Modelling the Mineralization of Formaldehyde by Treatment with Nitric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Experimental Design
3. Results and Discussion
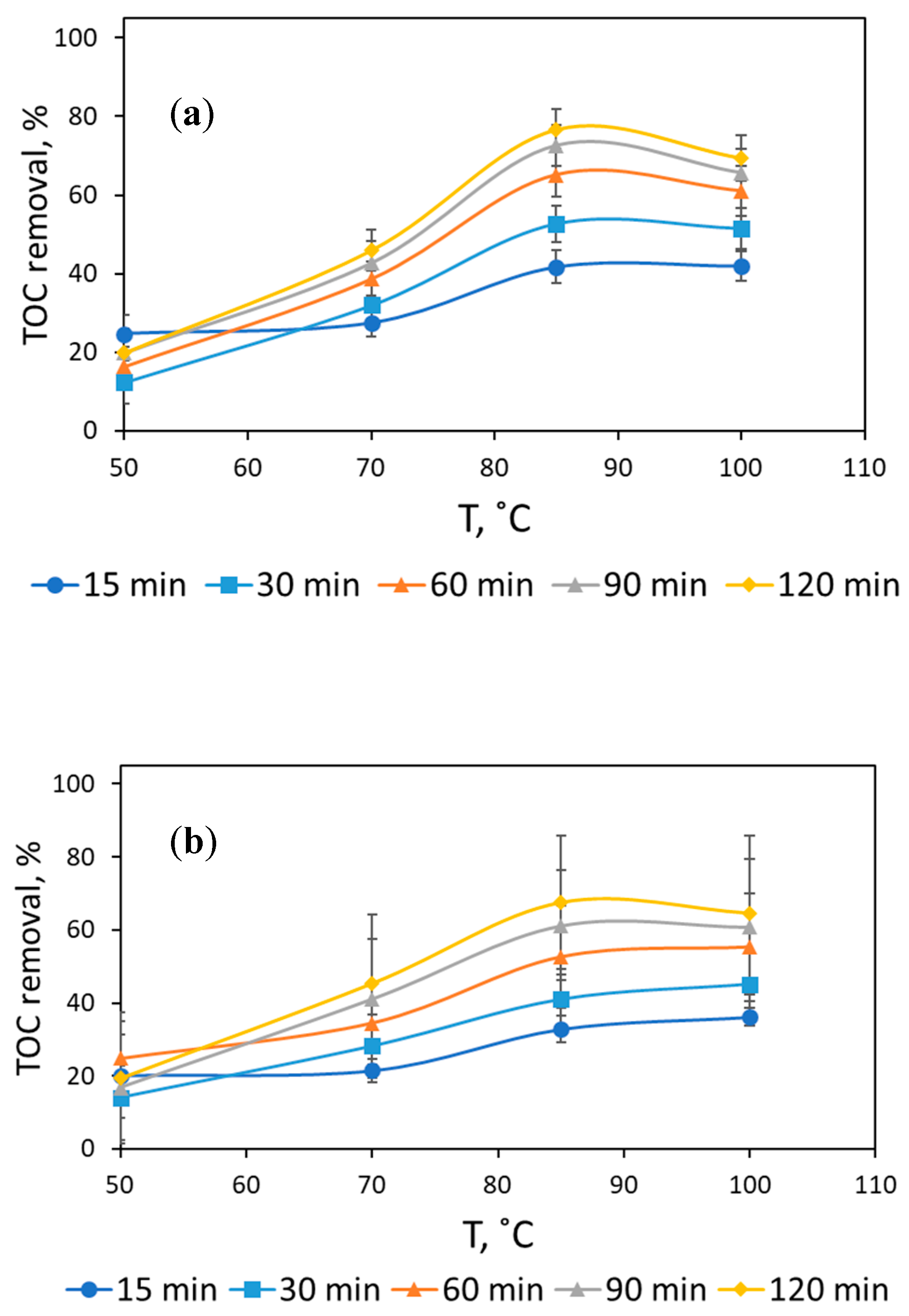
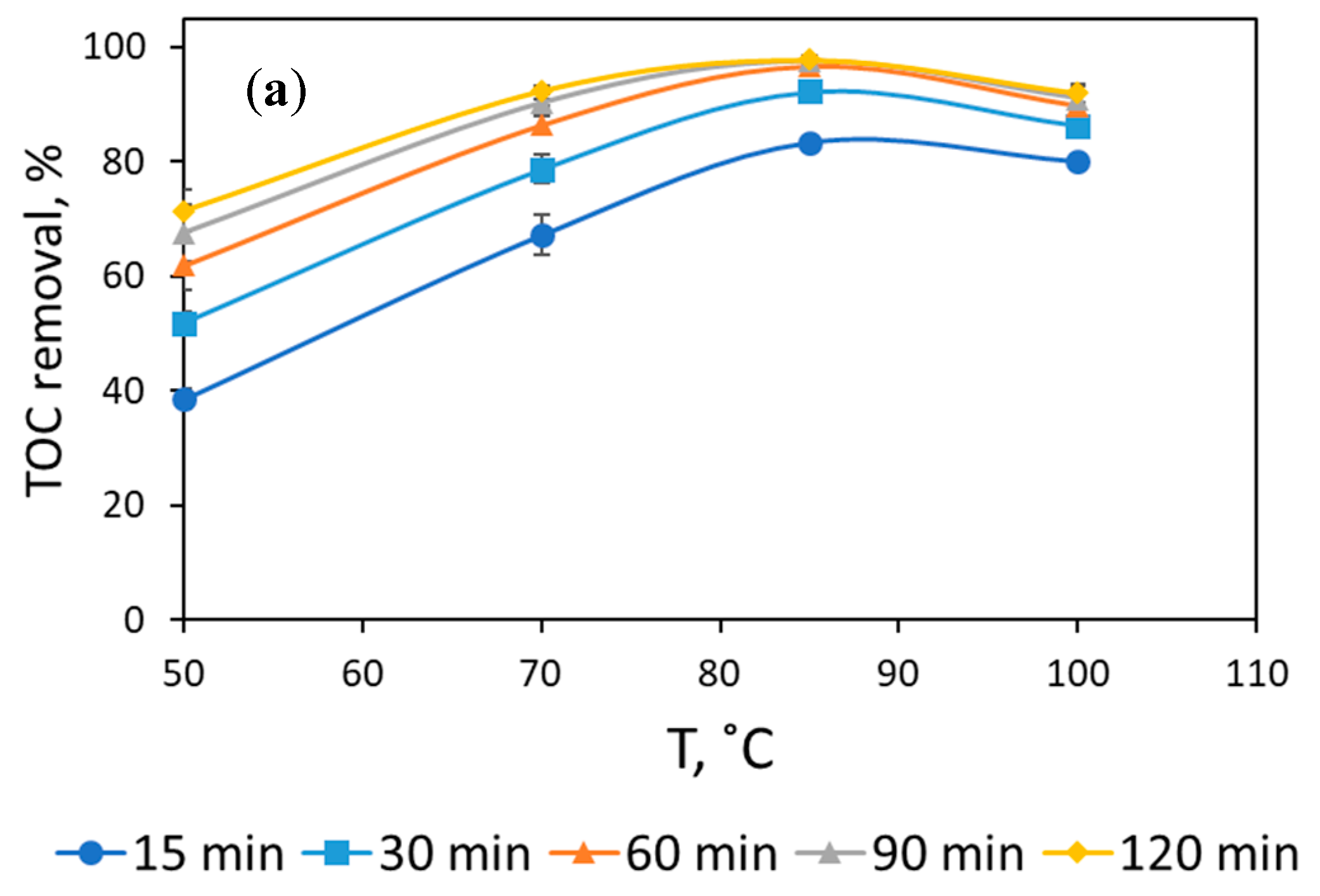
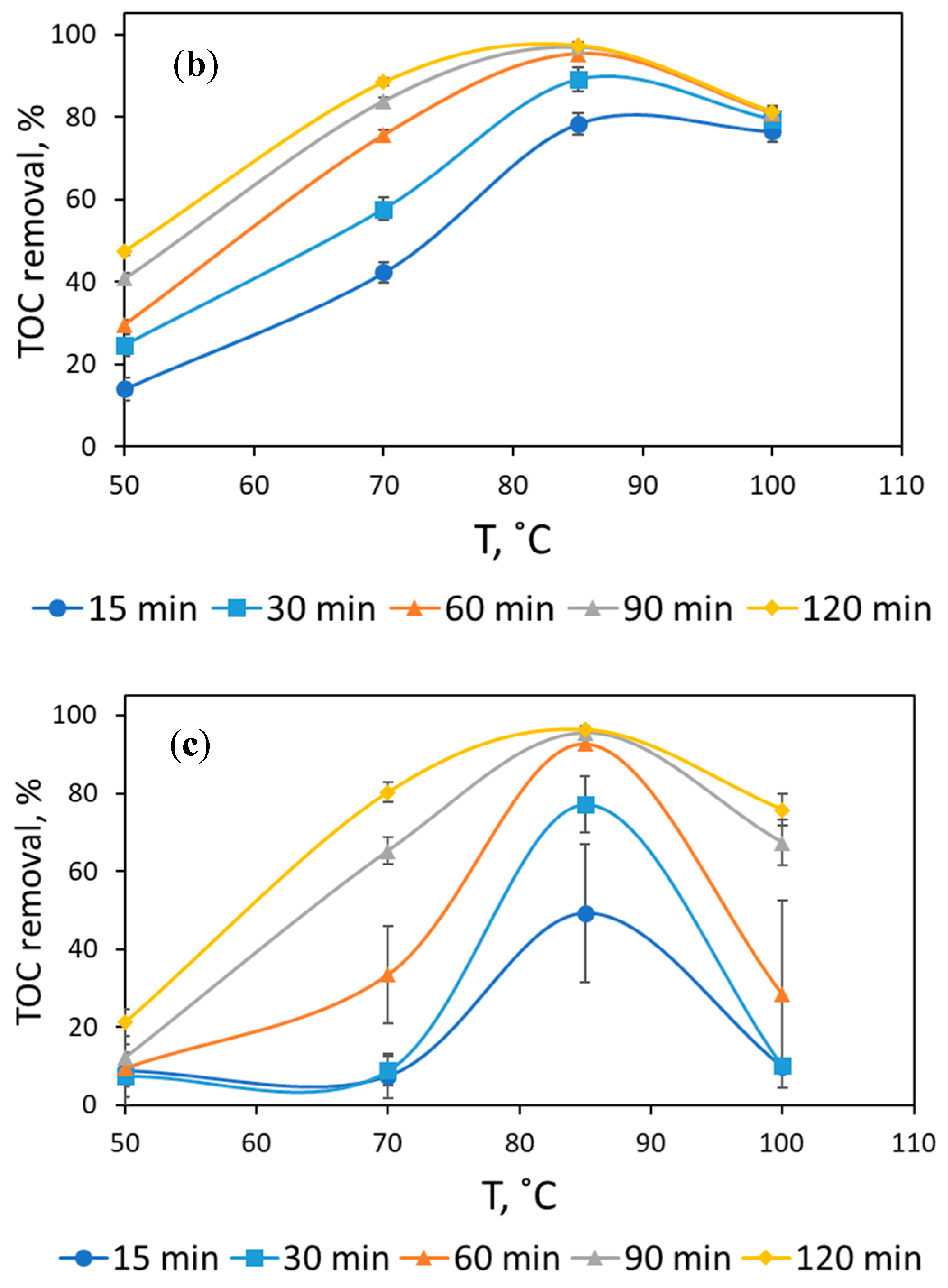
3.1. Assessment of Reaction Conditions to Mineralize Formaldehyde
3.2. Statistical Analysis
− 0.0033·NaNO22 + 0.26·NaNO2·HNO3 + 0.0037·NaNO2·T +
+ 8.81·HNO32 + 0.48·HNO3·T − 0.012·T2
− 0.0022·NaNO22 + 0.065·NaNO2·HNO3 − 0.0010·NaNO2·T +
+ 2.52·HNO32 + 0.42·HNO3·T − 0.015·T2
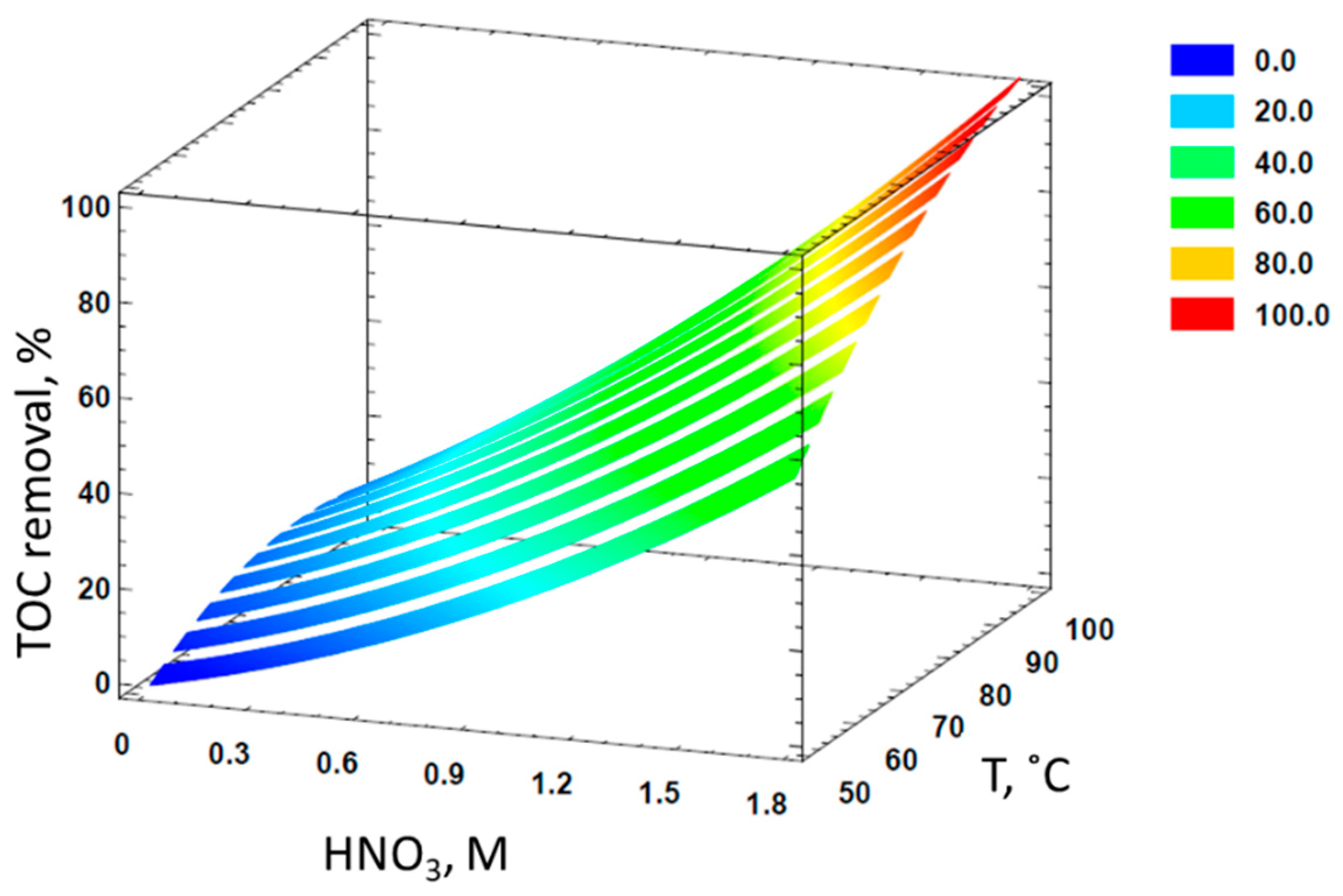
3.3. Response Surface Methodology to Model the Mineralization of Formaldehyde
NaNO2 + 2.52 · HNO32 − 0.022 · NaNO22
3.4. Assessment of the Influence of HNO3/CH2O Ratio
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, N.; Zaiat, M. Degradation of formaldehyde in anaerobic sequencing batch biofilm reactor (ASBBR). J. Hazard. Mater. 2009, 163, 777–782. [Google Scholar] [CrossRef]
- Garrido, J.; Méndez, R.; Lema, J. Treatment of wastewaters from a formaldehyde-urea adhesives factory. Water Sci. Technol. 2000, 42, 293–300. [Google Scholar] [CrossRef]
- Wieslander, G.; Norbäck, D.; Björnsson, E.; Janson, C.; Boman, G. Asthma and the indoor environment: The significance of emission of formaldehyde and volatile organic compounds from newly painted indoor surfaces. Int. Arch. Occup. Environ. Health 1996, 69, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M. Environmental Toxicants: Human Exposures and Their Health Effects, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000; ISBN 978-0-471-79335-9. [Google Scholar]
- Babu, B.R.; Parande, A.; Raghu, S.; Kumar, T.P. Textile technology. An Overview of Wastes Produced During Cotton Textile Processing and Effluent Treatment Methods. J. Cotton Sci. 1995, 11, 110–122. [Google Scholar]
- Carlson, R.M.; Smith, M.C.; Nedorost, S.T. Diagnosis and treatment of dermatitis due to formaldehyde resins in clothing. Dermatitis 2004, 15, 169–175. [Google Scholar]
- Costa, N.; Pereira, J.A.M.; Martins, J.M.; Ferra, J.; Cruz, P.; Magalhães, F.D.; Mendes, A.M.; Carvalho, L. Alternative to latent catalysts for curing UF resins used in the production of low formaldehyde emission wood-based panels. Int. J. Adhes. Adhes. 2012, 33, 56–60. [Google Scholar] [CrossRef]
- Celik, F.E.; Lawrence, H.; Bell, A.T. Synthesis of precursors to ethylene glycol from formaldehyde and methyl formate catalyzed by heteropoly acids. J. Mol. Catal. A Chem. 2008, 288, 87–96. [Google Scholar] [CrossRef]
- Guven, B.B.; Olaguer, E.P. Ambient formaldehyde source attribution in Houston during TexAQS II and TRAMP. Atmos. Environ. 2011, 45, 4272–4280. [Google Scholar] [CrossRef]
- Moussavi, G.; Yazdanbakhsh, A.; Heidarizad, M. The removal of formaldehyde from concentrated synthetic wastewater using O3/MgO/H2O2 process integrated with the biological treatment. J. Hazard. Mater. 2009, 171, 907–913. [Google Scholar] [CrossRef]
- Yamazaki, T.; Tsugawa, W.; Sode, K. Biodegradation of formaldehyde by a formaldehyde-resistant bacterium isolated from seawater. Appl. Biochem. Biotech. 2001, 91, 213–217. [Google Scholar] [CrossRef]
- Ezhilkumar, P.; Sivakumar, V.; Yogasri, A.; Thirumarimurugan, M. Biodegradation of formaldehyde using Bacillus subtilis in batch process. Int. J. Mater. Prod. Technol. 2017, 55, 296–307. [Google Scholar] [CrossRef]
- De Oliveira, S.V.W.B.; Moraes, E.; Adorno, M.; Varesche, M.; Foresti, E.; Zaiat, M. Formaldehyde degradation in an anaerobic packed-bed bioreactor. Water Res. 2004, 38, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Cloteaux, A.; Gerardin, F.; Thomas, D.; Midoux, N.; André, J.-C. Fixed bed photocatalytic reactor for formaldehyde degradation: Experimental and modeling study. Chem. Eng. J. 2014, 249, 121–129. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Xiao, S.; Zhan, X.; Li, J. Enhancement of photo-catalytic degradation of formaldehyde through loading anatase TiO2and silver nanoparticle films on wood substrates. RSC Adv. 2015, 5, 52985–52992. [Google Scholar] [CrossRef]
- Ubolchonlakate, K.; Sikong, L.; Tontai, T. Formaldehyde degradation by photocatalytic Ag-doped TiO2 film of glass fiber roving. J. Nanosci. Nanotechnol. 2010, 10, 7522–7525. [Google Scholar] [CrossRef]
- Hong, Q.; Sun, D.-Z.; Chi, G.-Q. Formaldehyde degradation by UV/TiO2/O3 process using continuous flow mode. J. Environ. Sci. 2007, 19, 1136–1140. [Google Scholar]
- Kajitvichyanukul, P.; Lu, M.-C.; Jamroensan, A. Formaldehyde degradation in the presence of methanol by photo-Fenton process. J. Environ. Manag. 2008, 86, 545–553. [Google Scholar] [CrossRef]
- Moussavi, G.; Bagheri, A.; Khavanin, A. The investigation of degradation and mineralization of high concentrations of formaldehyde in an electro-Fenton process combined with the biodegradation. J. Hazard. Mater. 2012, 237, 147–152. [Google Scholar] [CrossRef]
- Parisheva, Z.; Nusheva, L.; Danova, N. Advanced oxidation of solutions containing formaldehyde part 1. Combined effect of ozone and hydrogen peroxide. Environ. Prot. Eng. 2003, 29, 5–14. [Google Scholar]
- Healy, T.V. The Reaction of nitric acid with formaldehyde and with formic acid and its application to the removal of nitric acid from mixtures. J. Appl. Chem. 2007, 8, 553–561. [Google Scholar] [CrossRef]
- Mishra, S.; Lawrence, F.; Sreenivasan, R.; Pandey, N.; Mallika, C.; Koganti, S.B.; Mudali, U.K. Development of a continuous homogeneous process for denitration by treatment with formaldehyde. J. Radioanal. Nucl. Chem. 2010, 285, 687–695. [Google Scholar] [CrossRef]
- Kumar, S.V.; Nadkarni, M.N.; Mayankutty, P.C.; Pillai, N.S.; Shinde, S.S. Destruction of Nitric Acid in Purex Process Streams by Formaldehyde Treatment; Bhabha Atomic Research Centre: Trombay, Mumbai, 1974.
- Horváth, M.; Lengyel, I.; Bazsa, G. Kinetics and mechanism of autocatalytic oxidation of formaldehyde by nitric acid. Int. J. Chem. Kinet. 1988, 20, 687–697. [Google Scholar] [CrossRef]
- Sugaya, N.; Nakagawa, T.; Sakurai, K.; Morita, M.; Onodera, S. Analysis of Aldehydes in Water by Head Space-GC/MS. J. Health Sci. 2001, 47, 21–27. [Google Scholar] [CrossRef][Green Version]
- Álava, A.F.B.; Lucas, L.M.G.; Villamar, J.D.C.; Acebo, M.R.A.; Toala, T.O.M.; Parrales, T.M.M. Elementos de Química Básica; 3Ciencias: Alicante, Spain, 2018. [Google Scholar]
- Liu, Y.; Wang, J.; Zheng, Y.; Wang, A. Adsorption of methylene blue by kapok fiber treated by sodium chlorite optimized with response surface methodology. Chem. Eng. J. 2012, 184, 248–255. [Google Scholar] [CrossRef]

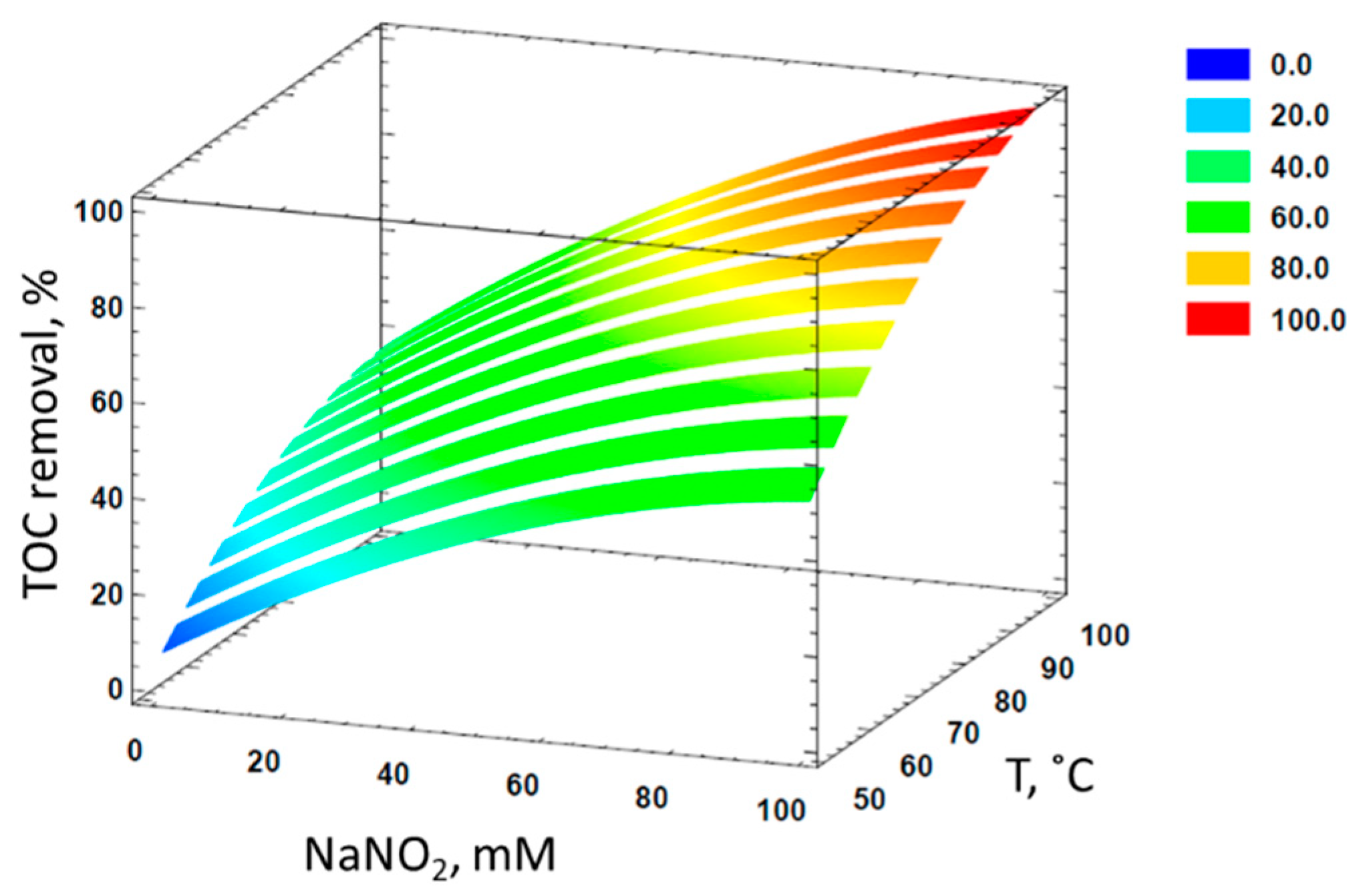
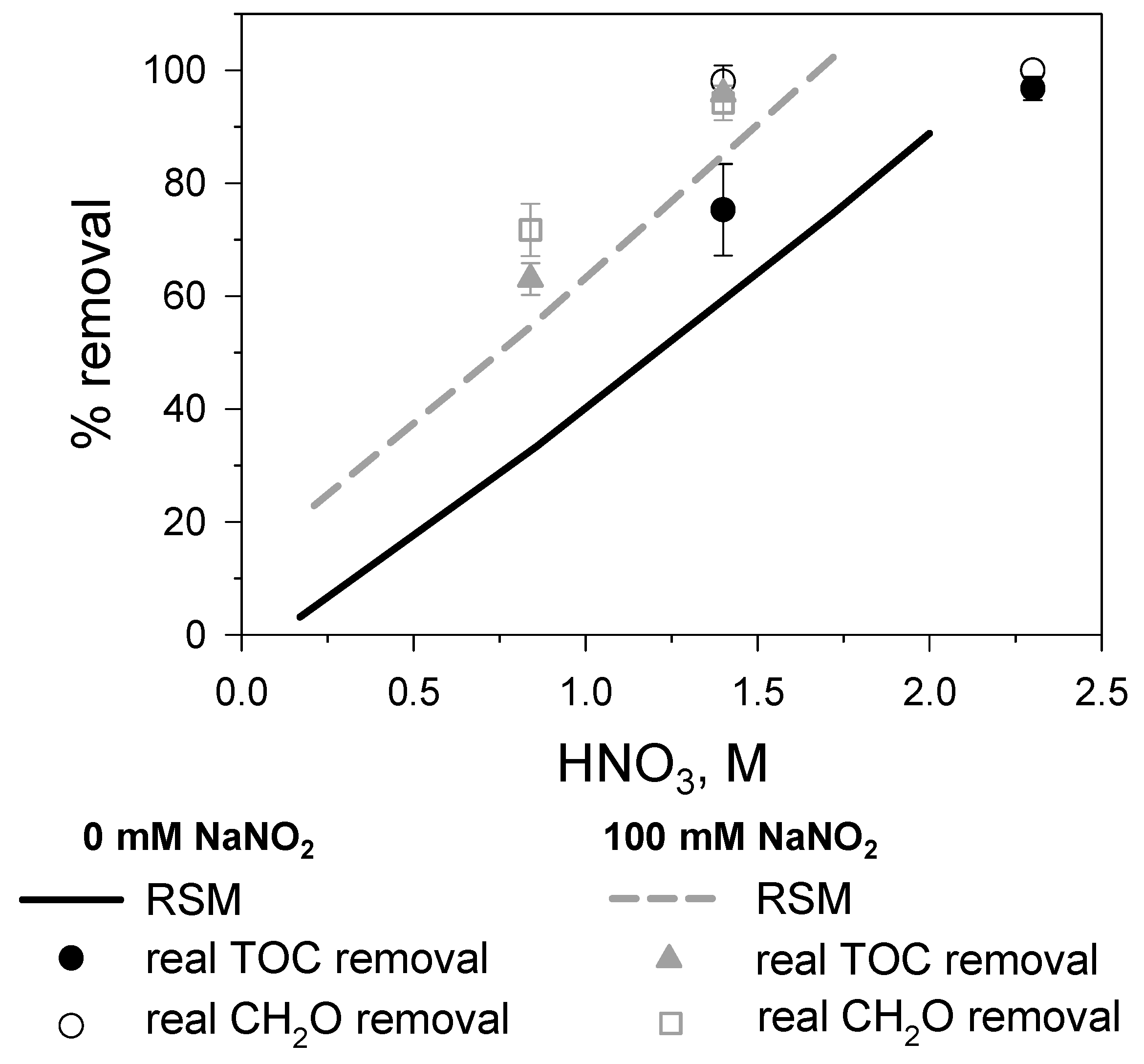
| NaNO2, mM | HNO3, M | T, °C | Y1,experimental, % | Y1,predicted, % | Y2,experimental, % | Y2,predicted, % |
|---|---|---|---|---|---|---|
| 0 | 0.17 | 50 | 0.7 | −2.5 | 1.4 | −16.0 |
| 0 | 0.17 | 70 | 0.7 | 3.3 | 2.8 | −0.4 |
| 0 | 0.17 | 85 | 5.6 | 1.0 | 4.9 | 3.1 |
| 0 | 0.17 | 100 | 2.8 | −6.9 | 7.6 | −0.2 |
| 0 | 0.86 | 50 | 2.1 | −4.3 | 0.0 | 4.2 |
| 0 | 0.86 | 70 | 0.7 | 8.1 | 1.4 | 25.5 |
| 0 | 0.86 | 85 | 0 | 10.7 | 2.1 | 33.5 |
| 0 | 0.86 | 100 | 4.2 | 7.8 | 40.0 | 34.5 |
| 0 | 1.72 | 50 | 7.5 | 5.2 | 21.2 | 32.6 |
| 0 | 1.72 | 70 | 8.9 | 25.8 | 80.2 | 61.2 |
| 0 | 1.72 | 85 | 77.2 | 34.6 | 96.3 | 74.6 |
| 0 | 1.72 | 100 | 10.2 | 37.8 | 75.8 | 81.1 |
| 50 | 0.17 | 50 | 8.9 | 7.1 | 7.5 | 0.1 |
| 50 | 0.17 | 70 | 8.3 | 16.5 | 5.5 | 14.5 |
| 50 | 0.17 | 85 | 5 | 17.0 | 11.4 | 17.4 |
| 50 | 0.17 | 100 | 10.9 | 11.9 | 3.1 | 13.3 |
| 50 | 0.86 | 50 | 14.1 | 14.4 | 19.5 | 22.4 |
| 50 | 0.86 | 70 | 28.2 | 30.5 | 45.4 | 42.7 |
| 50 | 0.86 | 85 | 41 | 35.9 | 67.4 | 49.9 |
| 50 | 0.86 | 100 | 45.1 | 35.7 | 64.5 | 50.2 |
| 50 | 1.72 | 50 | 24.6 | 35.3 | 47.4 | 53.6 |
| 50 | 1.72 | 70 | 57.7 | 59.5 | 88.4 | 81.2 |
| 50 | 1.72 | 85 | 89 | 71.2 | 97.3 | 93.8 |
| 50 | 1.72 | 100 | 79.4 | 77.1 | 81.2 | 99.6 |
| 100 | 0.17 | 50 | 10.5 | 0.2 | 10.5 | 5.3 |
| 100 | 0.17 | 70 | 11.2 | 13.4 | 9.1 | 18.7 |
| 100 | 0.17 | 85 | 10.5 | 16.6 | 10.5 | 20.8 |
| 100 | 0.17 | 100 | 16.8 | 14.2 | 18.2 | 15.9 |
| 100 | 0.86 | 50 | 12.3 | 16.7 | 19.9 | 29.9 |
| 100 | 0.86 | 70 | 31.9 | 36.4 | 46.0 | 49.2 |
| 100 | 0.86 | 85 | 52.7 | 44.6 | 76.8 | 55.6 |
| 100 | 0.86 | 100 | 51.4 | 47.2 | 69.5 | 55.1 |
| 100 | 1.72 | 50 | 51.8 | 48.9 | 71.5 | 63.9 |
| 100 | 1.72 | 70 | 78.7 | 76.9 | 92.4 | 90.4 |
| 100 | 1.72 | 85 | 92.2 | 91.2 | 97.8 | 102.3 |
| 100 | 1.72 | 100 | 86.4 | 100.0 | 92.0 | 107.2 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| x1: NaNO2 concentration | 6272.5 | 1 | 6272.5 | 36.46 | 0 | Significant |
| x2: HNO3 concentration | 13,034.8 | 1 | 13,034.8 | 75.77 | 0 | Significant |
| x3: Temperature | 2665.5 | 1 | 2665.5 | 15.49 | 0.0006 | Significant |
| x12 | 541.2 | 1 | 541.2 | 3.15 | 0.0878 | |
| x1·x2 | 1688.8 | 1 | 1688.8 | 9.82 | 0.0042 | Significant |
| x1·x3 | 279.0 | 1 | 279.0 | 1.62 | 0.2141 | |
| x22 | 217.5 | 1 | 217.5 | 1.26 | 0.2711 | |
| x2·x3 | 1131.3 | 1 | 1131.3 | 6.58 | 0.0165 | Significant |
| x32 | 450.3 | 1 | 450.3 | 2.62 | 0.1177 | |
| Total error | 4472.7 | 26 | 172.0 | |||
| Total (cor.) | 31,483.7 | 35 | ||||
| Square-R = 85.79% | ||||||
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| x1: NaNO2 concentration | 3344.6 | 1 | 3344.6 | 15.72 | 0.0005 | Significant |
| x2: HNO3 concentration | 29,209.4 | 1 | 29,209.4 | 137.26 | 0 | Significant |
| x3: Temperature | 4294.7 | 1 | 4294.7 | 20.18 | 0.0001 | Significant |
| x12 | 232.2 | 1 | 232.2 | 1.09 | 0.3058 | |
| x1·x2 | 101.4 | 1 | 101.4 | 0.48 | 0.4962 | |
| x1·x3 | 21.8 | 1 | 21.8 | 0.10 | 0.7515 | |
| x22 | 17.8 | 1 | 17.8 | 0.08 | 0.7746 | |
| x2·x3 | 884.4 | 1 | 884.4 | 4.16 | 0.0518 | |
| x32 | 675.9 | 1 | 675.9 | 3.18 | 0.0864 | |
| Total error | 5532.8 | 26 | 212.8 | |||
| Total (cor.) | 45,265.1 | 35 | ||||
| Square R = 87.78% | ||||||
| HNO3, M | T, °C | NaNO2, mM | CH2O, mg/L | HNO3/CH2O Molar Ratio | % CH2O Removed |
|---|---|---|---|---|---|
| 5.7 | 100 | 0 | 1465 | 117 | 100 |
| 5.7 | 85 | 0 | 1737 | 99 | 100 |
| 5.7 | 70 | 0 | 1737 | 99 | 100 |
| 4.35 | 85 | 0 | 1363 | 96 | 100 |
| 4.35 | 85 | 0 | 1460 | 89 | 100 |
| 4.35 | 70 | 0 | 927 | 141 | 95.3 |
| 2.3 | 100 | 0 | 1205 | 57 | 100 |
| 2.3 | 85 | 0 | 1778 | 39 | 100 |
| 2.3 | 70 | 0 | 1416 | 49 | 100 |
| 1.7 | 85 | 100 | 2204 | 22 | 97.7 |
| 1.7 | 85 | 100 | 2326 | 22 | 92.4 |
| 1.4 | 85 | 0 | 1768 | 24 | 100 |
| 1.4 | 70 | 0 | 2001 | 21 | 100 |
| 0.91 | 85 | 100 | 2278 | 12 | 68.4 |
| 0.91 | 70 | 100 | 2278 | 12 | 58.7 |
| 0.20 | 100 | 100 | 3920 | 1.5 | 77.3 |
| 0.20 | 70 | 100 | 3920 | 1.5 | 77.8 |
| 0.176 | 85 | 0 | 2399 | 2.2 | 20 |
| 0.176 | 70 | 0 | 2399 | 2.2 | 11 |
| 0.066 | 70 | 0 | 1922 | 1.0 | 29.1 |
| 0.066 | 85 | 0 | 1922 | 1.0 | 13 |
| HNO3, M | T, °C | NaNO2, mM | CH2O, mg/L | HNO3/CH2O Molar Ratio | % CH2O Removed |
|---|---|---|---|---|---|
| 5.5 | 100 | 0 | 43,600 | 3.8 | 99.9 |
| 2.4 | 100 | 0 | 43,600 | 1.7 | 99.8 |
| 1.5 | 100 | 0 | 43,600 | 1.0 | 98.4 |
| 0.66 | 100 | 0 | 43,600 | 0.5 | 98.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merayo, N.; Balea, A.; Tejera, J.; Garrido-Escudero, A.; Negro, C.; Blanco, A. Modelling the Mineralization of Formaldehyde by Treatment with Nitric Acid. Water 2020, 12, 1567. https://doi.org/10.3390/w12061567
Merayo N, Balea A, Tejera J, Garrido-Escudero A, Negro C, Blanco A. Modelling the Mineralization of Formaldehyde by Treatment with Nitric Acid. Water. 2020; 12(6):1567. https://doi.org/10.3390/w12061567
Chicago/Turabian StyleMerayo, Noemi, Ana Balea, Javier Tejera, Amalio Garrido-Escudero, Carlos Negro, and Angeles Blanco. 2020. "Modelling the Mineralization of Formaldehyde by Treatment with Nitric Acid" Water 12, no. 6: 1567. https://doi.org/10.3390/w12061567
APA StyleMerayo, N., Balea, A., Tejera, J., Garrido-Escudero, A., Negro, C., & Blanco, A. (2020). Modelling the Mineralization of Formaldehyde by Treatment with Nitric Acid. Water, 12(6), 1567. https://doi.org/10.3390/w12061567








