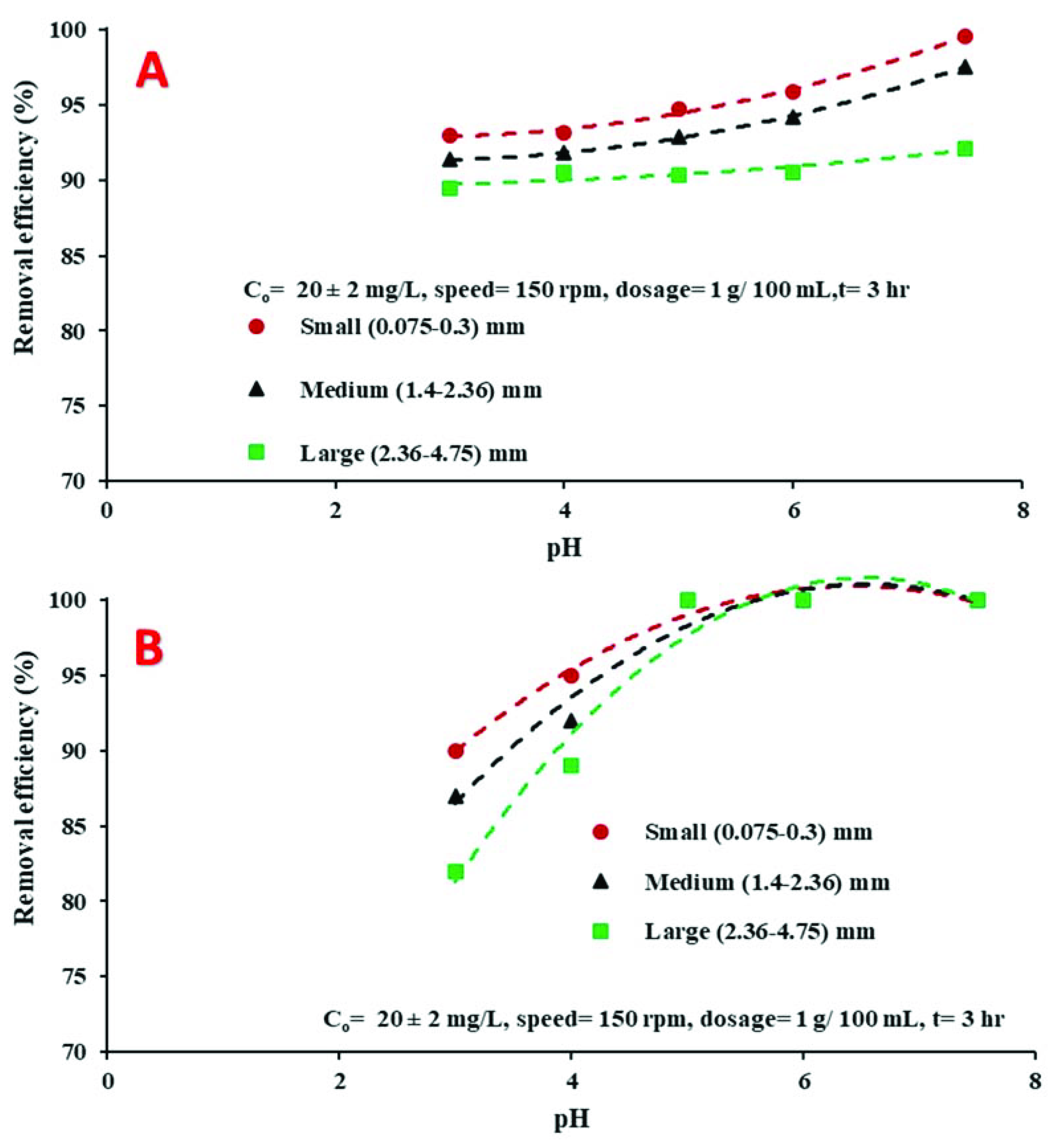
4.3.3. Influence of Initial pH
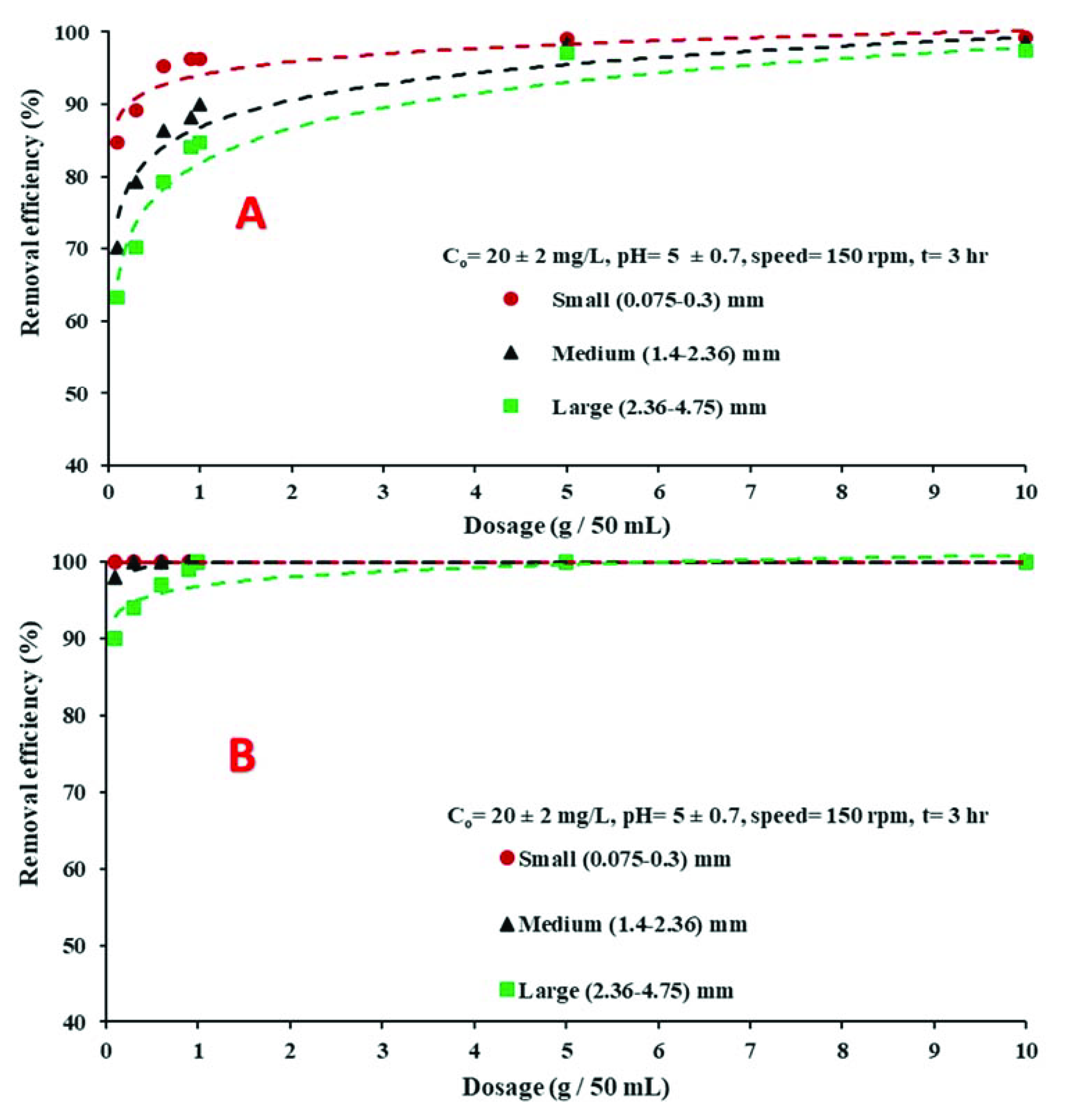
4.3.4. Influence of Adsorbent Doses
4.4. Sorption Kinetics
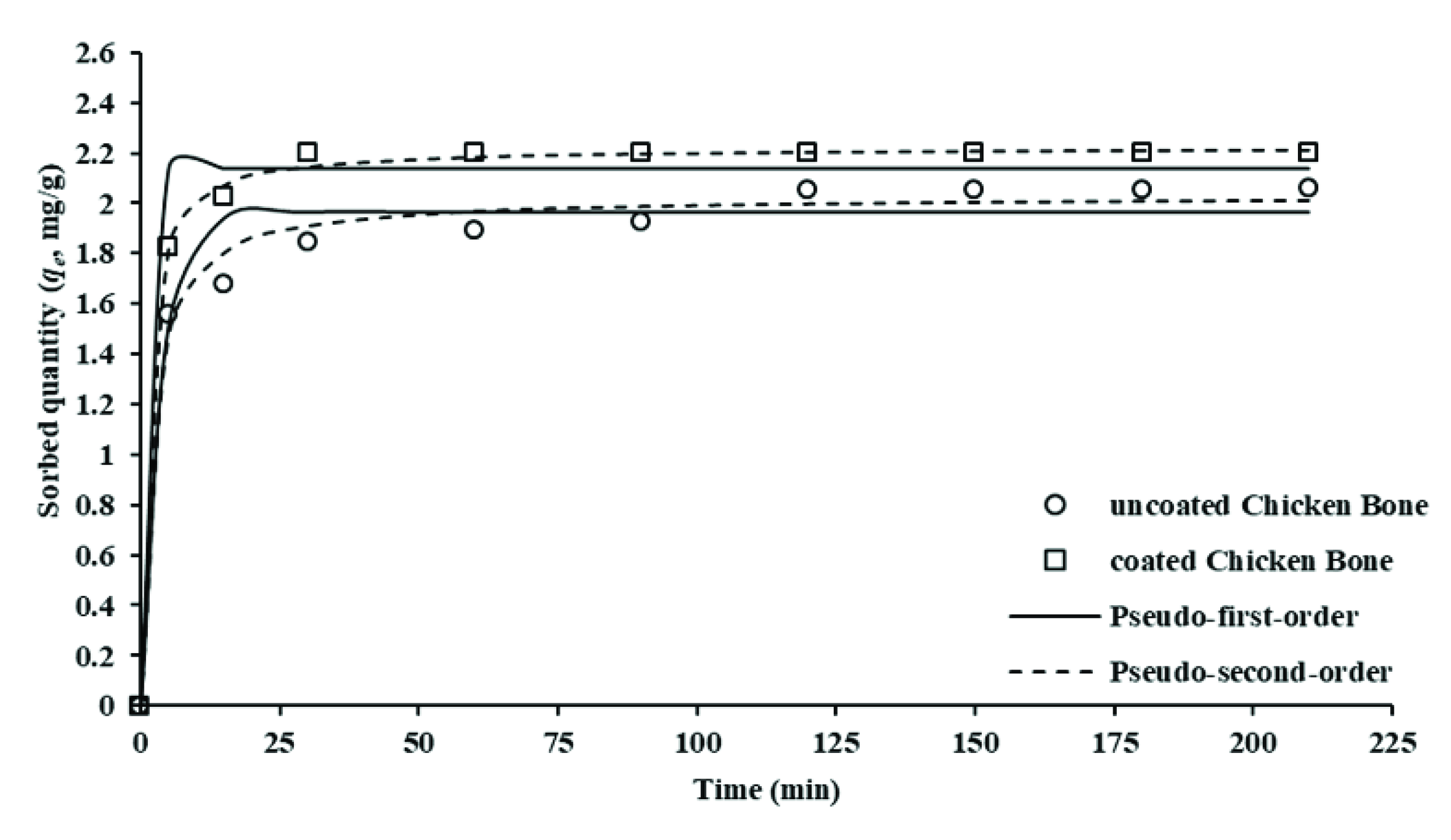
4.4.1. Pseudo-First Order Kinetic Model
4.4.2. Pseudo-Second Order Kinetic Model
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, S.; Majhi, P.K.; Kothari, R.; Singh, R.P. Industrial wastewater footprinting: A need for water security in Indian context. In Environmental Concerns and Sustainable Development; Springer: Berlin, Germany, 2020; pp. 197–212. [Google Scholar]
- Hashim, K.S.; AlKhaddar, R.; Shaw, A.; Kot, P.; Al-Jumeily, D.; Alwash, R.; Aljefery, M.H. Electrocoagulation as an eco-friendly River water treatment method. In Advances in Water Resources Engineering and Management; Springer: Berlin, Germany, 2020; pp. 219–235. [Google Scholar]
- Omran, I.I.; Al-Saati, N.H.; Hashim, K.S.; Al-Saati, Z.N.; Kot, P.; Khaddar, R.A.; Al-Jumeily, D.; Shaw, A.; Ruddock, F.; Aljefery, M.; et al. Assessment of heavy metal pollution in the Great Al-Mussaib irrigation channel. Desalin. Water Treat. 2019, 168, 165–174. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Mousazadeh, M.; Mokhtari, N.; Jamali, H.A.; Makkiabadi, M.; Naghdali, Z.; Hashim, K.S.; Ghanbari, R. Simultaneous removal of phenol and linear alkylbenzene sulfonate from automotive service station wastewater: Optimization of coupled electrochemical and physical processes. Sep. Sci. Technol. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Aljerf, L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Abdulhadi, B.A.; Kot, P.; Hashim, K.S.; Shaw, A.; Khaddar, R.A. Influence of current density and electrodes spacing on reactive red 120 dye removal from dyed water using electrocoagulation/electroflotation (EC/EF) process. In Proceedings of the First International Conference on Civil and Environmental Engineering Technologies (ICCEET), Al-Najaf, Iraq, 23–24 April 2019; pp. 12–22. [Google Scholar]
- Zubaidi, S.; Al-Bugharbee, H.; Ortega Martorell, S.; Gharghan, S.; Olier, I.; Hashim, K.; Al-Bdairi, N.; Kot, P. A Novel Methodology for Prediction Urban Water Demand by Wavelet Denoising and Adaptive Neuro-Fuzzy Inference System Approach. Water 2020, 12, 1628. [Google Scholar] [CrossRef]
- Zubaidi, S.L.; Ortega-Martorell, S.; Al-Bugharbee, H.; Olier, I.; Hashim, K.S.; Gharghan, S.K.; Kot, P.; Al-Khaddar, R. Urban Water Demand Prediction for a City that Suffers from Climate Change and Population Growth: Gauteng Province case study. Water 2020, 12, 1885. [Google Scholar] [CrossRef]
- Alattabi, A.W.; Harris, C.; Alkhaddar, R.; Alzeyadi, A.; Hashim, K. Treatment of Residential Complexes’ Wastewater using Environmentally Friendly Technology. Procedia Eng. 2017, 196, 792–799. [Google Scholar] [CrossRef]
- Hashim, K.S.; Ali, S.S.M.; AlRifaie, J.K.; Kot, P.; Shaw, A.; Al Khaddar, R.; Idowu, I.; Gkantou, M. Escherichia coli inactivation using a hybrid ultrasonic—Electrocoagulation reactor. Chemosphere 2020, 247, 125868–125875. [Google Scholar] [CrossRef]
- Sun, D.; Hong, X.; Wu, K.; Hui, K.; Du, Y.; Hui, K. Simultaneous removal of ammonia and phosphate by electro-oxidation and electrocoagulation using RuO2–IrO2/Ti and microscale zero-valent iron composite electrode. Water Res. 2020, 169, 115239. [Google Scholar]
- Ryecroft, S.P.; Shaw, A.; Fergus, P.; Kot, P.; Hashim, K.; Conway, L. A Novel Gesomin Detection Method Based on Microwave Spectroscopy. In Proceedings of the 12th International Conference on Developments in eSystems Engineering (DeSE), Kazan, Russia, 7–10 October 2019; pp. 429–433. [Google Scholar]
- Al-Saati, N.H.; Hussein, T.K.; Abbas, M.H.; Hashim, K.; Al-Saati, Z.N.; Kot, P.; Sadique, M.; Aljefery, M.H.; Carnacina, I. Statistical modelling of turbidity removal applied to non-toxic natural coagulants in water treatment: A case study. Desalin. Water Treat. 2019, 150, 406–412. [Google Scholar] [CrossRef]
- Hashim, K.S.; Al-Saati, N.H.; Alquzweeni, S.S.; Zubaidi, S.L.; Kot, P.; Kraidi, L.; Hussein, A.H.; Alkhaddar, R.; Shaw, A.; Alwash, R. Decolourization of dye solutions by electrocoagulation: An investigation of the effect of operational parameters. In Proceedings of the First International Conference on Civil and Environmental Engineering Technologies (ICCEET), Al-Najaf, Iraq, 23–24 April 2019; pp. 25–32. [Google Scholar]
- Hashim, K.S.; Hussein, A.H.; Zubaidi, S.L.; Kot, P.; Kraidi, L.; Alkhaddar, R.; Shaw, A.; Alwash, R. Effect of initial pH value on the removal of reactive black dye from water by electrocoagulation (EC) method. In Proceedings of the 2nd International Scientific Conference, Al Diwaniyah, Iraq, 23–24 June 2019; pp. 12–22. [Google Scholar]
- Hashim, K.S.; Shaw, A.; Al Khaddar, R.; Pedrola, M.O.; Phipps, D. Iron removal, energy consumption and operating cost of electrocoagulation of drinking water using a new flow column reactor. J. Environ. Manag. 2017, 189, 98–108. [Google Scholar] [CrossRef]
- Doggaz, A.; Attoura, A.; Mostefa, M.L.P.; Côme, K.; Tlili, M.; Lapicque, F. Removal of heavy metals by electrocoagulation from hydrogenocarbonate-containing waters: Compared cases of divalent iron and zinc cations. J. Water Process Eng. 2019, 29, 100796. [Google Scholar] [CrossRef]
- Rahi, M.A.; Faisal, A.A.; Naji, L.A.; Almuktar, S.A.; Abed, S.N.; Scholz, M. Biochemical performance modelling of non-vegetated and vegetated vertical subsurface-flow constructed wetlands treating municipal wastewater in hot and dry climate. J. Water Process Eng. 2020, 33, 101003. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Toda, M.; Otani, M.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of Arsenic (III) Ion from Aqueous Media Using Complex Nickel-Aluminum and Nickel-Aluminum-Zirconium Hydroxides. Water 2020, 12, 1697. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hakami, M.; Zacharof, M.-P.; Abu Homod, H.; Alsadun, A. Mercury, Arsenic and Lead Removal by Air Gap Membrane Distillation: Experimental Study. Water 2020, 12, 1574. [Google Scholar] [CrossRef]
- Mohora, E.; Rončević, S.; Agbaba, J.; Zrnić, K.; Tubić, A.; Adžemović, M.; Dalmacija, B. Effects of combined Fe-Al electrodes and groundwater temperature on arsenic removal by electrocoagulation. Environ. Prot. Eng. 2019, 45, 5–18. [Google Scholar] [CrossRef]
- Hashim, K.S.; Al-Saati, N.H.; Hussein, A.H.; Al-Saati, Z.N. An investigation into the level of heavy metals leaching from canal-dreged sediment: A case study metals leaching from dreged sediment. In Proceedings of the First International Conference on Materials Engineering & Science, Istanbul, Turkey, 22–24 April 2018; pp. 12–22. [Google Scholar]
- Bakulski, K.M.; Seo, Y.A.; Hickman, R.C.; Brandt, D.; Vadari, H.S.; Hu, H.; KyunPark, S. Heavy Metals Exposure and Alzheimer’s Disease and Related Dementias. J. Alzheimer’s Dis. 2020, 1, 1–28. [Google Scholar] [CrossRef]
- Fu, X.; Li, L.; Yang, G.; Xu, X.; He, L.; Zhao, Z. Removal of Trace Thallium from Industrial Wastewater by Fe0-Electrocoagulation. Water 2020, 12, 163. [Google Scholar] [CrossRef]
- Chamanchia, M.; Vaferib, B.; Jalili, I. A comparative experimental study of the removal of heavy metals using low cost natural adsorbents and commerical activated carbon. Int. J. 2012, 3, 55–60. [Google Scholar]
- Shareef, K.M. Sorbents for contaminants uptake from aqueous solutions. Part I: Heavy metals. World J. Agric. Sci. 2009, 5, 819–831. [Google Scholar]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Skoulou, V.; Zabaniotou, A. Investigation of agricultural and animal wastes in Greece and their allocation to potential application for energy production. Renew. Sustain. Energy Rev. 2007, 11, 1698–1719. [Google Scholar] [CrossRef]
- Abdulredha, M.; Rafid, A.; Jordan, D.; Hashim, K. The development of a waste management system in Kerbala during major pilgrimage events: Determination of solid waste composition. Procedia Eng. 2017, 196, 779–784. [Google Scholar] [CrossRef]
- Abdulredha, M.; Al Khaddar, R.; Jordan, D.; Kot, P.; Abdulridha, A.; Hashim, K. Estimating solid waste generation by hospitality industry during major festivals: A quantification model based on multiple regression. Waste Manag. 2018, 77, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Ladas, D. Meat waste treatment methods and potential uses. Int. J. Food Sci. Technol. 2008, 43, 543–559. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; Jiang, C.; Jiang, X.; Guan, Y. Simultaneous and efficient capture of inorganic nitrogen and heavy metals by polyporous layered double hydroxide and biochar composite for agricultural nonpoint pollution control. ACS Appl. Mater. Interfaces 2018, 10, 43013–43030. [Google Scholar] [CrossRef]
- Kizilkaya, B.; Tekınay, A.A. Utilization to remove Pb (II) ions from aqueous environments using waste fish bones by ion exchange. J. Chem. 2014, 739273, 1–12. [Google Scholar] [CrossRef]
- Ahmed, D.N.; Naji, L.A.; Faisal, A.A.; Al-Ansari, N.; Naushad, M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Naji, L.A.; Jassam, S.H.; Yaseen, M.J.; Faisal, A.A.; Al-Ansari, N. Modification of Langmuir model for simulating initial pH and temperature effects on sorption process. Sep. Sci. Technol. 2019, 23, 1–8. [Google Scholar] [CrossRef]
- Faisal, A.A.; Al-Wakel, S.F.; Assi, H.A.; Naji, L.A.; Naushad, M. Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J. Water Process Eng. 2020, 33, 101112. [Google Scholar] [CrossRef]
- Hashim, K.; Kot, P.; Zubaid, S.; Alwash, R.; Al Khaddar, R.; Shaw, A.; Al-Jumeily, D.; Aljefery, M. Energy efficient electrocoagulation using baffle-plates electrodes for efficient Escherichia Coli removal from wastewater. J. Water Process Eng. 2020, 33, 101079–101086. [Google Scholar] [CrossRef]
- Hashim, K.S.; Khaddar, R.A.; Jasim, N.; Shaw, A.; Phipps, D.; Kota, P.; Pedrola, M.O.; Alattabi, A.W.; Abdulredha, M.; Alawsh, R. Electrocoagulation as a green technology for phosphate removal from River water. Sep. Purif. Technol. 2019, 210, 135–144. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Ng, D.H. Synthesis of a 3D hierarchical structure of γ-AlO (OH)/Mg–Al-LDH/C and its performance in organic dyes and antibiotics adsorption. J. Mater. Chem. A 2015, 3, 21106–21115. [Google Scholar] [CrossRef]
- Shen, Y.; Li, H.; Zhu, W.; Ho, S.-H.; Yuan, W.; Chen, J.; Xie, Y. Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour. Technol. 2017, 244, 1031–1038. [Google Scholar] [CrossRef]
- Parvathi, K.; Nagendran, R.; Nareshkumar, R. Lead biosorption onto waste beer yeast by-product: A means to decontaminate effluent generated from battery manufacturing industry. Electron. J. Biotechnol. 2007, 10, 92–105. [Google Scholar] [CrossRef][Green Version]
- Znad, H.; Frangeskides, Z. Chicken drumstick bones as an efficient biosorbent for copper (II) removal from aqueous solution. Desalin. Water Treat. 2014, 52, 1560–1570. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Banat, F.; Mohai, F. Sorption of copper and nickel by spent animal bones. Chemosphere 1999, 39, 2087–2096. [Google Scholar] [CrossRef]
- Amarasinghe, B.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Puranik, P.; Modak, J.; Paknikar, K. A comparative study of the mass transfer kinetics of metal biosorption by microbial biomass. Hydrometallurgy 1999, 52, 189–197. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetensk. Handingarl 1898, 24, 1–39. [Google Scholar]
- Khamizov, R.K.; Sveshnikova, D.; Kucherova, A.; Sinyaeva, L. Kinetic model of batch sorption processes: Comparing calculated and experimental data. Russ. J. Phys. Chem. A 2018, 92, 2032–2038. [Google Scholar] [CrossRef]
- Komy, Z.R.; Shaker, A.M.; Heggy, S.E.; El-Sayed, M.E. Kinetic study for copper adsorption onto soil minerals in the absence and presence of humic acid. Chemosphere 2014, 99, 117–124. [Google Scholar] [CrossRef] [PubMed]

| Model | Parameter | Uncoated Adsorbents | Coated Adsorbents |
|---|---|---|---|
| Pseudo-first order | qe | 1.965 | 2.139 |
| k1 | 0.294 | 3.539 | |
| R2 | 0.964 | 0.968 | |
| SSE | 0.131 | 0.137 | |
| Pseudo-second order | qe | 2.030 | 2.224 |
| k2 | 0.261 | 0.404 | |
| R2 | 0.987 | 0.999 | |
| SSE | 0.047 | 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alquzweeni, S.S.; Alkizwini, R.S. Removal of Cadmium from Contaminated Water Using Coated Chicken Bones with Double-Layer Hydroxide (Mg/Fe-LDH). Water 2020, 12, 2303. https://doi.org/10.3390/w12082303
Alquzweeni SS, Alkizwini RS. Removal of Cadmium from Contaminated Water Using Coated Chicken Bones with Double-Layer Hydroxide (Mg/Fe-LDH). Water. 2020; 12(8):2303. https://doi.org/10.3390/w12082303
Chicago/Turabian StyleAlquzweeni, Saif S., and Rasha S. Alkizwini. 2020. "Removal of Cadmium from Contaminated Water Using Coated Chicken Bones with Double-Layer Hydroxide (Mg/Fe-LDH)" Water 12, no. 8: 2303. https://doi.org/10.3390/w12082303
APA StyleAlquzweeni, S. S., & Alkizwini, R. S. (2020). Removal of Cadmium from Contaminated Water Using Coated Chicken Bones with Double-Layer Hydroxide (Mg/Fe-LDH). Water, 12(8), 2303. https://doi.org/10.3390/w12082303




