1. Introduction
Transport is a key point for the overall economy and its efficient services are essential in a globalized world. For this reason, the past decade has seen a wide development of roads and railways for improving it. Tunnel construction (i.e., for underground or subsoil roads) can play an important role in minimizing surface impacts, in reducing urban congestion and in avoiding deforestation in the rural areas [
1,
2,
3] and, in some cases, tunnels are necessary for completing railing and road webs when a mountain area is present. However, tunneling excavation projects require the assessment of their sustainability before, during and after their implementation.
In the last few years, tunnel mechanized excavation with EPB-TBMs (Earth Pressure Balance-Tunnel Boring Machines) made it possible to perform more efficient, rapid and safe works. The use of foaming agents (FAs) and additives, such as anti-clogging or anti-wear polymers, as lubricants is necessary for enhancing the mechanized excavation process with EPB-TBMs, reducing the hard rock abrasiveness and facilitating excavated material transportation [
4,
5]. FAs are generally water solutions of anionic surfactants and are typically used in tunneling excavations between 0.1 and 3 L/m
3 of soil [
6]. Sodium lauryl ether sulfate (SLES) is the main component of most FAs, in percentages variable from 5 to 50%. In some cases, other minor components are also present [
7,
8].
The management of many millions of tons of excavated soil mixed to FAs containing SLES residues (spoil material) can be an environmental issue for both the excavation industry and society if it is not well planned. In fact, if spoil material cannot be reused, it has to be treated as a waste [
9]. On the contrary, its possible reuse as a by-product for industrial (aggregate for concrete, building and infrastructure construction) or green area use (e.g., land covering, public green areas, including with artificial ponds) and has the undoubted advantages of lowering project costs, recycling a non-renewable natural resource (soil), and avoiding occupying large areas for waste disposal [
8]. On the other hand, SLES and other minor chemicals occurring in soil debris may pose an environmental risk at concentrations which are toxic for biota [
6,
10,
11,
12]. Although at the FA amounts generally used in tunneling SLES residual concentrations in the spoil material are not toxic for terrestrial organisms, they can be potentially hazardous for aquatic ones because of their sensitivity to surfactant presence [
6,
12,
13]. In particular, if at the final destination site (e.g., renaturation areas or refilling of roads and quarries close to water bodies) aquatic contamination is possible, the leaching of SLES from soil into underlying aquifers or nearby rivers has to be taken into consideration in the tunnel project plan [
14]. Consequently, a correct evaluation of SLES residual concentrations and ecotoxicity of spoil material is definitely required for assessing its possible reuse and protect human and ecosystem health [
15].
Recent studies conducted in microcosm experiments using real soil from excavation sites, showed that the anionic surfactant SLES is degradable in spoil material and its depletion can occur during the temporary storage at the construction site. These studies showed that SLES can be degraded by environmental bacteria, with variable rates which depend on site-specific biotic (bacterial abundance) and abiotic (e.g., soil type, temperature) factors [
6,
8,
16]. In principle, whenever possible, the storage in temporary deposit areas of spoil material makes the natural degradation of SLES possible and, consequently, reduces or blanks its possible toxic effects on the environment. The use of ecotoxicological tests on target organisms is an effective approach for assessing the storage time required for spoil material before establishing any reuse of spoil material, in order to avoid potential environmental risks [
6,
12,
13,
17]. Moreover, the ecotoxicological responses make it possible to evaluate the overall effects of a chemical or a mixture of chemicals occurring in soil treated with FAs, including minor or unknown ones [
18,
19,
20,
21].
The aim of this work was the evaluation of the environmental fate and possible effects of a foaming agent in soil debris from an underground EPB-TBM excavation. The foaming agent was tested at different real concentrations on four different soils alone or together with a polymer. For this purpose, the soils were collected from an excavation area and were conditioned with the least ecotoxic foaming agent (consisting of a water solution of SLES at 5–10% of the product) previously selected using a toxicity screening with the bacterium
V. fischeri. The FA conditioned soils were maintained for 28 days in laboratory microcosms for simulating spoil material storage at the construction site. Soil sub-samples were collected from each microcosm to carry out chemical (SLES residual concentration in soils and elutriates), microbiological (microbial abundance) and ecotoxicological (a battery of tests using five different species) analyses. The tests were performed both on conditioned soils (with the ostracod
Heterocypris incongruens and the worm
Eisenia foetida) and the corresponding soil water extracts (with the luminescent bacteria
Vibrio fischeri, the plant
Lepidium sativum and the fish embryo
Danio rerio). In this study, the following hypothesis were tested: (i) If the concentration of SLES in the soil water extracts was lower/equal to 2 mg/L, soil debris was not toxic for any organism tested and could be used as a by-product; (ii) The
Vibrio fischeri test was the most sensitive to the anionic surfactant [
17].
4. Discussion
The present work shows the application of an ecological approach for the evaluation of environmental fate and the effects of foaming agents in soil debris from underground EPB-TBM excavation. It combined the analysis of SLES and the performances of ecotoxicological tests at different times (0, 7, 14 and 28 days) in both FA-conditioned soils and the corresponding elutriates, simulating the storage of the spoil material at the construction site.
The anionic surfactant decreased in soils at different rates (DT
50 ranged from 9 to 25 days). Since the overall experimental conditions (temperature, light, pH values) were similar. The fact that SLES was degraded more (S4 + F, S4 + F + P and S2 + F) in some soils than in others (S1 + F and S3 + F + P) can be ascribed to the different soil lithological characteristics and, above all, the microbial abundance. In fact, a positive correlation was found between average microbial numbers and SLES half-lives (DT
50s), confirming the role of microorganisms in degrading this anionic surfactant [
6,
8,
16]. The slowest disappearance rate in S1 + F can be also ascribed to the low water content of this soil (15% lower than in the other ones), which is known to influence biodegradation.
Following the SLES decrease in soils, lower amounts of surfactant were gradually also found in the corresponding elutriates. Elutriates were obtained in accordance with a standard procedure [
27], which is used for simulating the leaching of a substance from soil to water and representing the worst scenario (no degradation) at day 0. In reality, SLES was leached from soil to water even at concentrations quite low at day 0 and negligible from day 7. Moreover, the percentage of SLES washed out from soils was not constant and comprised the range 8–28%, indicating a high adsorption capacity of the surfactant in these soils, which is ascribable to their high fine fraction percentages. The overall results are in accordance with Finizio et al. [
6], which affirm that SLES concentration in elutriates depends not only on the surfactant amount in soils, but also on their adsorption capacities, which, in turn, are influenced by soil texture and mineralogical composition [
8]. The different adsorption capacity of each soil indicates the usefulness of performing site-specific studies for evaluating SLES mobilization in the aqueous phase. In fact, previous studies showed that foaming agents at the amounts used in tunneling operations are generally not toxic to terrestrial organisms, but can have a potential impact on the aquatic compartment, which is known to be very sensitive to any anionic surfactant residues [
6,
7,
12,
52].
As mentioned above, the results of the ecotoxicological tests at day 0 represent a scenario which excludes any SLES degradation in soils, by taking into account instantaneous leaching or run-off processes, simulating SLES transfer directly into the aquatic compartment.
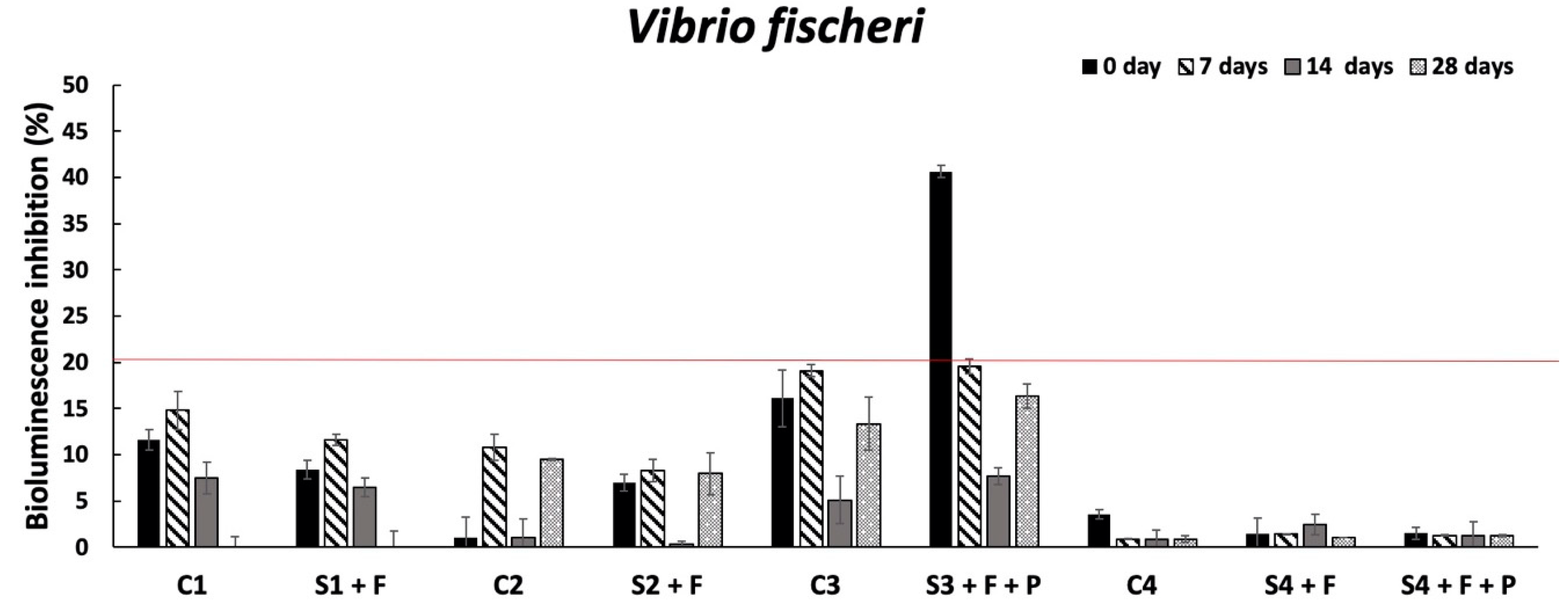
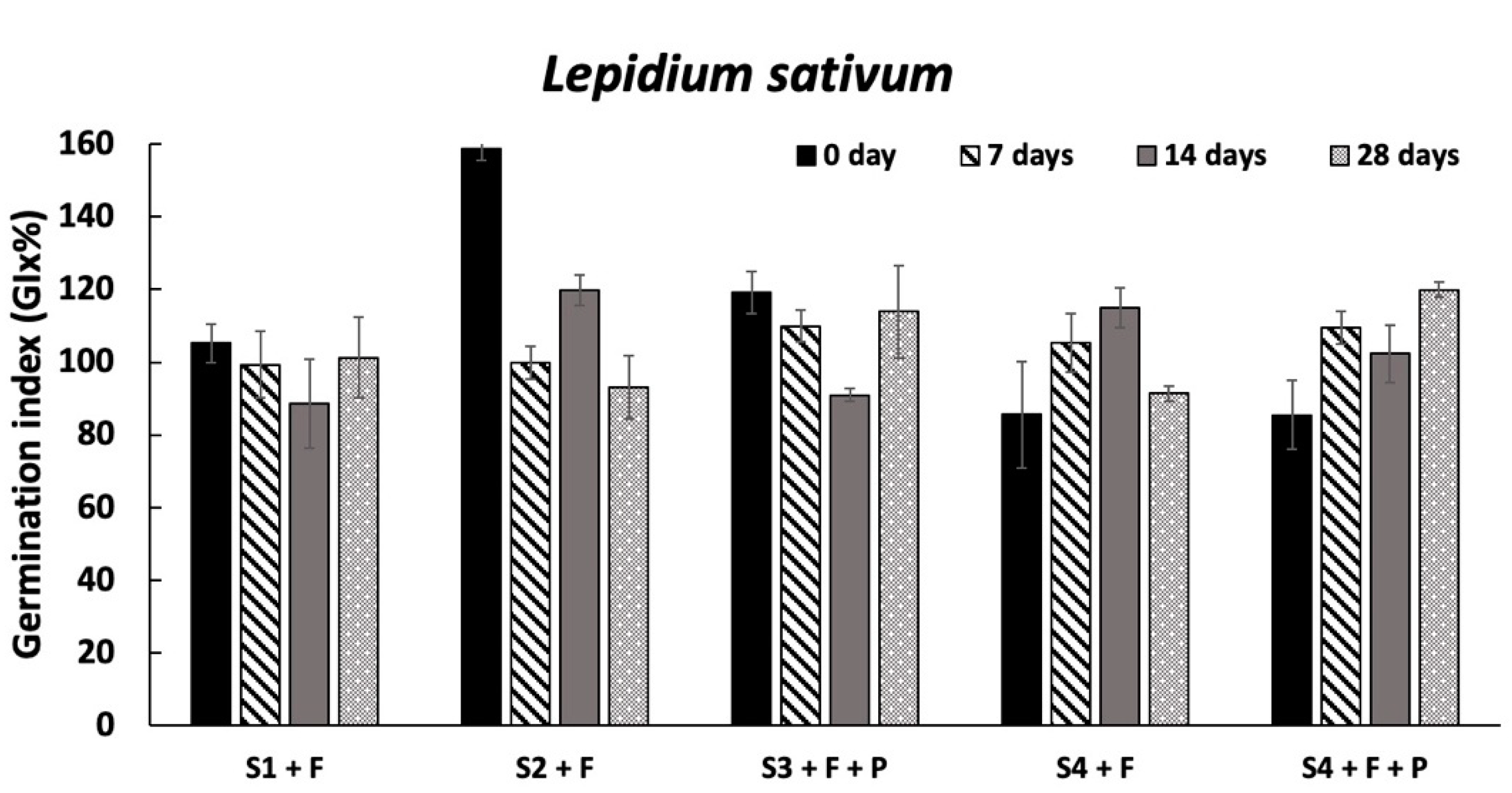
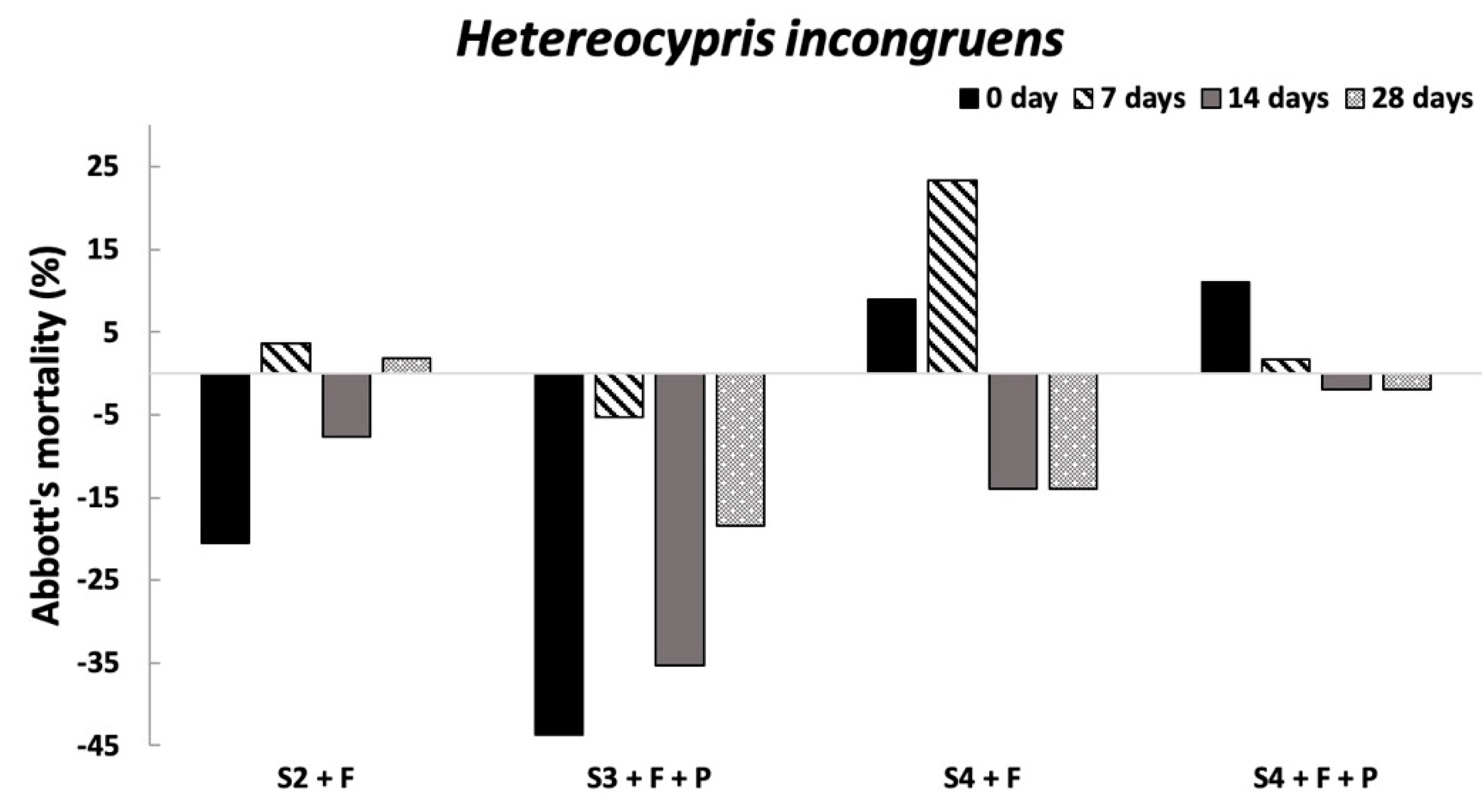
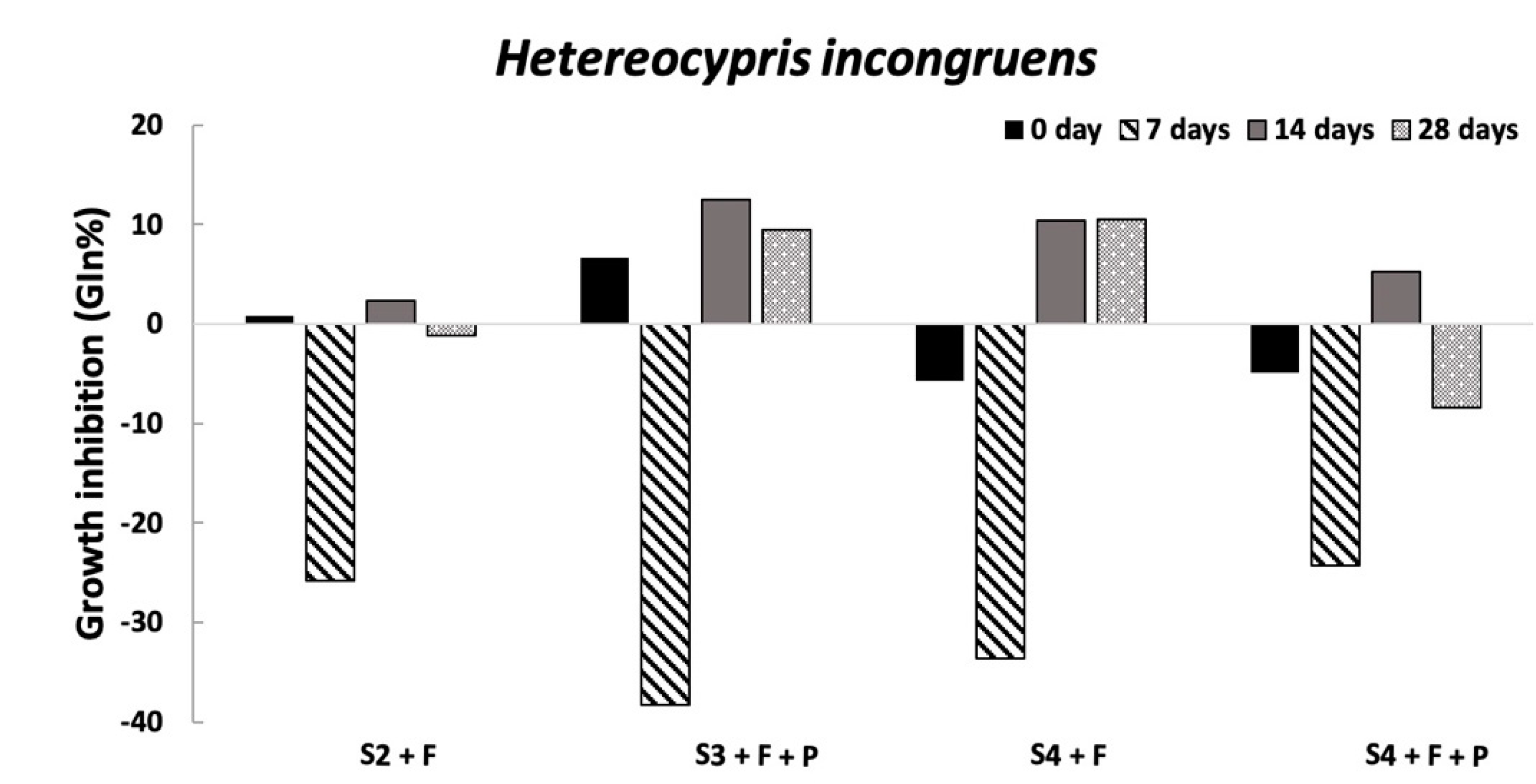
Overall, the ecotoxicological results evidenced the absence of harmful effects on the organisms already tested at the conditioning time, supporting the first hypothesis that if SLES in soil elutriates is not higher than 2 mg/L [
17], soil debris can be re-used as a safe by-product. At the concentrations used in these experiments (from 52.2 to 105.8 mg/kg), the spoil material did not cause any acute or sub-chronic effect on the organisms tested. Although, in some cases (e.g.,
H. incongruens in all conditioned soils and experimental times and
L. sativum in S2 + F), an increase in the organism survival and growth was observed, these results cannot be considered significant because the values were in the range of those observed in the standard reference sediment. Interestingly, as assumed, in the second hypothesis, the most sensitive organism to SLES residues was confirmed to be the bacterium
V. fischeri. In fact, at day 0 in S3 + F + P, where the highest SLES concentration was detected both in the soil (105.8 mg/kg) and in the corresponding elutriate (2.6 mg/L), a harmful effect on the bacterial bioluminescence (40% inhibition) was recorded. Anyway, this effect was transient and, in the following sampling (7 days), in line with SLES decrease in the elutriate (1.45 mg/L), it was never observed. These results are in accordance with previous ones, which showed 2 mg/L as a toxic SLES threshold in spoil material for this bacterium [
17]. In fact, the authors proved this concentration value for a significant toxic effect (>20%) occurring on
V. fischeri in soil elutriates and proposed the bioluminescence inhibition test as a suitable tool for evaluating the environmental compatibility of foaming agent-treated soils during the excavation phase. This bacterium was demonstrated to be an effective test for real matrices in other works [
53] and its reliability lies in its reproducibility [
54] and high sensitivity to several contaminants.
However, although SLES concentration in elutriates, which negatively affects the V. fischeri bacterium, is currently known, its corresponding value in soil cannot be generally established because, as mentioned above, the soil adsorption capacity can vary by several orders of magnitude among different soils. This suggests the need for site-specific studies for evaluating SLES in elutriates and confirms that the use of biological assays is the most reliable approach to assess the environmental impact of chemicals. In fact, ecotoxicological responses reflect the overall effects of contaminants, also considering additive, antagonistic and synergistic effects, and taking into account the bioavailable fraction of all the chemicals in solid or semi-solid samples, such as soil debris. Moreover, the use of ecotoxicological tests on site-specific environmental matrices (soil and water extract) makes it possible to overcome the legislative gaps in this context. In fact, currently neither at EU level nor in Italy (Italian Decree 161/2012 and 120/2017) are there specific threshold limits in environmental regulations for spoil material containing additive components (e.g., SLES) used for excavation.
For these reasons, knowledge on SLES’s environmental fate in realistic use conditions, together with studies on the ecotoxicological effects on terrestrial and aquatic compartments, need to be performed to ensure a correct management of the large quantity of soil debris produced during excavation works and to support the decision-making processes of stakeholders involved in tunneling projects (as performed in this specific case-study).