Integrated Monitoring with Moss-Bag and Mussel Transplants in Reservoirs
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Design
2.2. Analytical Procedures
2.3. Contamination Factors and Metal Pollution Index
- CF < 2, normal condition (blue)
- 2 ≤ CF < 6, suspected pollution (green)
- 6 ≤ CF < 18, certain pollution (yellow)
- 18 ≤ CF < 54, strong pollution (orange)
- 54 ≤ CF, extreme pollution (red).
- MPI ≤ 1—unpolluted by 6 metals (blue);
- 1 ≤ MPI ≤ 2.1—low contamination (green);
- 2.1 ≤ MPI ≤ 4.5—moderate contamination (yellow);
- 4.5 ≤ MPI ≤ 6.5—strong contamination (orange);
- MPI ≥ 6.5—heavy contamination (red).
2.4. Statistical Analysis
3. Results and Discussion
3.1. Water
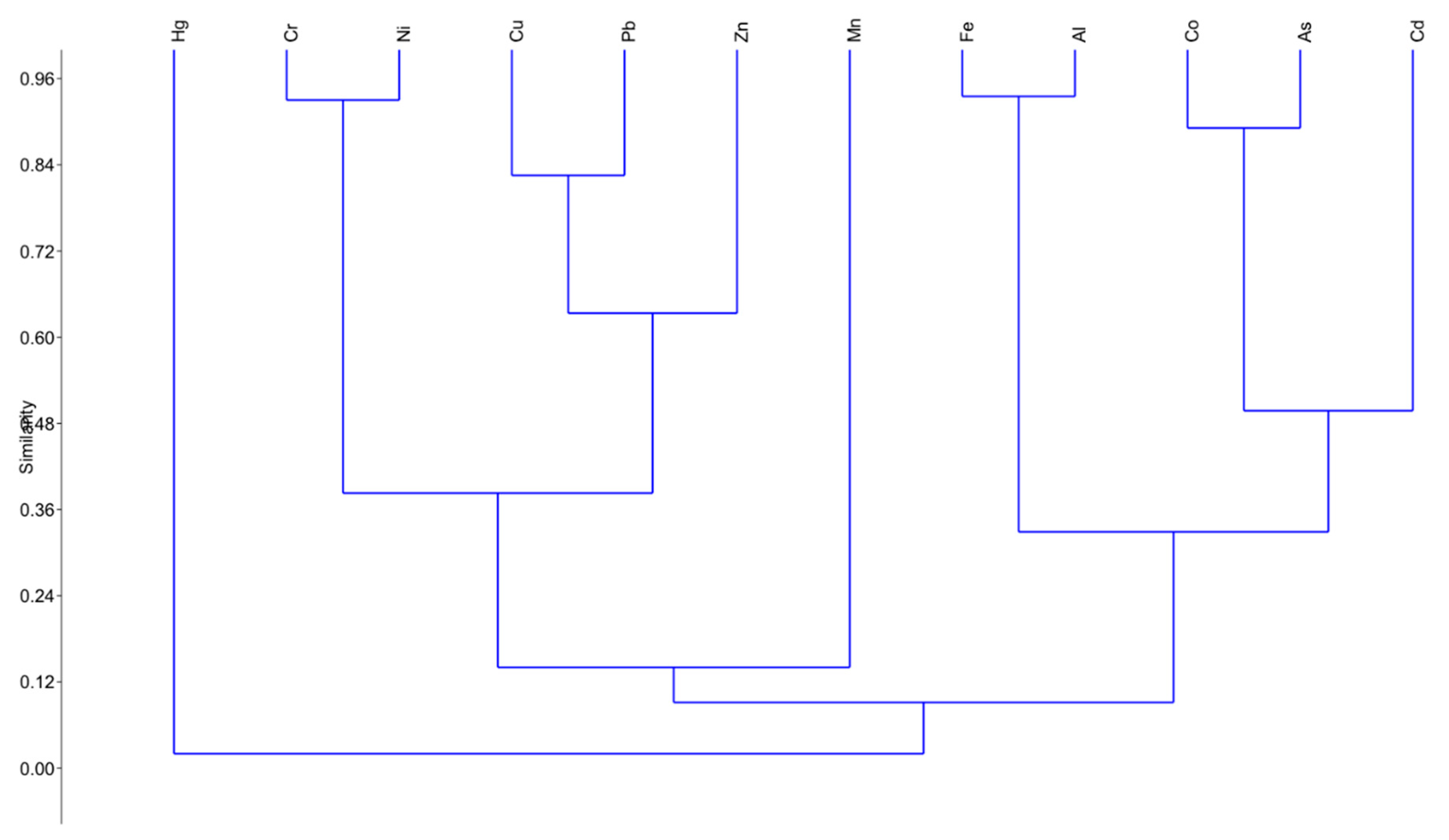
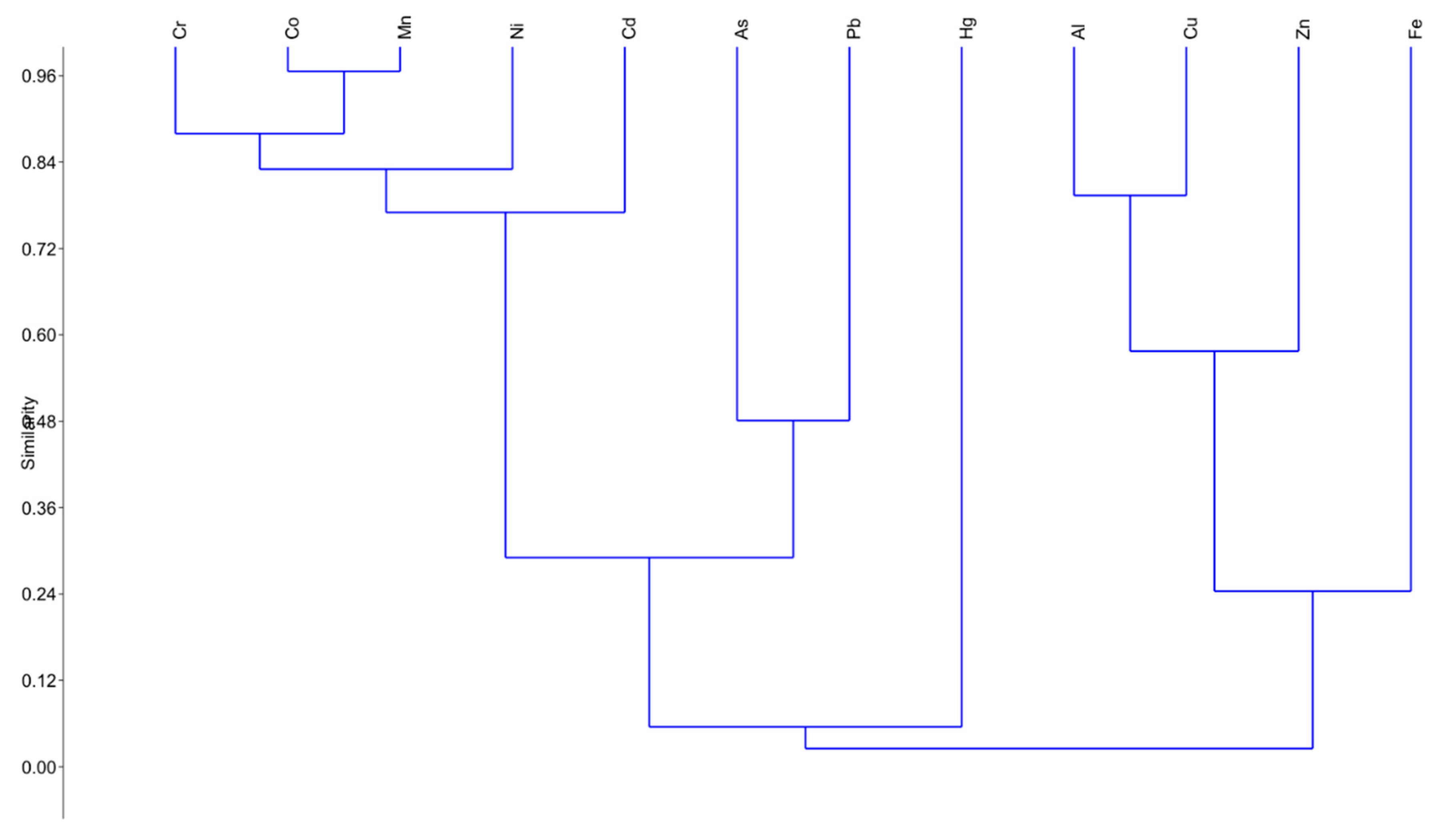
3.2. Moss-Bags
3.3. Mussels
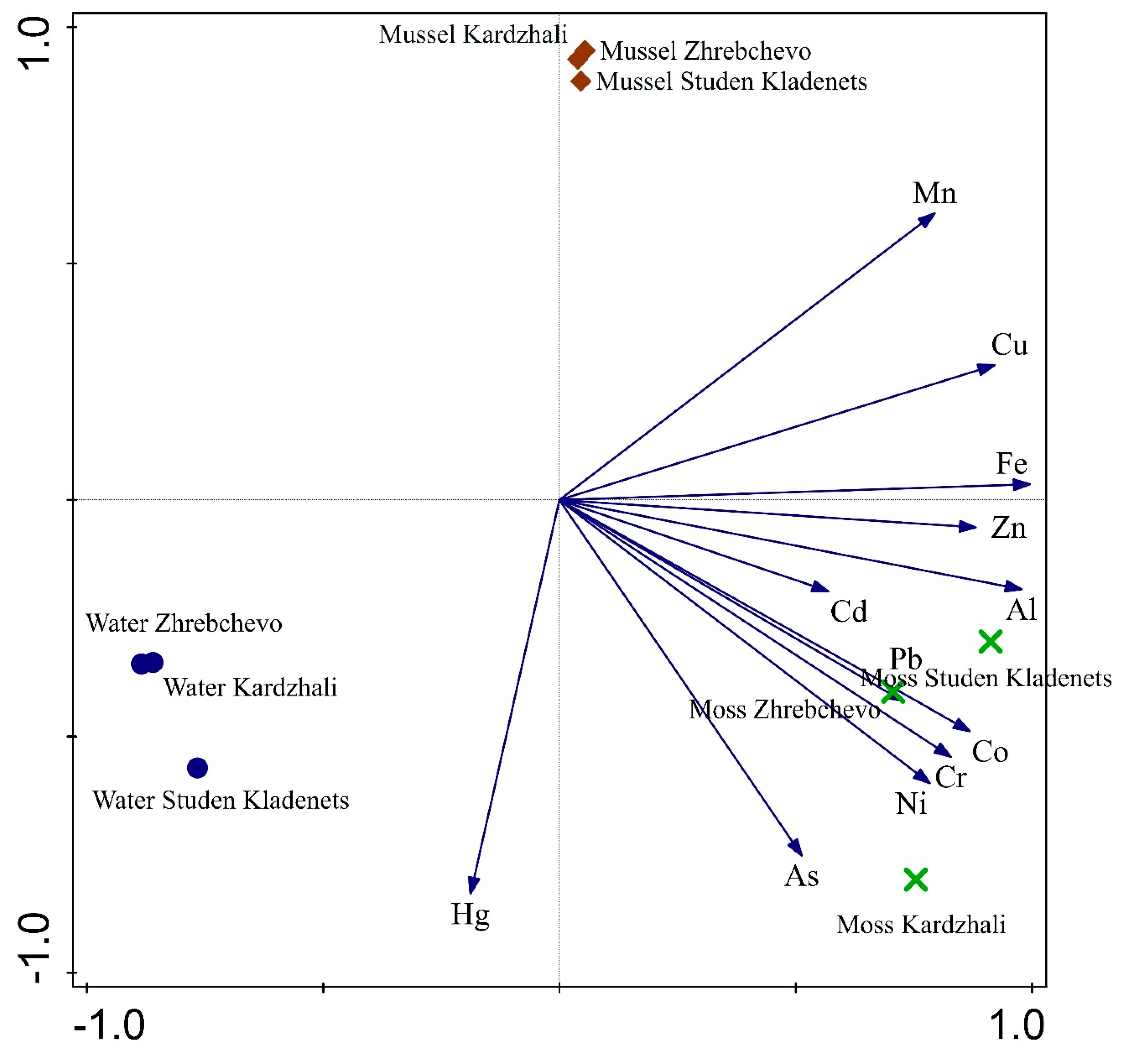
3.4. Integrated Transplant Monitoring
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Núñez, R.; García, M.Á.; Alonso, J.; Melgar, M.J. Arsenic, cadmium and lead in fresh and processed tuna marketed in Galicia (NW Spain): Risk assessment of dietary exposure. Sci. Total Environ. 2018, 627, 322–331. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 14. [Google Scholar] [CrossRef]
- Wania, F.; Dugani, C.B. Assessing the long-range transport potential of polybrominated diphenyl ethers: A comparison of four multimedia models. Environ. Toxicol. Chem. 2003, 22, 1252–1261. [Google Scholar] [CrossRef]
- Ondarza, P.M.; Gonzalez, M.; Fillmann, G.; Miglioranza, K.S.B. PBDEs, PCBs and organochlorine pesticides distribution in edible fish from Negro River basin, Argentinean Patagonia. Chemosphere 2014, 94, 135–142. [Google Scholar] [CrossRef]
- De Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- De Wit, C.A.; Herzke, D.; Vorkamp, K. Brominated flame retardants in the Arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408, 2885–2918. [Google Scholar] [CrossRef]
- Zeng, L.; Lam, J.C.W.; Chen, H.; Du, B.; Leung, K.M.Y.; Lam, P.K.S. Tracking dietary sources of short- and medium-chain chlorinated paraffins in marine mammals through a subtropical marine food web. Environ. Sci. Technol. 2017, 51, 9543–9552. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, H.; Geng, N.; Ren, X.; Giesy, J.P.; Luo, Y.; Xing, L.; Wu, P.; Yu, Z.; Chen, J. Short-chain chlorinated paraffins (SCCPs) disrupt hepatic fatty acid metabolism in liver of male rat via interacting with peroxisome proliferatoractivated receptor α (PPARα). Ecotoxicol. Environ. Saf. 2019, 181, 164–171. [Google Scholar] [CrossRef]
- Bayen, S.; Obbard, J.P.; Thomas, G.O. Chlorinated paraffins: A review of analysis and environmental occurrence. Environ. Int. 2006, 32, 915–929. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, N.; Cui, Y.; Jiang, W.; Lina, W.; Zhenhua, W.; Chen, X.; Jiang, L.; Ding, L. Short-chain chlorinated paraffin (SCCP) pollution from a CP production plant in China: Dispersion, congener patterns and health risk assessment. Chemosphere 2018, 211, 456–464. [Google Scholar] [CrossRef]
- Law, R.J.; Covaci, A.; Harrad, S.; Herzke, D.; Abdallah, M.A.; Fernie, K.; Toms, L.M.; Takigami, H. Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environ. Int. 2014, 65, 147–158. [Google Scholar] [CrossRef]
- Houde, M.; Muir, D.C.; Tomy, G.T.; Whittle, D.M.; Teixeira, C.; Moore, S. Bioaccumulation and trophic magnification of short- and medium-chain chlorinated paraffins in food webs from Lake Ontario and Lake Michigan. Environ. Sci. Technol. 2008, 42, 3893–3899. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gao, L.; Xia, D.; Qiao, L. Bioaccumulation and biomagnification of short and medium chain polychlorinated paraffins in different species of fish from Liaodong Bay, North China. Sci. Rep. 2017, 7, 10749. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, X.; He, T.; Wang, J.; Zhen, W.; Li, P.; Yongzhong, L.; Sanganyado, E.; Liu, W. Integrated assessment of heavy metal pollution using transplanted mussels in eastern Guangdong, China. Environ. Pollut. 2018, 243, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Schulze, S.; Zahn, D.; Montes, R.; Rodil, R.; Quintana, J.B.; Knepper, T.P.; Reemtsma, T.; Berger, U. Occurrence of emerging persistent and mobile organic contaminants in European water samples. Water Res. 2019, 153, 80–90. [Google Scholar] [CrossRef]
- Kesavan, K.; Murugan, A.; Venkatesan, V.; Vijay Kumar, B.S. Heavy metal accumulation in molluscs and sediment from uppanar estuary, southeast coast of India. Thalassas 2013, 29, 15–21. [Google Scholar]
- Kumar, V.; Sinha, A.K.; Rodrigues, P.P.; Mubiana, V.K.; Blust, R.; De Boeck, G. Linking environmental heavy metal concentrations and salinity gradients with metal accumulation and their effects: A case study in 3 mussel species of Vitoria estuary and Espírito Santo bay, Southeast Brazil. Sci. Total Environ. 2015, 523, 1–15. [Google Scholar] [CrossRef]
- Azizi, G.; Akodad, M.; Baghour, M.; Layachi, M.; Moumen, A. The use of Mytilus spp. mussels as bioindicators of heavy metal pollution in the coastal environment. A review. JMES 2018, 9, 1170–1181. [Google Scholar]
- Debén, S.; Fernández, J.A.; Carballeira, A.; Aboal, J.R. Using devitalized moss for active biomonitoring of water pollution. Environ. Pollut. 2016, 210, 315–322. [Google Scholar] [CrossRef]
- Benson-Evans, K.; Williams, P.F. Transplanting aquatic bryophytes to assess river pollution. J. Bryol. 1976, 9, 81–91. [Google Scholar] [CrossRef]
- Figueira, R.; Ribeiro, T. Transplants of aquatic mosses as biomonitors of metals released by a mine effluent. Environ. Pollut. 2005, 136, 293–301. [Google Scholar] [CrossRef]
- Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. A comparison of native and transplanted Fontinalis antipyretica Hedw. as biomonitor of water polluted with heavy metals. Sci. Total Environ. 2005, 341, 97–107. [Google Scholar] [CrossRef]
- Gecheva, G.; Yurukova, L. Water pollutant monitoring with aquatic bryophytes: A review. Environ. Chem. Lett. 2014, 12, 49–61. [Google Scholar] [CrossRef]
- Kucuksezgin, F.; Pazi, I.; Yucel-Gier, G.; Akcali, B.; Galgani, F. Monitoring of heavy metal and organic compound levels along the Eastern Aegean coast with transplanted mussels. Chemosphere 2013, 93, 1511–1518. [Google Scholar] [CrossRef]
- Goldberg, E.D. The Mussel Watch-a first step in global marine monitoring. Mar. Pollut. Bull. 1975, 6, 111–114. [Google Scholar] [CrossRef]
- Goldberg, E.D. The Mussel Watch concept. Environ. Monit. Assess. 1986, 7, 91–103. [Google Scholar] [CrossRef]
- Martin, M. State Mussel Watch: Toxics surveillance in California. Mar. Pollut. Bull. 1985, 16, 140–146. [Google Scholar] [CrossRef]
- Lauenstein, G.G.; Robertson, A.; O’Connor, T.P. Comparison of trace metal data in mussels and oysters from a mussel watch programme of the 1970s with those from a 1980s programme. Mar. Pollut. Bull. 1990, 21, 440–447. [Google Scholar] [CrossRef]
- Sparks, C.; Odendaal, J.; Snyman, R. An analysis of historical Mussel Watch Programme data from the west coast of the Cape Peninsula, Cape Town. Mar. Pollut. Bull. 2014, 87, 374–380. [Google Scholar] [CrossRef]
- Farrington, J.W.; Tripp, B.W.; Tanabe, S.; Subramanian, A.; Sericano, J.L.; Wade, T.L.; Knap, A.H.; Edward, D. Goldberg’s proposal of “the mussel watch”: Reflections after 40 years. Mar. Pollut. Bull. 2016, 110, 501–510. [Google Scholar] [CrossRef]
- Robinson, C.D.; Webster, L.; Martínez-Gomez, C.; Burgeot, T.; Gubbins, M.J.; Thain, J.E.; Vethaak, A.D.; Mcintosh, A.D.; Hylland, K. Assessment of contaminant concentrations in sediments, fish and mussels sampled from the North Atlantic and European regional seas within the ICON project. Mar. Environ. Res. 2017, 124, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Watters, G.T. Synthesis and review of the expanding range of the Asian freshwater mussel Anodonta woodiana (Lea, 1834) (Bivalvia: Unionidae). Veliger 1997, 40, 152–156. [Google Scholar]
- Popa, O.P.; Bartakova, V.; Bryja, J.; Reichard, M.; Popa, L.O. Characterization of nine microsatellite markers and development of multiplex PCRs for the Chinese huge mussel Anodonta (Sinanodonta) woodiana Lea, 1834 (Mollusca, Bivalvia). Biochem. Syst. Ecol. 2015, 60, 234–237. [Google Scholar] [CrossRef]
- Soroka, M.; Urbanska, M.; Andrzejewski, W. Chinese pond mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia): Origin of the Polish population and GenBank data. J. Limnol. 2014, 73, 454–458. [Google Scholar] [CrossRef][Green Version]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaja, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. Camb. Philos. Soc. 2016, 92, 572–607. [Google Scholar] [CrossRef]
- Kolarević, S.; Knežević-Vukčević, J.; Paunović, M.; Kračun, M. Monitoring of DNA damage in haemocytes of freshwater mussel Sinanodonta woodiana sampled from the Velika Morava River in Serbia with the comet assay. Chemosphere 2013, 93, 243–251. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Gan, J. Trace element accumulation in bivalve mussels Anodonta woodiana from Taihu Lake, China. Arch. Environ. Contam. Toxicol. 2010, 59, 593–601. [Google Scholar] [CrossRef]
- Uno, S.; Shiraishi, H.; Hatakeyama, S.; Otsuki, A.; Koyama, J. Accumulative characteristics of pesticide residues in organs of bivalves (Anodonta woodiana and Corbicula leana) under natural conditions. Arch. Environ. Contam. Toxicol. 2001, 40, 35–47. [Google Scholar]
- Woznicki, P.; Lewandowska, R.; Brzuzan, P.; Ziomek, E.; Bardega, R. The level of DNA damage and the frequency of micronuclei in haemolymph of freshwater mussels (Anadonta woodiana) exposed to benzo[a]pyrene. Acta Toxicol. 2004, 12, 41–45. [Google Scholar]
- Yancheva, V.; Mollov, I.; Velcheva, I.; Georgieva, E.; Stoyanova, S. Effects of cadmium (Cd) on the lysosomal membrane stability and respiration rate of two freshwater mollusks under ex situ exposure: Preliminary data. South West J. Hortic. Biol. Environ. 2016, 7, 27–34. [Google Scholar]
- Yancheva, V.; Mollov, I.; Velcheva, I.; Georgieva, E.; Stoyanova, S. Heavy metal effects on the lysosomal membrane stability and respiratory rate in Chinese Pond Mussel (Sinanodonta woodiana) under ex situ exposure: Preliminary data. Biharean Biol. 2016, 10, 55–57. [Google Scholar]
- Chen, X.B.; Liu, H.B.; Su, Y.P.; Yang, J. Morphological development and growth of the freshwater mussel Anodonta woodiana from early juvenile to adult. Invertebr. Reprod. Dev. 2015, 59, 131–140. [Google Scholar] [CrossRef]
- Cenci, R.M. The use of aquatic moss (Fontinalis antipyretica) as monitor of contamination in standing and running waters: Limits and advantages. J. Limnol. 2000, 60, 53–61. [Google Scholar] [CrossRef]
- Mersch, J.; Johansson, L. Transplanted aquatic mosses and freshwater mussels to investigate the trace metal contamination in the rivers Meurthe and Plaine, France. Environ. Technol. 1993, 14, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S.; Bernard, I.; Pouvreau, S.; Maurer, D.; Schaal, G.; Ganthy, F.; Cominassi, L.; Allain, G.; Sautour, B.; David, V. Spatial patterns in the condition index of the wild Pacific oyster Crassostrea gigas in a macrotidal coastal ecosystem: Influence of tidal processes and beyond. J. Sea Res. 2017, 119, 28–36. [Google Scholar] [CrossRef]
- Rosseland, B.O.; Massabuau, J.C.; Grimalt, J.; Hofer, R.; Lackner, R.; Raddum, G.; Rognerud, S.; Vives, I. Fish Ecotoxicology: European Mountain Lake Ecosystems Regionalisation, Diagnostic and Socio–Economic Evaluation—Fish Sampling Manual for Live Fish; Norwegian Institute for Water Research: Oslo, Norway, 2003. [Google Scholar]
- Velcheva, I. Zinc content in the organs and tissues of freshwater fish from the Kardjali and Studen Kladenets Dam Lakes in Bulgaria. Turk. J. Zool. 2006, 30, 1–7. [Google Scholar]
- Gribacheva, N.; Gecheva, G.; Stefanova, V. Air pollution monitoring with mosses in Western Rhodopes, Bulgaria. Bulg. Chem. Commun. 2019, 51, 256–260. [Google Scholar] [CrossRef]
- Mouvet, C. Rapport de contrat à l’Agence de l’Eau Rhin-Meuse et l’Agence de l’Eau Rhône-Méditerranée-Corse. In Métaux Lourds Et Mousses Aquatiques, Synthèse Méthodologique; Université de Metz Laboratoire d’Ecologie: Metz, France, 1986; p. 104. [Google Scholar]
- Gonçalves, E.P.R.; Boaventura, R.A.R.; Mouvet, C. Sediments and aquatic mosses as pollution indicators for heavy metals in the Ave river basin (Portugal). Sci. Total Environ. 1992, 114, 7–24. [Google Scholar] [CrossRef]
- Soares, H.M.V.M.; Boaventura, R.A.R.; Machado, A.A.S.C.; Esteves da Silva, J.C.G. Sediments as monitors of heavy metal contamination in the Ave river basin (Portugal): Multivariate analysis of data. Environ. Pollut. 1999, 105, 311–323. [Google Scholar] [CrossRef]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Communities 2000, 327, 1–72. [Google Scholar]
- Nikanorov, A.; Julidkov, A.; Pokarzevskii, A. Biomonitoping of Heavy Metals in Freshwater Ecosystems; Hydrometeoizdat: Leningrad, Russia, 1985; p. 144. (In Russian) [Google Scholar]
- Hammer, O.; Harper, D.; Ryan, P. PAST: PA leontological ST atistical software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Smilauer, P.; Budejovice, C. CANOCO 5, Ecological Multivariate Data Ordination Program; Biometris Wageninen-UR: Wageningen, The Netherlands, 2014. [Google Scholar]
- Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. Heavy metals in aquatic bryophytes from the Ore Mountains (Germany). Ecotoxicol. Environ. Saf. 2002, 52, 203–210. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Pisani, T.; Paoli, L.; Munzi, S.; Loppi, S. Bioacumulation and ultrastructural effects of Cd, Cu, Pb and Zn in the moss Scorpiurum circinatum (Brid.) Fleisch & Loeske. Environ. Pollut. 2012, 166, 208–211. [Google Scholar] [PubMed]
- Camizuli, E.; Monna, F.; Scheifler, R.; Amiotte-Suchet, P.; Losno, R.; Beis, P.; Bohard, B.; Chateau, C.; Alibert, P. Impact of trace metals from past mining on the aquatic ecosystem: A multi-proxy approach in the Morvan (France). Environ. Res. 2014, 134, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.K.; Cheng, W.H.; Karami, A.; Ismail, A. Health risk assessments of heavy metal exposure via consumption of marine mussels collected from anthropogenic sites. Sci. Total Environ. 2016, 553, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Layachi, M.; Akodad, M.; Yáñez-Ruiz, D.R.; Martín-García, A.I.; Baghour, M.; Mesfioui, A.; Skalli, A.; Moumen, A. Seasonal variations of heavy metals content in mussels (Mytilus galloprovincialis) from Cala Iris offshore (Northern Morocco). Mar. Pollut. Bull. 2018, 137, 688–694. [Google Scholar] [CrossRef]
- Debruyn, A.M.; Meloche, L.M.; Lowe, C.J. Patterns of bioaccumulation of polybrominated diphenyl ether and polychlorinated biphenyl congeners in marine mussels. Environ. Sci. Technol. 2009, 43, 3700–3704. [Google Scholar] [CrossRef]
- Booij, K.; Zegers, B.N.; Boon, J.P. Levels of some polybrominated diphenyl ether (PBDE) flame retardants along the Dutch coast as derived from their accumulation in SPMDs and blue mussels (Mytilus edulis). Chemosphere 2002, 46, 683–688. [Google Scholar] [CrossRef]
- Sun, R.; Luo, X.; Tang, B.; Chen, L.; Liu, Y.; Maia, B. Bioaccumulation of short chain chlorinated paraffins in a typical freshwater food web contaminated by e-waste in south china: Bioaccumulation factors, tissue distribution, and trophic transfer. Environ. Pollut. 2017, 222, 165–174. [Google Scholar] [CrossRef]
- Ma, X.; Chen, C.; Zhang, H.; Gao, Y.; Wang, Z.; Yao, Z.; Chen, J.; Chen, J. Congener-specific distribution and bioaccumulation of short-chain chlorinated paraffins in sediments and bivalves of the Bohai Sea, China. Mar. Pollut. Bull. 2014, 79, 299–304. [Google Scholar] [CrossRef]
- Vázquez, M.D.; López, J.; Carballeira, A. Uptake of heavy metals to the extracellular and intracellular compartments in three species of aquatic bryophyte. Ecotoxicol. Environ. Saf. 1999, 44, 12–24. [Google Scholar] [CrossRef] [PubMed]
- García-Álvaro, M.A.; Martínez-Abaigar, J.; Núñez-Olivera, E.; Beaucourt, N. Element concentrations and enrichment ratios in the aquatic moss Rhynchostegium riparioides along the River Iregua (La Riojia, Northern Spain). Bryologist 2000, 103, 518–533. [Google Scholar] [CrossRef]
- Mariussen, E.; Steinnes, E.; Breivik, K.; Nygård, T.; Schlabach, M.; Kålås, J.A. Spatial patterns of polybrominated diphenyl ethers (PBDEs) in mosses, herbivores and a carnivore from the Norwegian terrestrial biota. Sci. Total Environ. 2008, 404, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, H.; Hansson, K.; Potter, A.; Friedrichsen, J.; Brorström-Lundén, E. Persistant Organic Pollutants in Swedish Mosses; Swedish Environmental Research Institute: Stockholm, Sweden, 2016; p. 34. [Google Scholar]

| Water | Moss | Mussels | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Background | 1 | 2 | 3 | Background | 1 | 2 | 3 | Background | 1 | 2 | 3 | ||||
| Al | mg L−1 | <0.005 | 0.11 | 0.03 | 0.05 | % | 0.16 | 1.45 | 1.34 | 1.33 | mg kg−1 | 14.4 | 36.6 | 35.7 | 36.9 |
| RSD% | - | 7.6 | 13.3 | 11.4 | RSD% | 8.9 | 5.6 | 6.7 | 5.0 | RSD% | 11.1 | 6.2 | 5.7 | 6.9 | |
| As | µg L−1 | <1 | 1.2 | 5.2 | <1 | mg kg−1 | 1.3 | 8.0 | 5.3 | 4.3 | mg kg−1 | 0.37 | 0.80 | 0.63 | 1.15 |
| RSD% | - | 9.4 | 4.9 | - | RSD% | 5.2 | 6.8 | 8.1 | 15 | RSD% | 6.4 | 7.5 | 9.3 | 8.8 | |
| Ca | n.a. | % | 0.75 | 1.4 | 1.8 | 4.3 | % | 0.89 | 1.4 | 1.9 | 2.0 | ||||
| RSD% | 2.9 | 2.9 | 1.9 | 1.0 | RSD% | 3.1 | 2.1 | 2.4 | 2.3 | ||||||
| Cd | µg L−1 | <0.1 | <0.1 | 0.32 | <0.1 | mg kg−1 | 0.07 | 0.53 | 9.1 | 0.31 | mg kg−1 | 0.09 | 0.17 | 0.38 | 0.19 |
| RSD% | - | - | 7.9 | - | RSD% | 21 | 15.6 | 4.8 | 14.9 | RSD% | 10 | 6.0 | 5.6 | 13 | |
| Co | µg L−1 | <0.01 | <0.01 | 0.26 | <0.01 | mg kg−1 | 1.6 | 7.1 | 8.0 | 4.8 | mg kg−1 | 0.14 | 0.20 | 0.21 | 0.27 |
| RSD% | - | - | 5.4 | - | RSD% | 3.1 | 2.1 | 3.6 | 5.3 | RSD% | 6.0 | 7.1 | 5.8 | 7.4 | |
| Cr | µg L−1 | 0.06 | 0.18 | 0.06 | 0.13 | mg kg−1 | 3.9 | 66 | 25 | 21 | mg kg−1 | 0.15 | 0.22 | 0.14 | 0.19 |
| RSD% | 7.1 | 4.1 | 6.9 | 4.2 | RSD% | 2.5 | 6.7 | 9.7 | 5.3 | RSD% | 3.4 | 3.2 | 11.3 | 8.6 | |
| Cu | µg L−1 | 0.1 | 1.7 | 1.8 | 0.3 | mg kg−1 | 10 | 34 | 282 | 53 | mg kg−1 | 5.320 | 63 | 31.4 | 25.4 |
| RSD% | 8.9 | 6.2 | 5.7 | 7.3 | RSD% | 4.5 | 6.8 | 3.7 | 2.1 | RSD% | 4.9 | 3.4 | 3.9 | 4.3 | |
| Fe | µg L−1 | <0.1 | <0.1 | <0.1 | <0.1 | % | 0.23 | 1.2 | 1.2 | 1.2 | mg kg−1 | 170 | 212 | 196 | 294 |
| RSD% | - | - | - | - | RSD% | 3.1 | 3.2 | 2.3 | 2.5 | RSD% | 6.4 | 3.4 | 5.0 | 3.4 | |
| Hg | µg L−1 | <0.05 | <0.05 | <0.05 | <0.05 | mg kg−1 | 0.02 | 0.03 | 0.05 | 0.06 | mg kg−1 | 0.004 | 0.007 | 0.009 | 0.009 |
| RSD% | - | - | - | - | RSD% | 11.0 | 6.4 | 5.7 | 8.3 | RSD% | 16 | 12 | 11 | 12 | |
| K | n.a. | % | 1.01 | 0.22 | 0.57 | 0.65 | mg kg−1 | 211 | 229 | 234 | 201 | ||||
| RSD% | 2.4 | 2.2 | 2.8 | 3.8 | RSD% | 4.2 | 4.0 | 5.5 | 4.7 | ||||||
| Mg | mg L−1 | 3.4 | 2.6 | 3.6 | 12.1 | % | 0.20 | 0.60 | 0.42 | 0.52 | mg kg−1 | 268 | 454 | 450 | 659 |
| RSD% | 5.0 | 5.9 | 6.4 | 2.6 | RSD% | 1.9 | 2.1 | 2.6 | 1.8 | RSD% | 3.8 | 2.8 | 3.1 | 2.6 | |
| Mn | mg L−1 | 0.002 | 0.008 | 0.04 | 0.005 | mg kg−1 | 1000 | 204 | 1948 | 338 | % | 0.134 | 0.188 | 0.198 | 0.254 |
| RSD% | 6.8 | 5.2 | 3.3 | 5.3 | RSD% | 6.0 | 8.2 | 3.3 | 5.1 | RSD% | 7.5 | 5.0 | 5.6 | 4.0 | |
| Na | mg L−1 | 4.7 | 4.8 | 7.6 | 11.1 | mg kg−1 | 273 | 140 | 147 | 306 | mg kg−1 | 331 | 780 | 436 | 514 |
| RSD% | 3.9 | 3.6 | 2.1 | 2.7 | RSD% | 3.1 | 4.9 | 3.9 | 7.6 | RSD% | 3.6 | 3.0 | 3.1 | 3.0 | |
| Ni | µg L−1 | 0.1 | 0.4 | 0.6 | 0.4 | mg kg−1 | 3.7 | 74 | 21 | 17 | mg kg−1 | 0.14 | 0.37 | 0.17 | 0.17 |
| RSD% | 6.3 | 7.5 | 3.4 | 2.5 | RSD% | 6.5 | 2.6 | 4.7 | 2.3 | RSD% | 5.0 | 6.5 | 6.3 | 9.1 | |
| P | mg L−1 | <0.01 | 0.03 | 0.04 | <0.01 | % | 0.34 | 0.14 | 0.30 | 0.32 | mg kg−1 | 0.43 | 0.93 | 1.07 | 1.20 |
| RSD% | - | 9.2 | 8.2 | - | RSD% | 4.2 | 4.8 | 4.5 | 4.0 | RSD% | 6.4 | 6.0 | 5.1 | 5.7 | |
| Pb | µg L−1 | 0.1 | 0.6 | 18 | 0.3 | mg kg−1 | 4.4 | 37.0 | 383 | 18.9 | mg kg−1 | 1.6 | 1.7 | 4.0 | 1.9 |
| RSD% | 5.9 | 5.0 | 4.6 | 5.9 | RSD% | 6.0 | 6.7 | 4.7 | 2.1 | RSD% | 6.8 | 5.7 | 7.9 | 3.6 | |
| Zn | µg L−1 | <1 | <1 | 20 | <1 | mg kg−1 | 32 | 62 | 142 | 52 | mg kg−1 | 55.1 | 11.5 | 32.6 | 15.7 |
| RSD% | - | - | 4.8 | - | RSD% | 4.8 | 5.0 | 2.3 | 9.0 | RSD% | 6.2 | 3.6 | 4.7 | 3.8 | |
| BDE 28 | µg L−1 | <0.004 | 0.023 | 0.032 | <0.004 | µg kg−1 | 0.005 | <0.003 | <0.003 | 0.042 | µg kg−1 | 0.005 | <0.003 | 0.005 | <0.003 |
| ± | 0.006 | 0.009 | ± | 0.000 | 0.000 | ± | 0.000 | 0.000 | |||||||
| BDE 47 | µg L−1 | 0.005 | 0.012 | 0.005 | 0.005 | µg kg−1 | 0.008 | 0.005 | 0.019 | <0.003 | µg kg−1 | 0.005 | 0.005 | 0.005 | 0.005 |
| ± | 0.000 | 0.000 | 0.000 | 0.000 | ± | 0.000 | 0.000 | 0.000 | ± | 0.000 | 0.000 | 0.000 | 0.000 | ||
| BDE 99 | µg L−1 | 0.012 | 0.017 | 0.018 | <0.004 | µg kg−1 | <0.003 | 0.022 | 0.038 | 0.013 | µg kg−1 | 0.013 | 0.015 | 0.010 | <0.003 |
| ± | 0.000 | 0.000 | 0.000 | ± | 0.000 | 0.000 | 0.000 | ± | 0.000 | 0.000 | 0.000 | ||||
| BDE 100 | µg L−1 | 0.009 | <0.004 | <0.004 | <0.004 | µg kg−1 | 0.011 | 0.048 | 0.011 | <0.003 | µg kg−1 | 0.007 | <0.003 | <0.003 | <0.003 |
| ± | 0.000 | ± | 0.000 | 0.000 | 0.000 | ± | 0.000 | ||||||||
| BDE 153 | µg L−1 | 0.014 | 0.012 | 0.018 | 0.014 | µg kg−1 | 0.030 | <0.003 | 0.017 | 0.046 | µg kg−1 | 0.014 | 0.016 | 0.014 | <0.003 |
| ± | 0.000 | 0.000 | 0.000 | 0.000 | ± | 0.000 | 0.000 | 0.000 | ± | 0.000 | 0.000 | 0.000 | |||
| BDE 154 | µg L−1 | <0.004 | 0.010 | <0.004 | <0.004 | µg kg−1 | 0.010 | 0.013 | 0.014 | 0.014 | µg kg−1 | <0.003 | <0.003 | 0.010 | <0.003 |
| ± | 0.000 | ± | 0.000 | 0.000 | 0.000 | 0.000 | ± | 0.000 | |||||||
| SCCPs | µg L−1 | 0.58 | 0.86 | 1.2 | 3.9 | µg kg−1 | 4.6 | 5.4 | 6.0 | 9.2 | µg kg−1 | 7.4 | 0.56 | 6.1 | 0.22 |
| ± | 0.12 | 0.17 | 0.24 | 0.8 | ± | 1.4 | 1.6 | 1.8 | 2.7 | ± | 2.2 | 0.17 | 1.8 | 0.07 | |
| Station | Kardzhali | Studen Kladenets | Zhrebchevo |
|---|---|---|---|
| Al | 8.8 | 8.1 | 8.1 |
| As | 6.2 | 4.1 | 3.4 |
| Cd | 7.3 | 126 | 4.2 |
| Co | 4.5 | 5.1 | 3.1 |
| Cr | 17 | 6.4 | 5.4 |
| Cu | 3.3 | 28 | 5.2 |
| Fe | 5.5 | 5.5 | 5.2 |
| Mn | 0.2 | 1.9 | 0.3 |
| Ni | 20 | 5.8 | 4.5 |
| Pb | 8.4 | 87 | 4.3 |
| Zn | 1.9 | 4.4 | 1.6 |
| MPI | 10 | 90 | 4.4 |
| Kardzhali | Studen Kladenets | Zhrebchevo | |
|---|---|---|---|
| Al | 343 | 1064 | 771 |
| As | 668 | 122 | - |
| Cd | - | 1176 | - |
| Co | - | 822 | - |
| Cr | 1232 | 2345 | 1427 |
| Cu | 37,079 | 17,462 | 84,503 |
| Mg | 174 | 127 | 54 |
| Mn | 24 | 5 | 46 |
| Na | 162 | 57 | 46 |
| Ni | 928 | 287 | 415 |
| P | 28 | 29 | - |
| Pb | 2835 | 2247 | 6406 |
| Zn | - | 1629 | - |
| PBDEs | 0.46 | 0.59 | 0.54 |
| SCCPs | 0.05 | 7 | 0.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gecheva, G.; Yancheva, V.; Velcheva, I.; Georgieva, E.; Stoyanova, S.; Arnaudova, D.; Stefanova, V.; Georgieva, D.; Genina, V.; Todorova, B.; et al. Integrated Monitoring with Moss-Bag and Mussel Transplants in Reservoirs. Water 2020, 12, 1800. https://doi.org/10.3390/w12061800
Gecheva G, Yancheva V, Velcheva I, Georgieva E, Stoyanova S, Arnaudova D, Stefanova V, Georgieva D, Genina V, Todorova B, et al. Integrated Monitoring with Moss-Bag and Mussel Transplants in Reservoirs. Water. 2020; 12(6):1800. https://doi.org/10.3390/w12061800
Chicago/Turabian StyleGecheva, Gana, Vesela Yancheva, Iliana Velcheva, Elenka Georgieva, Stela Stoyanova, Desislava Arnaudova, Violeta Stefanova, Deyana Georgieva, Vesela Genina, Borislava Todorova, and et al. 2020. "Integrated Monitoring with Moss-Bag and Mussel Transplants in Reservoirs" Water 12, no. 6: 1800. https://doi.org/10.3390/w12061800
APA StyleGecheva, G., Yancheva, V., Velcheva, I., Georgieva, E., Stoyanova, S., Arnaudova, D., Stefanova, V., Georgieva, D., Genina, V., Todorova, B., & Mollov, I. (2020). Integrated Monitoring with Moss-Bag and Mussel Transplants in Reservoirs. Water, 12(6), 1800. https://doi.org/10.3390/w12061800









