Modeling and Analysis of Particle Deposition Processes on PVDF Membranes Using SEM Images and Image Generation by Auxiliary Classifier Generative Adversarial Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Membrane Filter
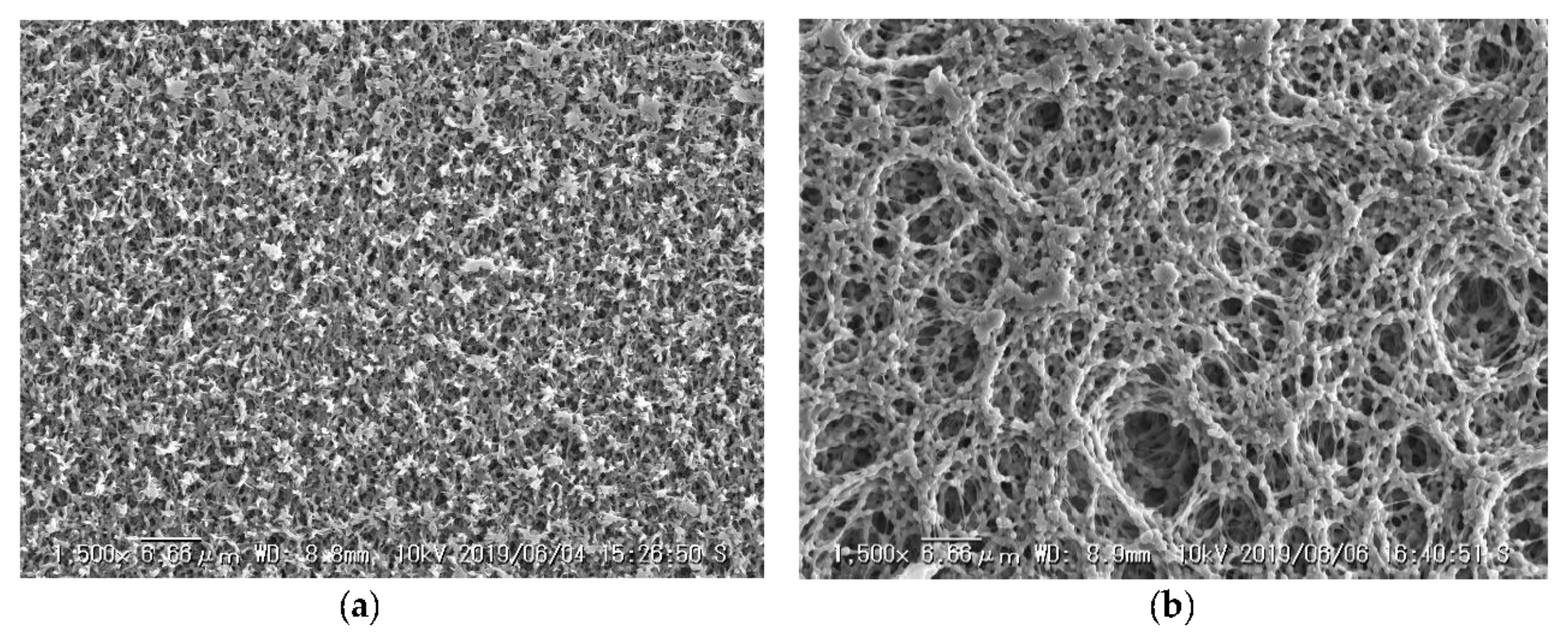
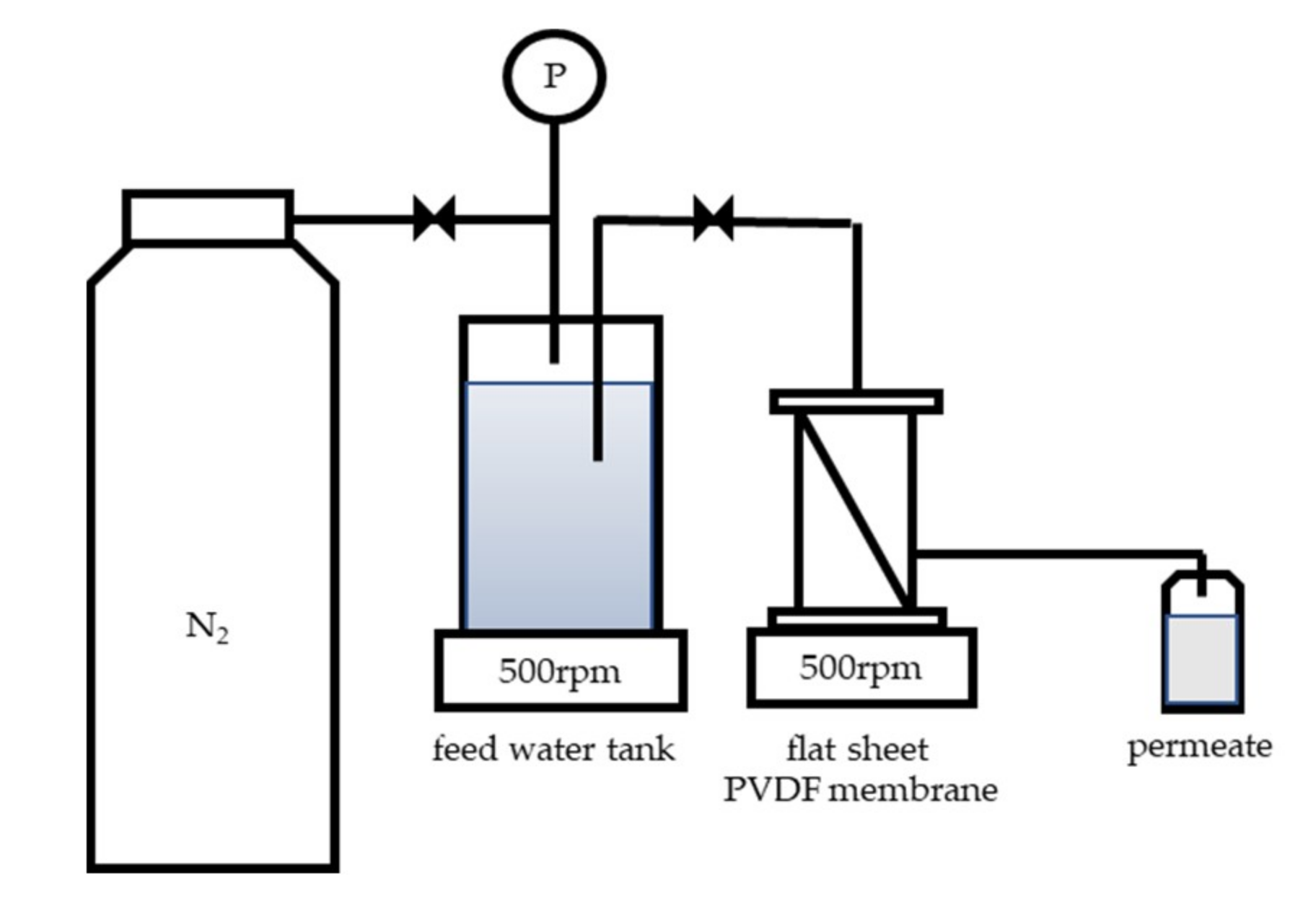
2.2. Filtration Experiments and Image Acquisition
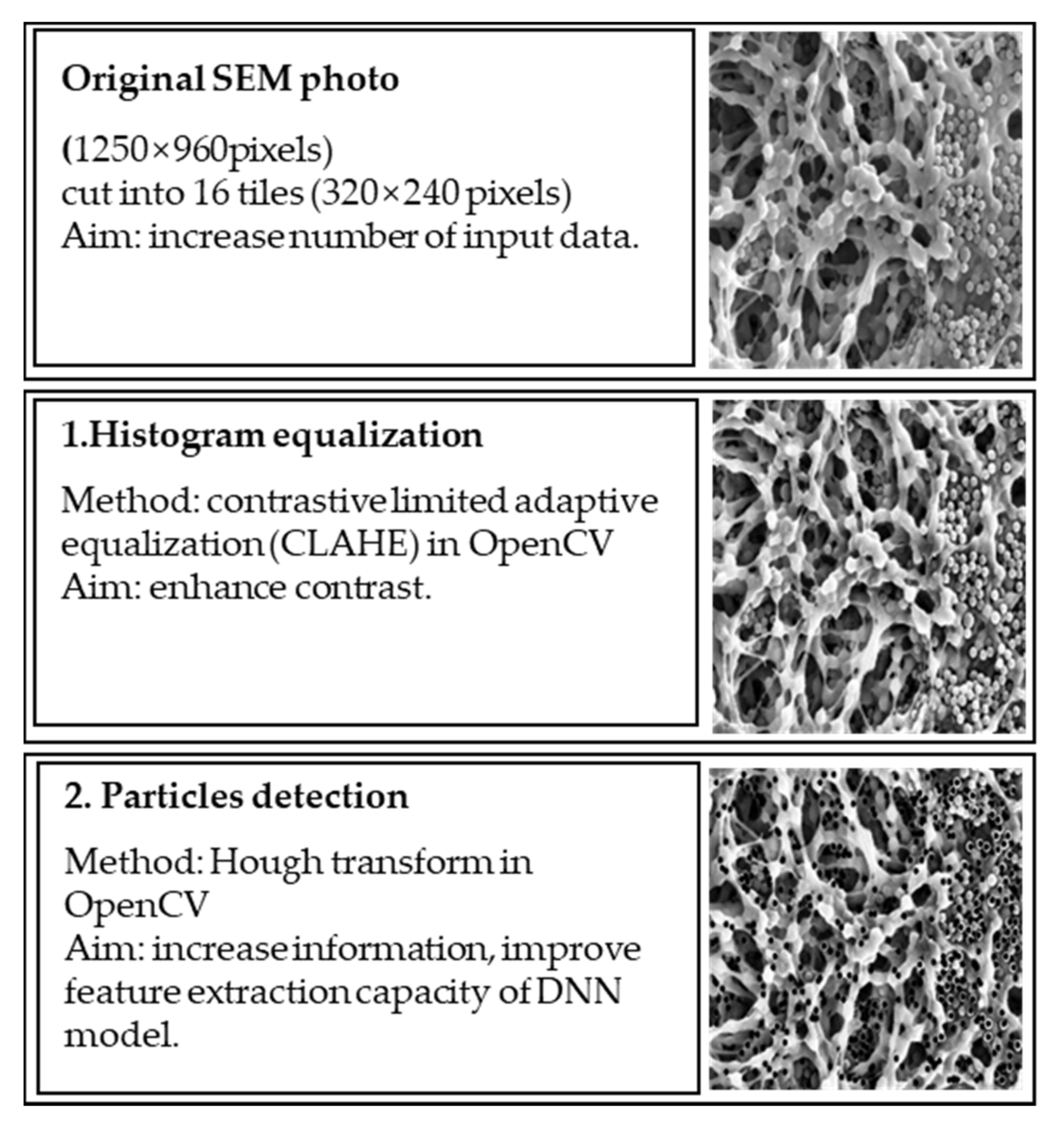
2.3. Image Preprocessing
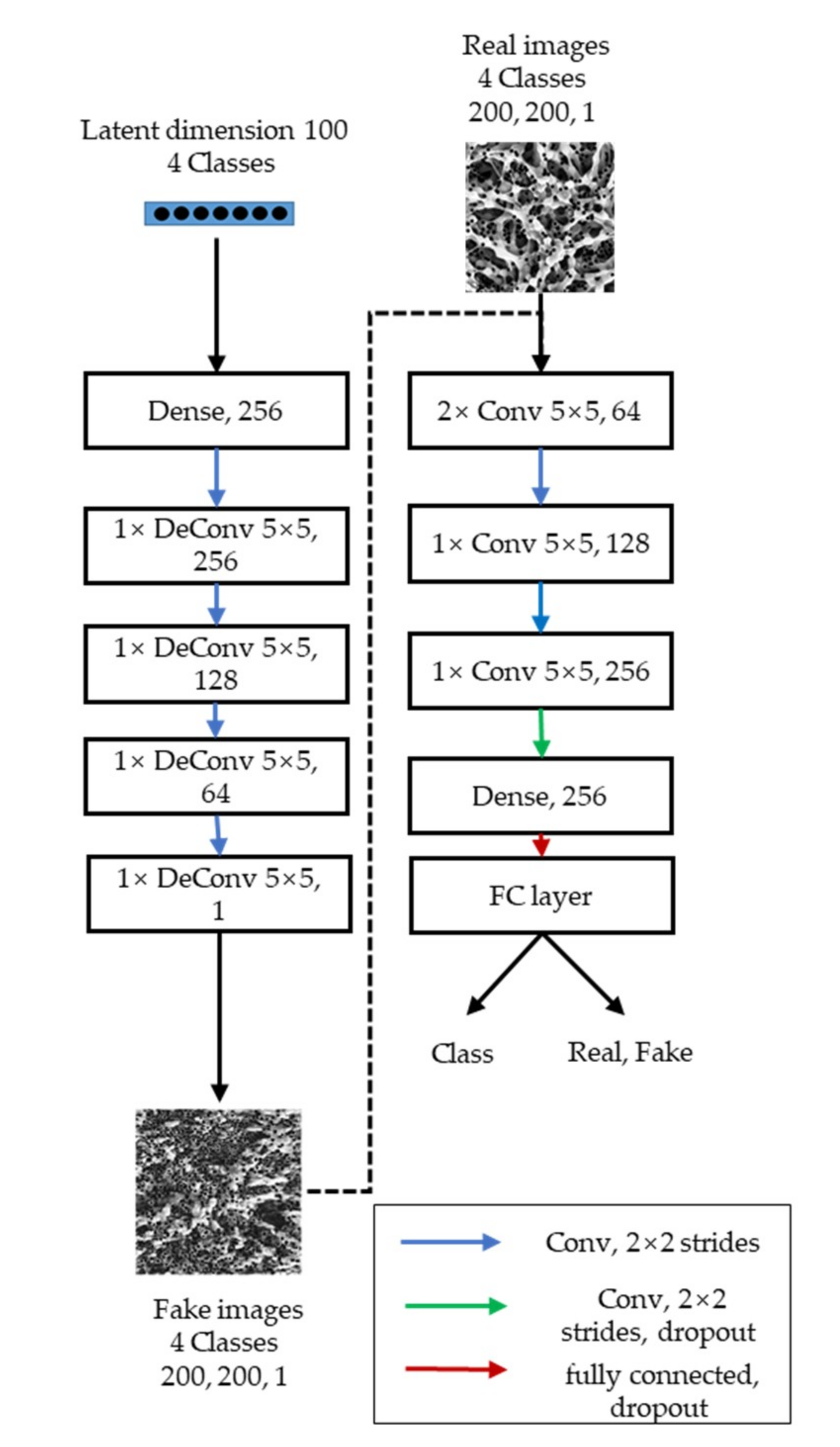
2.4. Particle Deposition Model Using ACGAN
2.5. Performance Evaluation
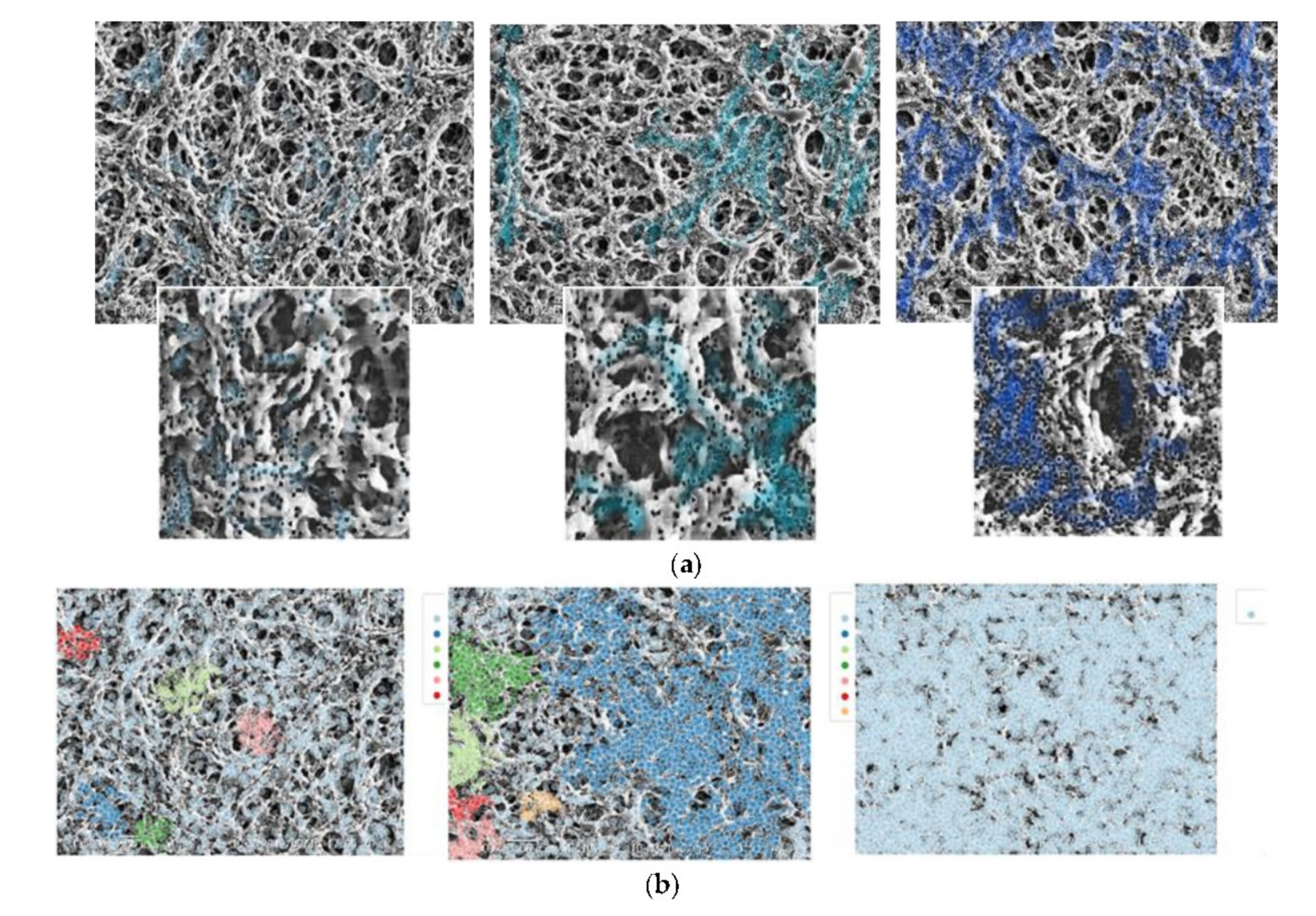
2.6. Density Clusters Identification
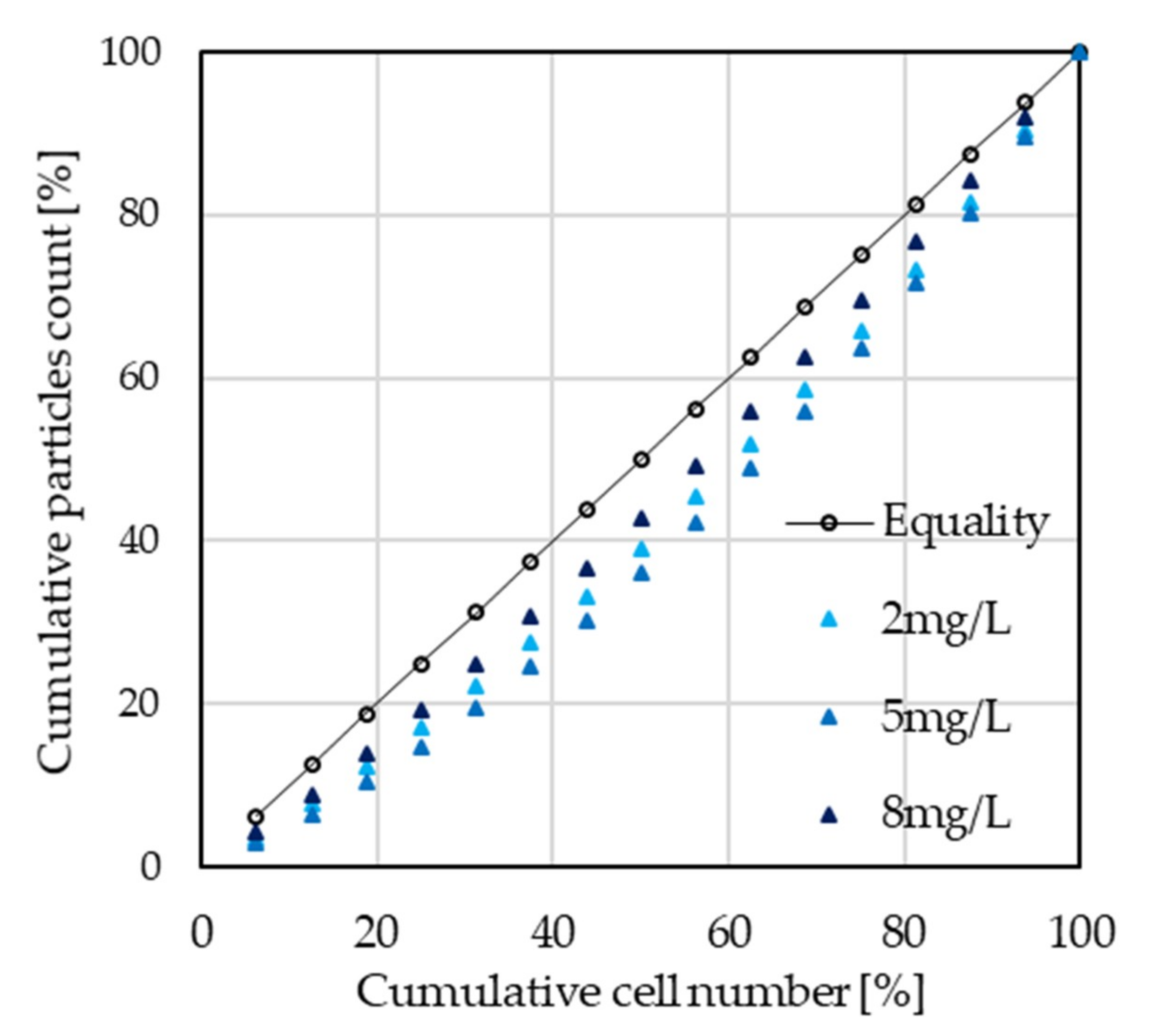
2.7. Gini Index Equality Distribution and ANOVA
3. Results
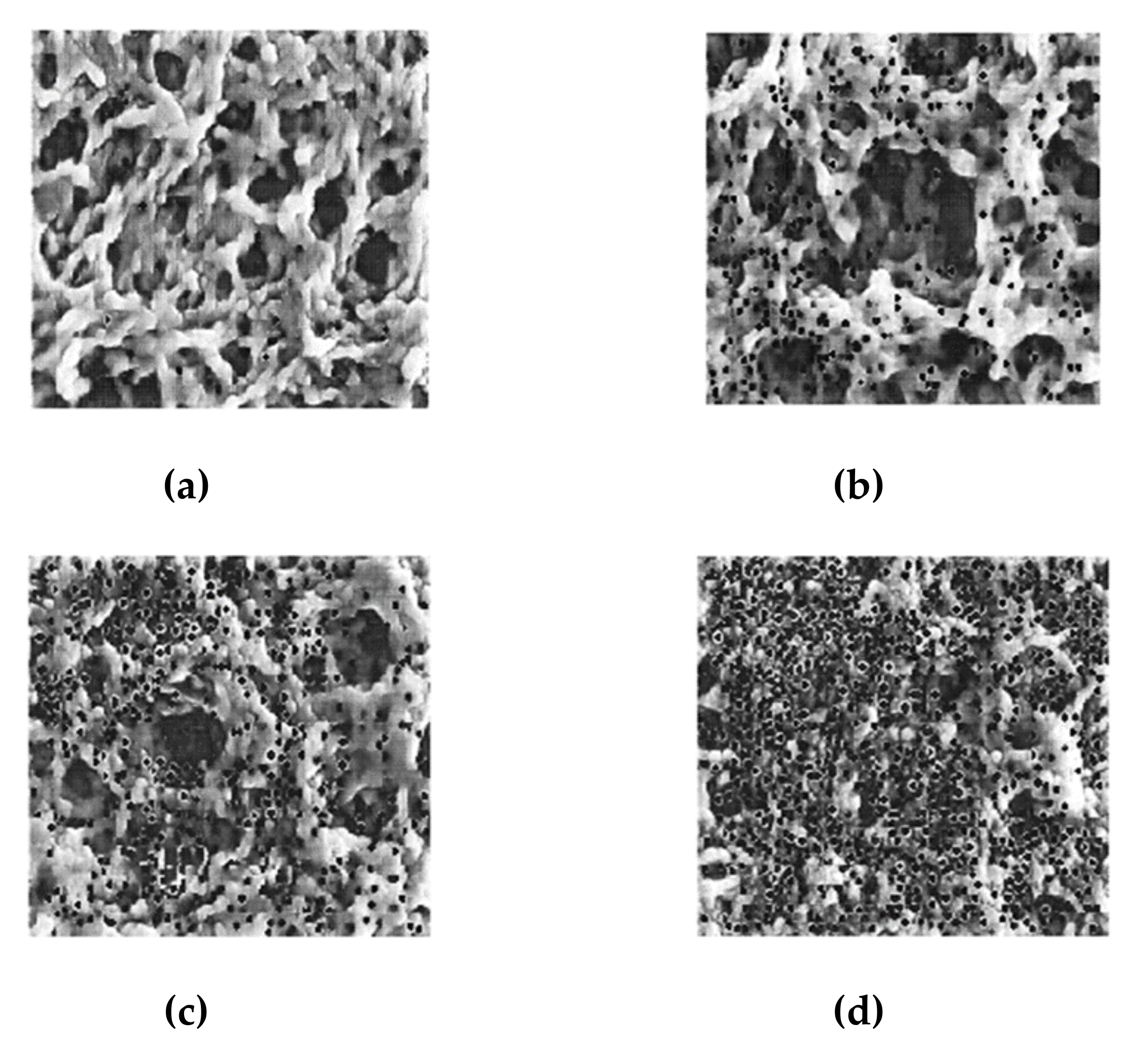
3.1. ACGAN Generated Images of PVDF Membranes with Deposited Particles
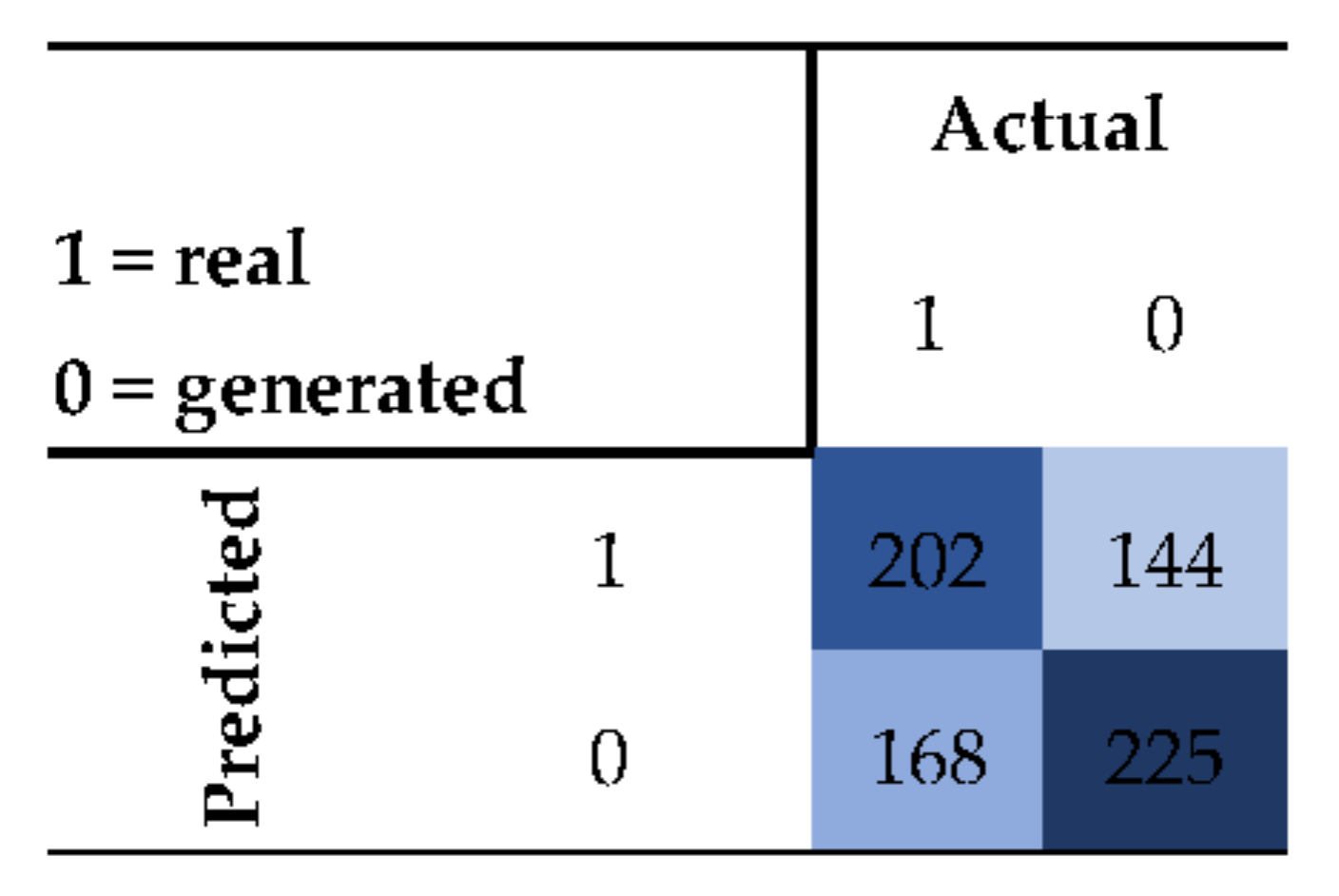
3.2. Evaluation of ACGAN Generated Images of PVDF Membranes with Deposited Particles
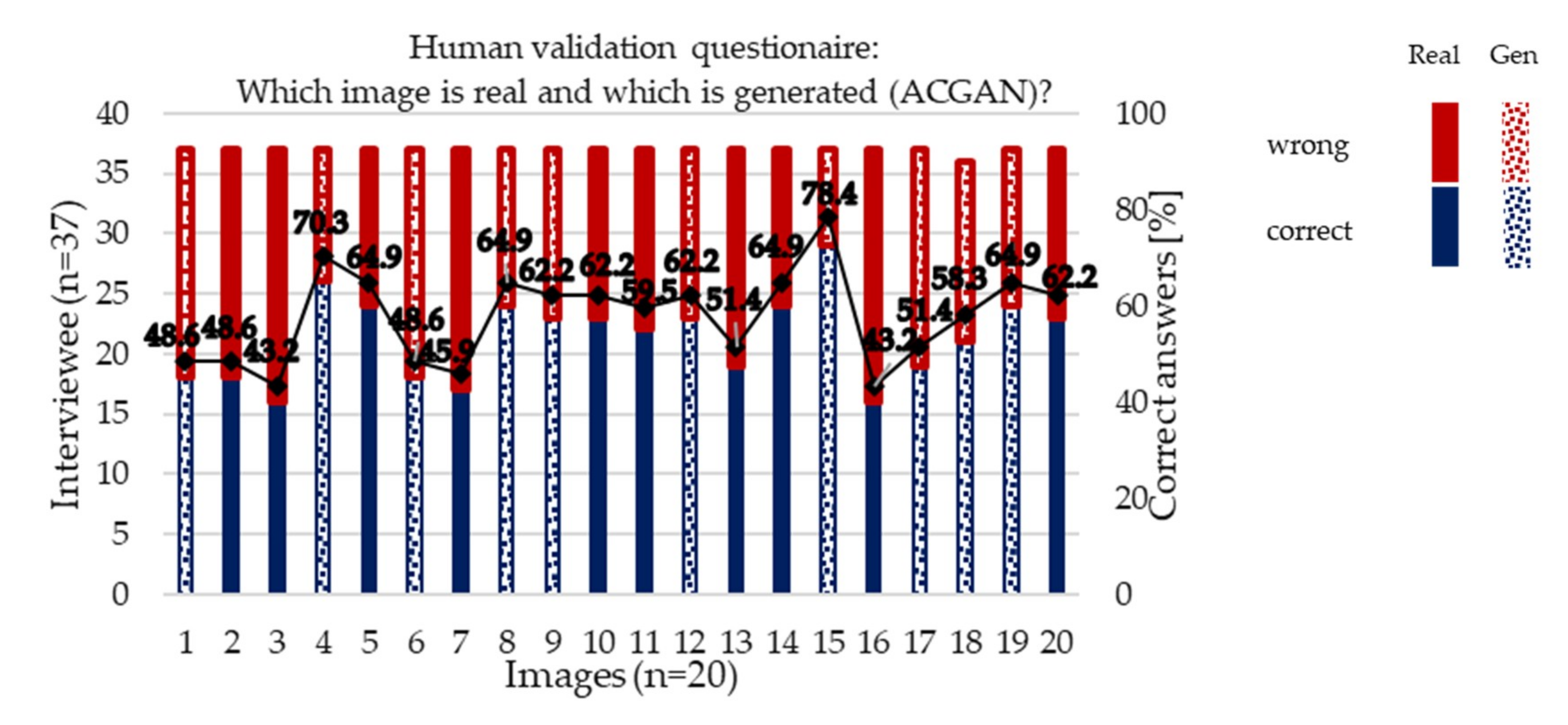
3.2.1. Human Validation
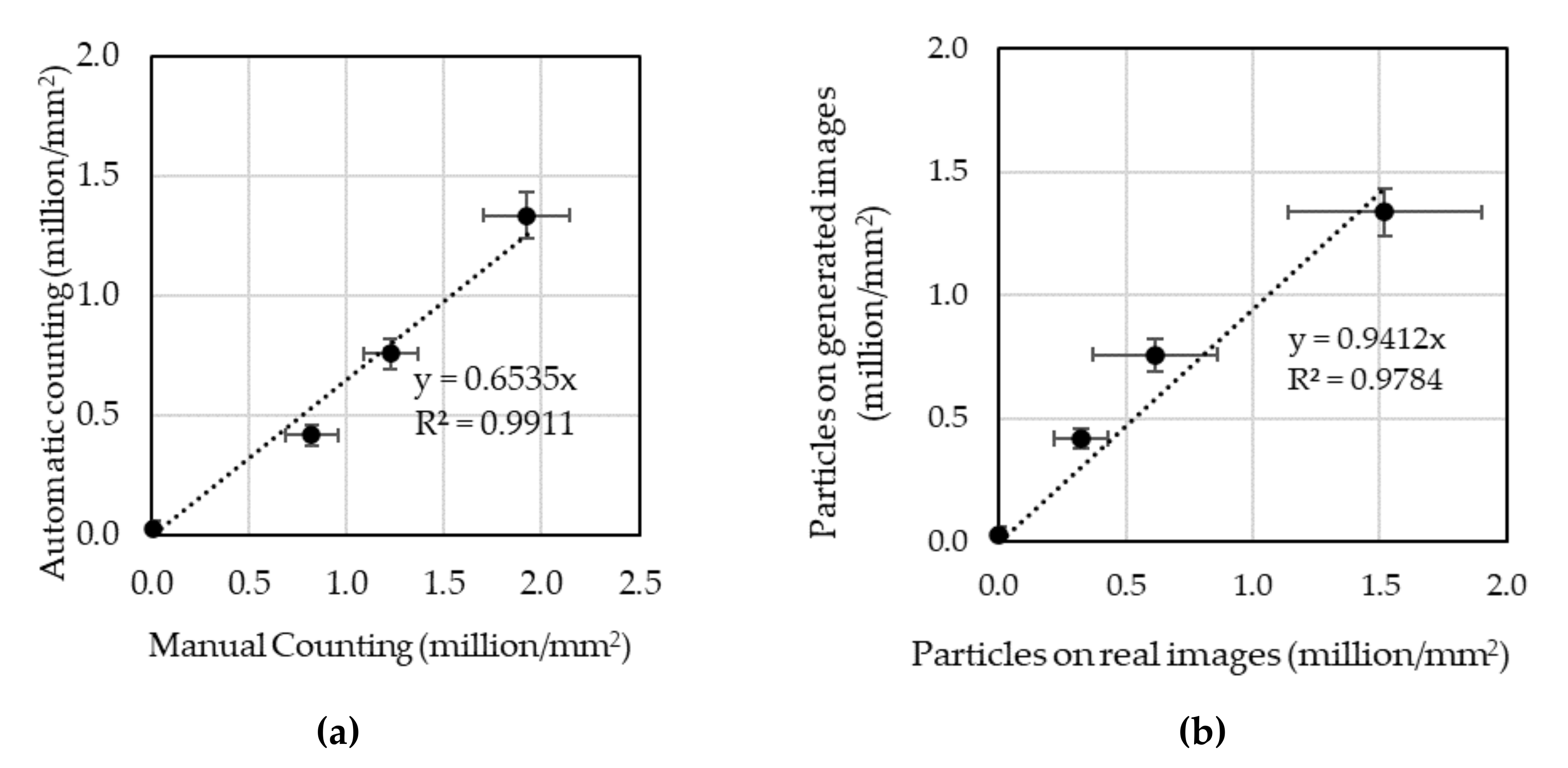
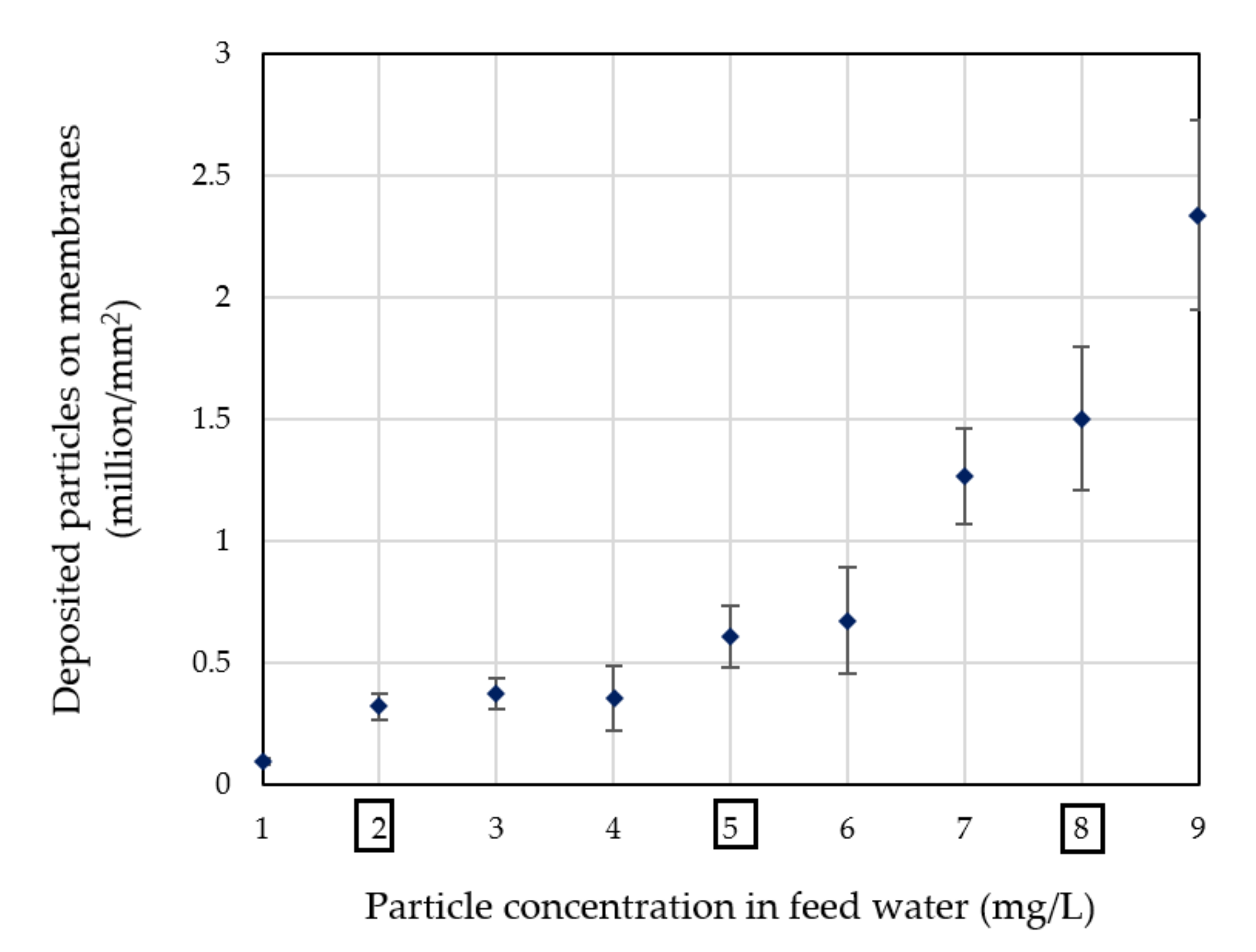
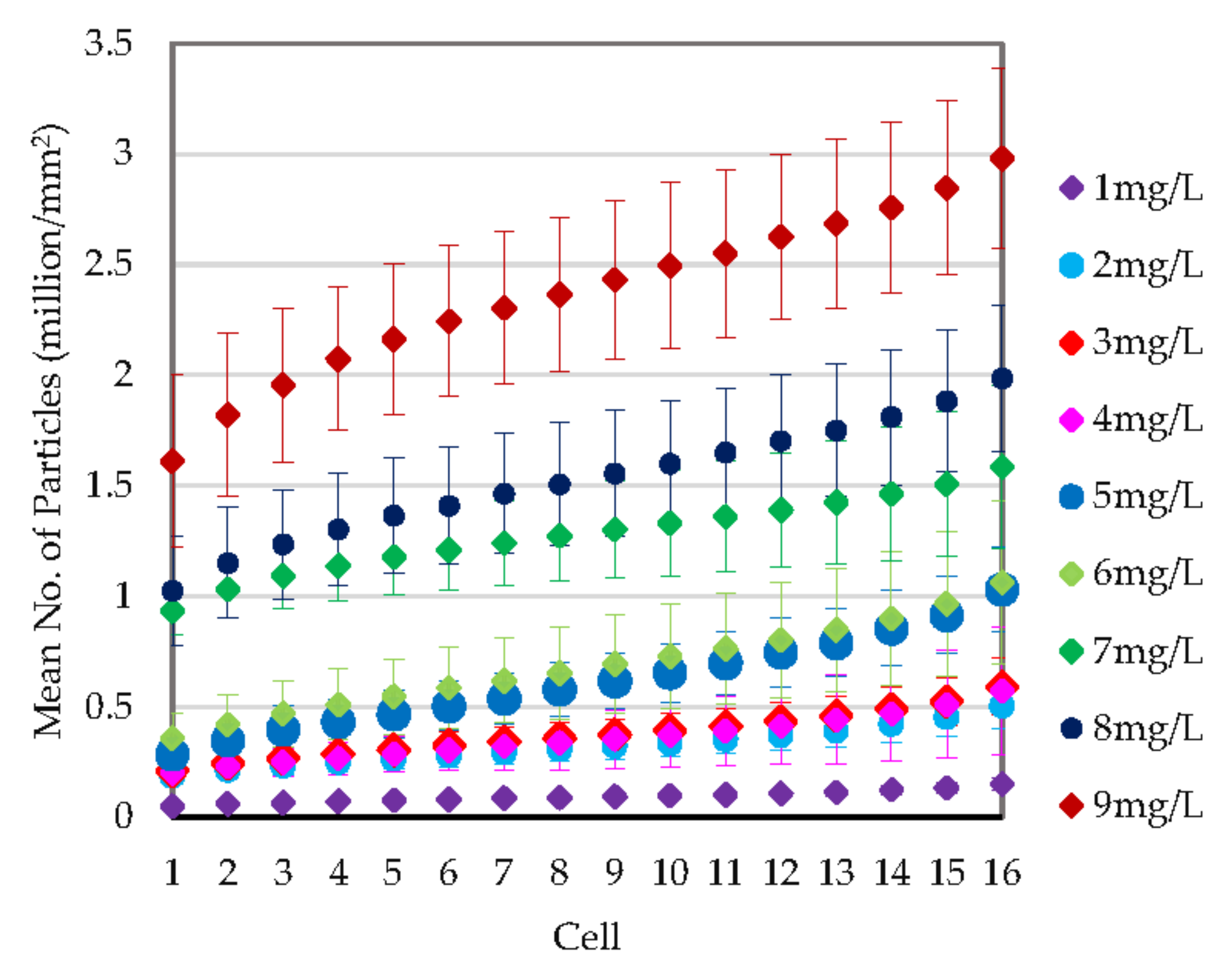
3.2.2. Quantitative Evaluation through Particle Counting
4. Discussion
4.1. Particle Deposition Patterns on Real and Generated Images
4.2. Particle Distribution Analysis through Gini Index Calculation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Operation | Kernel 1 | Stride 2 | Feature Map 3 | BN 4 | Dropout 5 | Activation Function 6 | |
|---|---|---|---|---|---|---|---|
| Discriminator | Convolution1 | 5 × 5 | 2 × 2 | 64 | Yes | LeakyReLU | |
| Convolution2 | 5 × 5 | 2 × 2 | 64 | Yes | LeakyReLU | ||
| Convolution3 | 5 × 5 | 2 × 2 | 128 | Yes | LeakyReLU | ||
| Convolution4 | 5 × 5 | 2 × 2 | 256 | Yes | 0.5 | LeakyReLU | |
| Dense | 256, 1 | Yes | 0.5 | Sigmoid (+Softmax) | |||
| Generator | Dense | 256 | Yes | LeakyReLU | |||
| Deconvolutional1 | 5 × 5 | 2 × 2 | 256 | Yes | LeakyReLU | ||
| Deconvolutional2 | 5 × 5 | 2 × 2 | 128 | Yes | LeakyReLU | ||
| Deconvolutional3 | 5 × 5 | 2 × 2 | 64 | Yes | LeakyReLU | ||
| Deconvolutional4 | 5 × 5 | 2 × 2 | 1 | Yes | tanh | ||
| Discriminator input | 200 × 200 × 1 | Classes | 4 | Samples | 7680 | ||
| Generator input | 10 × 10 × 1 | Output | 200 × 200 × 1 | Latent Space | 100 | ||
| Batch size | 30 | Iterations | 80,000 | Optimizer | Adam lr = 0.0002 | ||
References
- Alspach, B.; Adham, S.; Cooke, T.; Delphos, P.; Garcia-Aleman, J.; Jacangelo, J.; Karimi, A.; Pressman, J.; Schaefer, J.; Sethi, S. Microfiltration and ultrafiltration membranes for drinking water. J. Am. Water Work. Assoc. 2008, 100, 84–97. [Google Scholar]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Archilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, B. Microfiltration, ultrafiltration, nanofiltration, reverse osmosis, and forward osmosis. Fundam. Modeling Membr. Syst. 2018, 25–70. [Google Scholar]
- Singh, R.; Hoffmann, E.J.; Judd, S. Membrane Technology and Engineering for Water Purification; Membranes Technology ebook Collection; Elsevier: Amsterdam, Netherlands, 2008. [Google Scholar]
- Krupp, A.U.; Griffiths, M.; Please, C.P. Stochastic modelling of membrane filtration. Proc. R. Soc. 2017, 473, 20160948. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ngo, H.-H.; Li, J. A mini review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lohaus, J.; Perez, Y.M.; Wessling, M. What are the microscopic events of colloidal membrane fouling? J. Membr. Sci. 2018, 553, 90–98. [Google Scholar] [CrossRef]
- Griffiths, I.M.; Kumar, A.; Stewart, P.S. A combined network model for membrane fouling. J. Colloid Interface Sci. 2014, 432, 10–18. [Google Scholar] [CrossRef]
- Park, S.; Baek, S.-S.; Pyo, J.C.; Pachepsky, Y.; Park, J.; Cho, K.H. Deep neural networks for modeling fouling growth and flux decline during NF/RO membrane filtration. J. Membr. Sci. 2019, 587, 117164. [Google Scholar] [CrossRef]
- Shetty, G.R.; Chellam, S. Predicting membrane fouling during municipal drinking water nanofiltration using artificial neural networks. J. Membr. Sci. 2003, 217, 69–86. [Google Scholar] [CrossRef]
- Ando, T.; Akamatsu, K.; Fujita, M.; Nakao, S. Direct Simulation Model of Concentrated Particulate Flow in Pressure-Driven Dead-End Microfiltration. J. Chem. Eng. Jpn. 2010, 43, 815–828. [Google Scholar] [CrossRef][Green Version]
- Mino, Y.; Sakai, S.; Matsuyama, H. Simulations of particulate flow passing through membrane pore under dead-end and constant pressure filtration condition. Chem. Eng. Sci. 2018, 190, 68–76. [Google Scholar] [CrossRef]
- Abdoli, S.M.; Shafiei, S.; Raoof, A.; Ebadi, A.; Jafarzadeh, Y.; Aslannejad, H. Water Flux Reduction in Microfiltration Membranes: A Pore Network Study. Chem. Eng. Technol. 2018, 41, 1566–1576. [Google Scholar] [CrossRef]
- Xiong, Q.; Baychev, T.G.; Jivkov, A.P. Review of pore network modeling of porous media: Experimental characterizations, network constructions and applications to reactive transport. J. Contam. Hydrol. 2016, 192, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Beuscher, U. Modeling Sieving Filtration Using Multiple Layers of Parallel Pores. Chem. Eng. Technol. 2010, 33, 1377–1381. [Google Scholar] [CrossRef]
- Bowen, R.W.; Joes, M.G.; Welfoot, J.S.; Yousef, H.N.S. Predicting salt rejections at nanofiltration membranes using artificial neural networks. Desalination 2000, 129, 147–162. [Google Scholar] [CrossRef]
- Baeldung. Genetic Algorithms vs Neural Networks. Baeldung. 2020. Available online: https://www.baeldung.com/cs/genetic-algorithms-vs-neural-networks (accessed on 30 June 2020).
- Hua, F.; Fang, Z.; Qiu, T. Modeling Ethylene Cracking Process by Learning Convolutional Neural Network. Comput. Aided Chem. Eng. 2000, 44, 841–846. [Google Scholar]
- Liu, Q.-F.; Kim, S.-H. Evaluation of membrane fouling models based on bench-scale experiments: A comparison between constant flowrate blocking laws and artificial neural network (ANNs) model. J. Membr. Sci. 2008, 310, 393–401. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Bednarska, A.; Gradkowski, M. Scanning Electron Microscopy (SEM) in the Analysis of the Structure of Polymeric Nanofiltration Membranes. Probl. Eksploat.-Maint. 2016, 100, 119–128. [Google Scholar]
- De Rover, E.W.F.; Huisman, I.H. Microscopy as a tool for analysis of membrane failure and fouling. Desalination 2006, 207, 35–44. [Google Scholar] [CrossRef]
- Standfort, V.; Yan, K.; Pickhardt, P.J.; Summers, R.M. Data augmentation using generative adversarial networks (CycleGAN) to improve generalizability in CT segmentation tasks. Sci. Rep. 2019, 9, 16884. [Google Scholar] [CrossRef]
- Nicholson, C. A Beginner’s Guide to Generative Adversial Networks (GANs). 2019. Available online: https://skymind.ai/wiki/generative-adversarial-network-gan (accessed on 14 October 2019).
- Tokyo Metropolitan Waterworks, Water Quality Report. Available online: https://www.waterworks.metro.tokyo.jp/data/suigen/kekka30/j003-4.pdf (accessed on 30 June 2020).
- Bovik, A.C. Basic Gray Level Image Processing. In The Essential Guide to Image Processing; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Deshpande, A. A Beginner’s Guide to Understand Convolutional Neural Networks. 2016. Available online: https://adeshpande3.github.io/adeshpande3.github.io/A-Beginner’s-Guide-To-Understanding-Convolutional-Neural-Networks/ (accessed on 26 June 2019).
- Brownlee, J. Generative Adversarial Networks with Python: Deep Learning Generative Models for Image Synthesis and Image Translation; Machine Learning Mastery: Vermont, VIC, Australia, 2019. [Google Scholar]
- Odena, A.; Olah, C.; Shlens, J. Conditional Image Synthesis with Auxiliary Classifiers GANs. In Proceedings of the 34th International Conference on Machine Learning, Sydney, Australia, 6–11 August 2017. [Google Scholar]
- Radford, A.; Chintala, S. Unsupervised Learning with Deep Convolutional Generative Adversial Networks. Machine Learning; Cornell University: Ithaca, NY, USA, 2017. [Google Scholar]
- Brownlee, J. How to Code Algorithms from Scratch with Python. In What Is a Confusion Matrix in Machine Learning; Machine Learning Mastery: Vermont, VIC, Australia, 2019. [Google Scholar]
- Salton do Prado, K. How DBSCAN Works and Why Should We Use it. 2017. Available online: https://towardsdatascience.com/how-dbscan-works-and-why-should-i-use-it-443b4a191c80 (accessed on 20 April 2020).
- Wright Muelas, M.; Mughal, F.; O’Hagan, S.; Day, P.J.; Kell, D.B. The role and robustness of the Gini coefficient as an unbiased tool for the selection of Gini genes for normalizing expression profiling data. Sci. Rep. 2019, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.L.; Bai, R. Membrane fouling and cleaning in microfiltration of activated sludge wastewater. J. Membr. Sci. 2003, 216, 279–290. [Google Scholar] [CrossRef]
- Raschka, S.; Mirjalili, V. Python Machine Learning, 2nd ed.; Packt Publishing Ltd.: Birmingham, UK, 2017. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciatori, C.; Hashimoto, T.; Takizawa, S. Modeling and Analysis of Particle Deposition Processes on PVDF Membranes Using SEM Images and Image Generation by Auxiliary Classifier Generative Adversarial Networks. Water 2020, 12, 2225. https://doi.org/10.3390/w12082225
Cacciatori C, Hashimoto T, Takizawa S. Modeling and Analysis of Particle Deposition Processes on PVDF Membranes Using SEM Images and Image Generation by Auxiliary Classifier Generative Adversarial Networks. Water. 2020; 12(8):2225. https://doi.org/10.3390/w12082225
Chicago/Turabian StyleCacciatori, Caterina, Takashi Hashimoto, and Satoshi Takizawa. 2020. "Modeling and Analysis of Particle Deposition Processes on PVDF Membranes Using SEM Images and Image Generation by Auxiliary Classifier Generative Adversarial Networks" Water 12, no. 8: 2225. https://doi.org/10.3390/w12082225
APA StyleCacciatori, C., Hashimoto, T., & Takizawa, S. (2020). Modeling and Analysis of Particle Deposition Processes on PVDF Membranes Using SEM Images and Image Generation by Auxiliary Classifier Generative Adversarial Networks. Water, 12(8), 2225. https://doi.org/10.3390/w12082225







