Abstract
Phragmites australis (common reed) is one of the most extensively distributed species of emergent plant worldwide. The adaptive features of this plant show its competitive character. Owing to high intraspecific diversity of common reed, as well as its phenotypic plasticity, the plant shows a broad ecological amplitude. Moreover, the plant exhibits a high capacity for acclimatization to environmental conditions which are considered adverse. This plant has been used for many years in phytoremediation to purify various types of wastewater. Phragmites australis has a high ability to accumulate various nutrients, heavy metals, and micropollutants, and in this respect, it is superior to other aquatic plants. This review examines the existing literature on the biological and ecological properties of common reed, the use of common reed in wastewater treatment for removing pollutants and tolerance for metals, and in hydrophyte treatment systems. It seems vital to conduct further research on the physiology and biochemistry of the common reed, with the aim of increasing the plant’s efficiency for pollutants removal.
1. Introduction
Freshwater ecosystems (lakes, ponds, rivers, streams, and wetlands) cover only 2.5 percent of our planet [1] and play a pivotal role in providing a large array of services for a fast-growing human population [2], which is predicted to reach around 9.7 billion by 2050 [3].
However, a significant increase in pollution of aquatic ecosystems due to human activities associated with urbanization, industrial development, and the intensification of agricultural activities was observed in last five decades [4,5]. The decrease of surface water quality is connected with the negative influence of either point or non-point sources of water pollution. Pollutants originating from municipal and industrial wastewater, as well as surface runoffs from arable fields, roads, and highways, were divided into physical (solid material), biological (micro-organisms such as bacteria), and various different chemical pollutants [6,7,8]. The most dangerous to aquatic ecosystems is chemical pollution, which includes compounds of phosphorus (P) and nitrogen (N) (used as fertilizers and formed as a result of the breakdown of human and animal wastes); radioactive elements (e.g., strontium (Sr), caesium (Cs), and radon (Rn)); heavy metals (e.g., mercury (Hg), cadmium (Cd), lead (Pb), and chromium (Cr)); and natural (crude oil) and synthetic organic chemicals, such as pesticides and other persistent pollutants (e.g., detergents, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, pharmaceuticals, and personal-care products, nanoparticles) [9,10,11,12,13,14,15]. Although most aquatic ecosystems have a natural tendency to dilute pollution to some extent, severe contamination of aquatic ecosystems results in alterations in the fauna and flora of the community [16]. Therefore, particular attention is paid to actions aimed at reducing point and non-point pollution. A good solution is to use the next stage of wastewater treatment or protection of water reservoirs against surface runoff. A very interesting solution to this issue is provided by constructed wetlands (CWs). In many countries, different types of constructed wetlands have been created to improve water quality [17,18,19,20]. Regardless of flow type of the polluted water (surface or subsurface flow), CWs generally consist of the following: (1) an impermeable layer (generally clay or geomembrane), (2) a gravel layer that provides a substrate (i.e., an area that provides nutrients and enables development of microorganisms) for the root zone, and (3) an above-surface vegetation zone (emergent plant and aquatic plants) [21]. The impermeable layer prevents infiltration of wastes down into lower aquifers. The gravel layer and root zone are where water flows and bioremediation, as well as denitrification, take place. The aboveground vegetative layer accumulates nutrients and heavy metals in different tissues. Floating Treatment Wetlands (FTWs) constitute the latest version of CWs. These systems consist of a floating element (usually made of a plastic material) on which the plants are established and optically look like floating islands. As in the other CWs types, the plants develop a deep and dense root system within the underlying water column [20].
Constructed Wetlands have the following advantages when compared with the traditional sewage treatment methods:
- They are relatively inexpensive to construct and operate and are easy to maintain;
- They provide effective and ecologically friendly wastewater treatment;
- They can tolerate both great and small volumes of water and varying contaminant levels [22].
Plant species differ in their ability to extract biogenic compounds and metals from wastewater [23,24,25,26,27]. The common reed is a species that is very often used in these systems [28,29,30]. There are many reasons why reed is often chosen. Firstly, P. australis, as an emergent perennial plant, has a very wide geographical range that encompassed many climatic and ecological zones [31,32,33,34,35,36]. Reed is a typical swamp and aquatic plant species; it inhabits both aquatic and terrestrial ecosystems [37]. It is a cosmopolitan species, widespread in temperate and tropical regions around the world, except for Antarctica [33,34,35]. The natural range is difficult to determine due to the dilation of this species in many places in the world and its easy placement [38,39]. The plant is extensively distributed in North America, and, with the exception of Alaska, it can be found in all US states, Canadian provinces, and territories, except Nunavut and Yukon [40]. The common reed is a native plant to Puerto Rico and non-native to Hawaii [41]. In North America, the non-native common reed haplotype is extensively distributed [42]. Its occurrence extends from British Columbia to Quebec, and in the south, it is found throughout the contiguous United States [33]. The plant is common in Europe, North Asia, Central and South-West Asia (from the Mediterranean to Pakistan), East Asia, and in Australia [33,43,44]. Phragmites australis is found in a belt around the dense forest zone in tropical Africa, from Senegal east to Eritrea, as well as to the south of Ethiopia and Eritrea to Mozambique, Zimbabwe, Namibia, South Africa, and Swaziland. It also occurs in Madagascar [45]. The lineages and genotypes of P. australis are diverse both within and among populations. Moreover, genes from relatives from other phylogeographic regions and species can be incorporated into populations [31]. Phragmites australis is a cosmopolitan wetland grass which is classified as one species (Figure 1) but consists of three main phylogeographic groups [34,46].
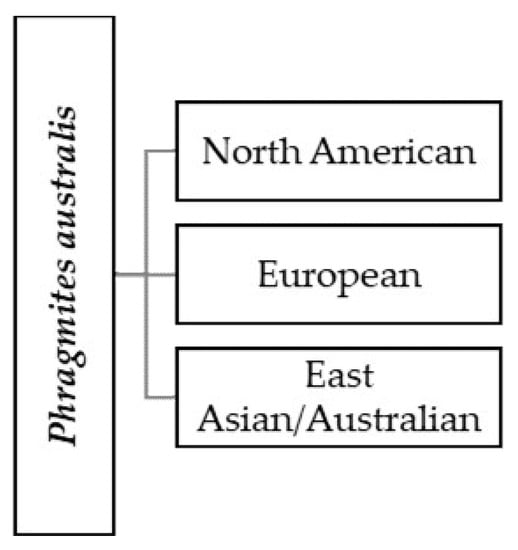
Figure 1.
Main phylogeographic groups, sourced from Reference [31].
Regardless of its geographical location, this species provides food and habitat for some organisms and serves to stabilize soils against erosion. Secondly, many biological features of this species predispose it to be used as a biological pollution filter [35]. In comparison with other species of emergent aquatic plants, P. australis has annual cane-like stems and is characterized by relatively high growth and mass (up to 6 m in height), show variations in diameter from 4 to 10 mm, and has long hollow internodes of 10–25 cm in length, as well as an extensive rhizome system. The perennial rhizomes have both horizontal and vertical components. The extension of the size of the clone is due to horizontal rhizomes, while the annual upright stems are due to vertical rhizomes. Rhizomes are characterized by an extensive aerenchymatous tissue. Its roots develop from rhizomes and other submerged parts of shoots. Rhizomes form the largest densities at a depth of 0.5 m. The lifespan of individual rhizomes is, on average, about six years, and they can grow within a radius of 10 m, at a rate of 1 per year. It grows well on various types of substrates, from sandy and gravel, through peat soils to various types of gyttia and mules. The leaves are smooth, alternate with narrow-lanceolate laminae 20–70 cm long and 1–5 cm wide. They are closely nerved, and they taper to long slender points [42]. The inflorescence is a terminal panicle, often 30 cm long, lax, with a color from dull purple to yellow, and the main branches bear many spikelets. The smooth branches usually have scattered groups of long silky hairs [47] (Figure 2).

Figure 2.
Phragmites australis (Photographers: Justyna Milke and Małgorzata Gałczyńska): (a) inflorescences in the form of panicles, (b) lanceolate leaves, (c) new green, young steams, (d) rhizomes
Thirdly, photosynthesis is the primary physiological process which determines plant growth and crop productivity and influences many other plant processes. There are three different plant systems in nature, viz., C3, C4, and crassulacean acid metabolism (CAM), characterized by CO2 trapping mechanisms. However, C4 and CAM plants are generally found to employ a C3 pathway to trap CO2 as the initial step. P. australis is a plant that exhibits photosynthetic properties of the C3 pathway [48,49]. It was also observed that P. australis exhibits characteristics of both C3 and C4 pathways, because its carbon anhydrase activity is typical of a C3 plant, while its phosphoenolpyruvate carboxylase activity ratio is indicative of a C4 plant [50,51]. Table 1 shows the P. australis survival strategy in two types of environmental conditions.

Table 1.
The C3–C4 ecotype of Phragmites australis. Modified from References [31,51].
The analysis of the information presented in Table 1 allows us to conclude that the phytoremediation process is more effective at low soil moisture than in a typical water environment (floating islands with P. australis). In conditions similar to the terrestrial environment, the plant is characterized by a greater increase in biomass, associated with an increased uptake of biogenic compounds, particularly N, and superiorly developed mycorrhiza, which supports the plant in the process of decontamination. The listed biological features of this species and its photosynthetic capacity for different mechanisms of CO2 trapping are related to relatively high growth, the possibility of obtaining high biomass in crop, and a highly developed root system—crucial adaptive properties of plants for phytoremediation of aquatic ecosystems.
Furthermore, even though Phragmites australis (Cav.) Trin. Ex Steud. is considered native to Europe, the adaptive features of this plant show its competitive character. Owing to high intraspecific diversity and phenotypic plasticity, the common reed shows an extensive ecological amplitude, as well as great acclimatization capacity to adverse environmental conditions. Phragmites australis grows in soils with various salinity [52,53,54,55], fertility [56], textures [57], and of different pH [28,58] and attains high productivity under different climatic conditions [10,32,59]. It is a highly adaptable emergent macrophyte, with a broad range of tolerance to flooding regimes [60,61,62,63,64,65]. Being a native species, P. australis shares many characteristics with invasive species [66,67,68,69,70].
The purpose of the present review article was to show the following: (1) The reed is a versatile and adaptable species and can therefore be implemented in constructed wetlands for phytoremediation in various geographical regions (wide geographical range, biological features of this species, and ecological background—the first section); (2) an overview of how well the reed can remove different pollutants from wastewater, in comparison to other aquatic species (the second section and fourth section); (3) presentation and summary analysis of the usefulness of applying the reed in different types of constructed wetlands to remove a number of frequently occurring pollutants (radar charts).
These characteristics aim at presenting the current research progress concerning the potential of P. australis for removing nutrients, heavy metals, and other chemical substances in wastewater treatment systems.
2. The Potential for Heavy Metal Absorption by Common Reeds Compared to Other Aquatic Plants
2.1. The Absorption of Heavy Metals by Plants
The contamination of aquatic environments is one of the most important global problems, because they are irreplaceable, and most of them, having exceeded certain concentrations of pollutants, have a toxic effect on living organisms. Reclamation of water ecosystem is the main priority for all ecologists worldwide. Even though metals are one of the largest categories of pollution, it is worth noting that they are perfectly removed by aquatic plants [71].
Metals with a high density (≥5 g/cm3) are often regarded as heavy metals. These metals are introduced into aquatic systems with agricultural runoff or industrial discharge. Increased levels of heavy metal contaminants in water have a negative effect on the ecological function of water, including recycling and primary production of nutrients. Moreover, the health of wildlife and humans is affected through bioaccumulation in the food chain, with the lasting impact of developing metal tolerance among certain organisms, even at a very low concentration [72]. Heavy metals are removed from the environment by aquatic plants through the following three processes [73,74]:
- (1)
- Plants attach the heavy metals to their cell wall;
- (2)
- The roots accumulate heavy metals and then translocate them to the shoots;
- (3)
- Hyperaccumulation (the ability to accumulate metals at very high concentrations in aboveground tissues, without phytotoxic effects).
Some heavy metals are needed for the upkeep and growth of aquatic plants. However, when the concentrations become excessive, the plant may be at a risk of heavy metal toxicity, both directly and indirectly [72]. Therefore, aquatic plants have developed defense mechanisms that allow toxic metals to survive [75,76,77]. Avoidance mechanisms constitute a strategy for extracting roots from the root cells out of the rhizosphere, e.g., compounds that heal metal ions [78] metal cations, including micronutrients, are primarily taken up by plant roots. According to Kushawha et al. [79], it is possible to attribute the cellular mechanism of detoxification and tolerance to metals to the following: (1) immobilization by mycorrhizal associations; (2) heavy metal restriction by binding to a plant cell wall (3) heavy metals chelation by root exudates, e.g., sugars and polysaccharides, organic and amino acids, peptides, and proteins; (4) reduced heavy metals influx by the plasma membrane; (5) active heavy metals efflux; (6) chelation by various ligands, i.e., phytochelatins, metallothioneins, and organic and amino acids.
In plants, heavy metals uptake takes place through the root system, but also through leaf blades. The easiest way for plants to take up metals from the soil is in the form of free ions, while the metals occurring in the form of complexes can be mobilized by active substances secreted by plant roots and then collected by plants [80,81]. The effectiveness of the phytoremediation process increases due to interactions between the plant’s roots and the microorganisms in the rhizosphere.
2.2. The Role of Microbial Interactions with Common Reed in Heavy Metal Uptake
To a great extent, plant growth and development depend on the activity of soil microorganisms found in the rhizosphere. These microorganisms influence the shaping of plants in various ways [82], and the mutualisms between the plants and their microbiome are common and facilitate plant invasion processes [83]. Phragmites australis is a macrophyte that is very productive, and its root zone is rich in dissolved oxygen [84] and organic carbon [85], providing suitable conditions for the colonization of microorganisms. Bacteria and mycorrhizal fungi found in the rhizosphere play an important role in phytoremediation trough degrading metals, organic pollutants, radionuclides, and xenobiotic compounds [83,84,85,86,87,88,89,90,91]. Soil microbes participate in mobilization of metals for plant uptake or immobilization of metals in the rhizosphere to restrict leaching. They help in these processes through acidification, chelation, and reduction of metals in the soil (for example, Pseudomonas fluorescens produces citric acid, but Rhodococcus sp. Reduces Arsenic (As) (VI) to As (III) [92]. For example, in the rhizosphere of P. communis, the most abundant acidophilic bacterium Gp6 and the dominant heterotrophic microorganism Gp7 were important members of soil microbes. Zhao et al. [89] pointed out that As and Nickel (Ni) promoted the growth and reproduction of Gp6 and Gp7. In turn, the dominant bacteria such as Gp6 and Longilinea were involved in metabolizing multiple carbohydrates and amino acid in the soil. Aerobic tissues in the stems of P. australis enable the roots to release oxygen and other primary and secondary metabolites into the rhizosphere [85,93], and they consequently create an oxygen-enriched sediment microhabitat. In their research, Chaturvedi et al. [86] have shown that the rhizosphere of P. australis contains many aerobic microorganisms, such as Microbacterium hydrocarbonoxydans, Achromobacter xylosoxidans, Alcaligens faecalis and species that belong to the genus Bacillus and Pseudomonas. Fifteen culturable bacterial species were grown on effluent-supplemented medium as a sole carbon source, resulting in the reduction of the levels of distillery effluent pollution with heavy metals. The latest research provides information on the impact of the winter or summer season on the diversity and composition of the microbiome [94], which, in addition to slowing cane vegetation, may additionally determine the rate of the phytoremediation process.
Arbuscular mycorrhizal fungi (AMF) also play a major role in decontamination of the rhizosphere. They are ubiquitous, obligatory plant symbionts. Fungi provide nutrition, particularly for plants. These microbes make plants more efficient in absorbing environmental resources, interact with indigenous microbes, and enhance the plant’s tolerance to stress, by promoting the secretion of glycoproteins into the rhizosphere. The expansion of the mycelium can greatly extend the area of influence of the rhizosphere. Increasing the rhizosphere means an increase in the bacterial population, which can also contribute to the bioremediation process. Arbuscular mycorrhizal fungi have been reported to occur in the association with wetland plants, too. The community structure of AFM is characteristic for specific plant species [90]. Huang et al. [95] suggested that AMF symbiosis with roots of P. australis can result in a marked tolerance to Cd via accumulating Cd with a shorter exposure treatment time. The decrease in phytotoxicity was mainly accomplished by increasing enzyme activities and levels of thiolic compounds in roots. In another research, Wu et al. [91] pointed out that AMF could effectively improve the growth and physiological activity of P. australis under copper stress. Excess copper accumulation in P. australis leads to a decrease in photosynthetic enzyme activity, yet the inoculation AFM of Rhizophagus irregularis can alleviate this adverse effect. Regardless of copper concentration, the response of P. australis after AMF addition is closely related to intracellular energy transfer. In turn, Malicka et al. [90] reported a negative affect by the presence of polycyclic aromatic hydrocarbons and phenol on the roots’ mycorrhizal colonization and AMF biomass in the soil.
2.3. Arrangement of Heavy Metals in Various Parts of Common Reed
The amount of metals taken in by plants is determined by the type of metal, their content in the soil, the forms in which they occur, and plant species [96]. Cell walls of individual root tissues form a barrier limiting migration of trace elements to the aboveground parts of plants. Bonanno et al. [97] reported that belowground organs were the primary areas of Cd, Cr, Copper (Cu), Hg, Manganese (Mn), Ni, Pb, and Zinc (Zn) accumulation. In particular, determined metal concentrations in P. australis organs show a decrease in the order of root > rhizome > leaf > stem.
Toxic effects of metals are associated with their excessive concentrations in the cell [98]. These concentrations cause disturbances in the functioning of membranes [99] in photosynthetic and mitochondrial electron transport, and also affect the inactivation of many enzymes involved in the regulation of basic cell metabolism, e.g., nitrate reductase [100], which in turn leads to a reduction in the energy balance of cells. Other specific effects include chlorosis and leaf necrosis, followed by traces of senescence and abscission, which lead to lower nutrient uptake and interfere with the biomass acquired [72].
Aquatic plants are natural absorbers of heavy metals and other nutrients; therefore, for many metals, there is a simple relationship between their content in the environment and the amount accumulated in plants [101]. In addition, based on biochemical composition, habitat, species, abundance, and environment, these macrophytes manifest the ability to absorb the said pollutants at various rates and with different efficiencies [102]. Heavy metals concentrations in individual parts of aquatic plants (roots, stem, and leaves) are varied and depend on the species, environmental conditions, metal uptake, transport mechanisms, and interactions between metals [103]. The rate of photosynthetic activity and plant growth play a role in removing small to medium amounts of pollutants during the implementation of phytoremediation technology [104].
Phragmites australis is one of the most studied aquatic plants for removing heavy metals because of its high potential for metal removal and fast growth, as well as its accumulation of metal in aboveground and belowground biomass [69]. This plant tends to release excessive metal ions by transpiration, reducing toxic concentrations in leaf tissues [105] It is also considered to be an "accumulator", that is, it accumulates metals in the roots [106]. Rzymski et al. [6] noted the accumulation of Cr, Cd, Cu, Cobalt (Co), Iron (Fe), Pb, Mn, Ni, and Zn in the roots of P. australis, as well as the translocation of Cd and Pb in the leaves. Peltier et al. [107] observed high accumulations of Zn and Mn in the roots of P. australis.
Table 2 shows concentrations of heavy metals in the organs of P. australis, in studied mesocosms and water ecosystems.

Table 2.
Heavy metal concentrations in the organs of P. australis (mg/kg, standard deviation SD).
Kastratović et al. [108] investigated the accumulation of Cd, Co, Cr, Cu, Mn, Ni, Pb, Zn, Sr, and V in sediment, water, and different organs of P. australis. The plants were collected from Lake Skadar, Montenegro, in different seasons of 2011. The concentrations of five (Cd, Cu, Mn, Zn, and Sr) out of the ten metals under analysis were found to be higher in the plant than in sediment during, as well as after, the growing season. At the same time, metal concentrations determined in the plants were found to be much higher than those identified in the water. This indicates that the sediment is the major source of the metals absorbed by the plant roots. Prica et al. [113] analyzed the concentrations of heavy metals (Fe, Mn, Ni, Zn, Pb, Cd, Co, and Cu) in P. australis plants spontaneously growing in shallow water of several mine tailing ponds. It was revealed that behavior of the metals within the plant and their toxicity are not merely a function of their total concentrations but also depend on the plant species and mechanisms involved in sequestration and translocation of particular metals within the plant. The study by Bonanno [111] showed bioaccumulation of trace elements in three wetland plants located around the worldwide: Typha domingensis, P. australis, and Arundo donax. The purpose of the study was to demonstrate which species shows superior potential for the removal and monitoring of the following elements: Al, As, Cd, Cr, Cu, Hg, Mn, Ni, Pb, and Zn. It was found that all species have the potential to be used as biomonitors of trace element contamination in sediment; however, only P. australis and A. donax exhibited a correlation with water. Many studies indicate that, in P. australis, the concentration of accumulated metals is higher in belowground organs than in ground organs. Other studies show the following order of metal accumulation: roots > leaves > stems [29,123,124]. In their research, Klink et al. [122] showed that the roots of the P. australis were correlated with the highest Mn, Fe, and Cu concentrations. The highest Pb, Zn, and Cd concentrations were identified in Typha latifolia roots. Despite the differences in the ability to accumulate trace metal between the studied species, the concentrations of Fe, Cu, Zn, Pb, and Ni in the P. australis and T. latifolia followed the accumulation pathway: roots > rhizomes > leaves > stems. Mn concentration decreased according to the following order: root > leaf > rhizome > stem. There are some common characteristics of wetland plants, e.g., high tolerance to toxic element levels, capacity of phytostabilization, and different element concentrations in various organs [97]. Despite some ecological and morphological similarities, different plant species respond differently to heavy metals exposure. This may result from an ability of a given species to accumulate and detoxify various metals rather than differences in their ecological and morpho-anatomical characteristics [115]. In the study by Chernykh et al. [121], the regularities of accumulation of heavy metals (Fe, Cu, Zn, Cd, and Pb) and As in various types of aquatic vegetation were studied, with respect to season and the levels of pollution of the Srepok River (Vietnam). The results show that the concentrations of Fe, Cu, Zn, As, Cd, and Pb in all studied areas of the river were higher in the roots of water hyacinth and common reed than in the stems.
The present review of the literature allowed us to develop a graph in which metal arrangement in common reed organs was marked (Figure 3). In accordance with environmental standards for waters in Poland (Regulation of the Minister of the Environment of July 21, 2016, on how to classify the status of surface water bodies and environmental quality standards for priority substances), the elements leading to deterioration of surface waters are marked in red.
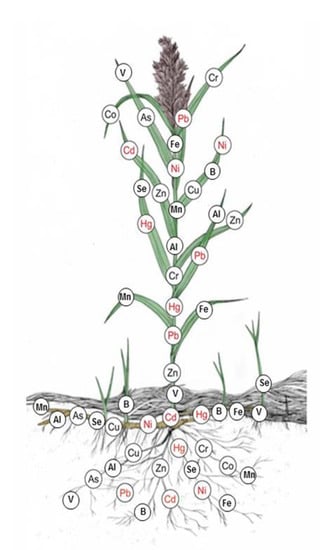
Figure 3.
Arrangement of heavy metals in various parts of P. australis, own study.
2.4. Comparison of Removal of Heavy Metals by Common Reed and Other Aquatic Plants
The review of scientific papers published between 1995 and 2020 was carried out in relation to three terms: phytoremediation, aquatic plants, and heavy metals found in the title and keywords of articles. The total number of records was 7617 (Figure 4). Phragmites australis appeared in 25.4% scientific papers. The selection of other species to compile the data in Table 3 was also based on the percentage share of other aquatic plant species in the phytoremediation of aquatic ecosystems contaminated with heavy metals. The list of plant species includes the diversity of their habitat (emergent: T. latifolia—14.2% all articles, Hippuris vulgaris—0.3%; submerged: Ceratophyllum demersum—9.3%; floating aquatic plants: Lemna minor—18.8%, Eichornia crassipes—18.6%, Pistia stratiotes—9.7%, Hydrocharis morsus-ranae—0.5%). Other species described in the literature accounted for 3.2%. Among the floating aquatic plants, there are data on a high capacity of H. morsus-ranae. This species, especially in Canada, creates compact floating mats and, since it is invasive, reduces the biodiversity of local aquatic ecosystems [127]. The fight against this species brings positive, effects because the mechanical removal of this plant from reservoirs contributes to reducing the concentration of heavy metals in water. Another species little described in the literature is H. vulgaris. Information on its heavy metal uptake capacity is given in Table 3, below, as this species can be used in the treatment of municipal wastewater not only in Europe, but also in Asian countries, such as China. In addition, this plant occurs in various forms, as terrestrial, wetland, and underwater.
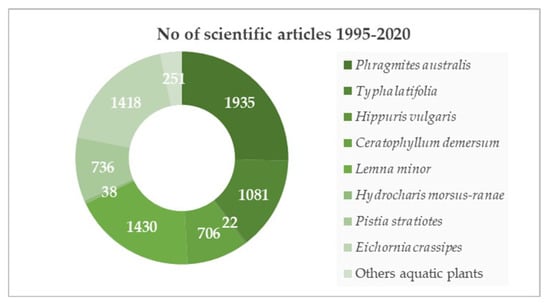
Figure 4.
Number of scientific articles from 1995 to 2020 on aquatic plants used in phytoremediation with respect to heavy metals.

Table 3.
Comparison of removal of metals by some water plants.
Despite the large number of publications on phytoremediation, it was not possible to document the effectiveness of wastewater treatment by several plant species for some metal concentrations in wastewater. Nevertheless, the data in Table 3 may provide useful information on the high degree of wastewater treatment, with varying metal concentrations through the common reed and other biofilters.
Depending on the concentration of heavy metals in polluted waters and information on the efficiency of their removal, it can be indicated, with some probability, which species of aquatic plants, under given conditions, are most suitable for phytoremediation. High efficiency of this process, over 90 percent gives the possibility of using only one species for water purification. The construction of multi-species biological systems is associated with many problems. One of them is the possibility of inter-species competition, which, over time, can lead to the dominance of one species. The next problem may be the ease of transferring pathogens between co-growing plant species. Moreover, not less important is having the knowledge and skills to perform various care treatments in relation to these species. The positive effect of using, for example, two or three species is the ability to reduce even very high metal concentrations in treated waters. By analyzing the combination of the efficiency of removing a given metal from water by selected plant species, it can be determined which of them, one by one, could be reduced by absorption of the concentration of this metal in water. For example, for Hg (Figure 5), such a sequence would look like the following:
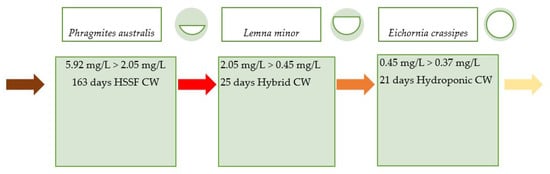
Figure 5.
Plant sequence as biosorbents and the effect of reducing Hg concentration in water.
In this combination, with a high level of pollution 5.92 mg Hg/L, the first species that could participate in phytoremediation would be P. australis (mean rate efficiency 65%), followed by L. minor (82.84%), and finally, after acidification of the environment to pH 5.5, E. crassipes (16.52%). The entire biosorption system could potentially remove this toxic metal to 0.37 mg/L, which is a 93.75% reduction in concentration. This hypothetical arrangement of consecutive plots of constructed wetland with the listed plant species and wastewater retention time is a proposal that requires testing. However, this gives grounds for the composition of constructed wetlands with a high degree of mercury removal in wastewater treatment.
The data in Table 3 do not indicate that P. australis has always been the most effective species for use in phytoremediation. Several scientific articles have been found in which the removal efficiency of this plant and other emerging aquatic plant species (P. australis, T. latifolia, Phalaris arundinacea, Vetiver zizanioides, Acorus calamus, Juncus effuses, and Helianthus annus) was compared at the same metal concentration. These emergent aquatic plants play a very important role in the removal of heavy metals from municipal or industrial wastewater [159,160,161,162,163,164,165]. The conducted researches indicate high efficiency for P. australis, but also for other species at the same metal concentrations. These data are presented in Figure 6 for eight heavy metals (nickel, cadmium, iron, chromium, lead, manganese, zinc, and copper). They indicate the general regularity that the higher metal concentration in the wastewater led to the lower its removal efficiency (Figure 6b,e). In addition, some of the data of rate removal of metals are very similar to each other, even though they show the results from different studies. This indicates the repeatability of the results obtained, and thus their reliability. Phragmites australis has a higher removal efficiency of Ni and Cd than T. latifolia and J. articulatis in all of these metal concentrations [159,160]. In the case of Pb and Fe, it occurs that the common reed removes these metals better at higher, than at a lower, concentrations (Figure 6c,d).
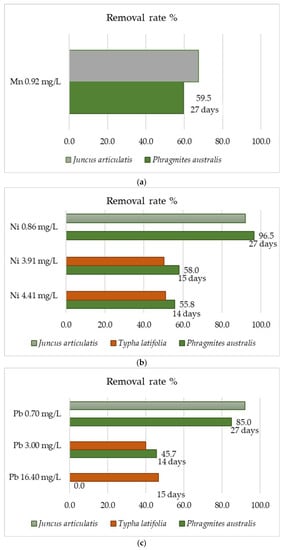
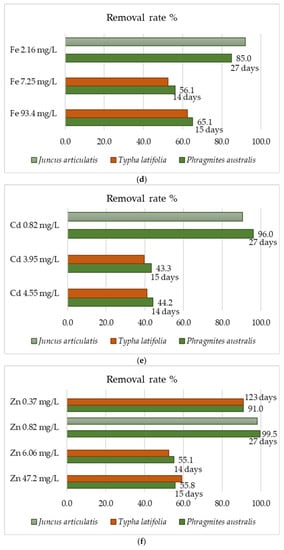
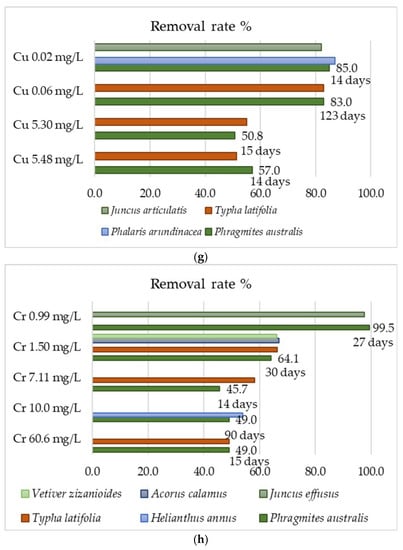
Figure 6.
Heavy metals removal rate % for P. australis and the others emergent aquatic plants: (a) Mn, (b) Ni, (c) Pb, (d) Fe, (e) Cd, (f) Zn, (g) Cu, (h) Cr.
3. Removal of Other Contaminants by the Common Reed
The widespread use of pharmaceuticals, medicines, and personal-care products is becoming an increasing threat to water management. Phragmites australis additionally removes salts and works well in phytodesalination of soil and water. The presence of drugs in the aquatic environment may have an adverse effect on organisms living in the aquatic and terrestrial environment and cause a decrease in the diversity of algae and immunization of organisms to antibacterial agents. Phragmites australis is also used for removing different types of compounds—silicone, dyes, pesticide, pharmaceuticals, personal-care products, and illicit drugs—from wastewater [15,166,167]. For example, P. australis degrades ibuprofen (IBP) from water after 21 days of exposure and is therefore suitable for use in constructed wetlands, for the purpose of cleaning wastewater effluents containing IBP [168]. Phragmites australis is used for phytoremediation of veterinary medicines, and their removal is 94% with respect to enrofloxacin and 75% for tetracycline observed from enriched water [169]. Lv et al. [170] found that P. australis was able to remove 96.1% of tebuconazole and 99.8% of imazalil from the aquatic medium. Pesticide removal from the hydroponic solution was not enantioselective. However, tebuconazole degradation was enantioselective both in the roots and shoots. Imazalil was also enantioselectively translocated and degraded inside Phragmites: R-imazalil translocated faster than S-imazalil. Jie-Ting et al. [14] showed that, by using P. australis in laboratory-simulated vertical wetland systems, it is possible to remove 36.9% of polycyclic aromatic hydrocarbons in continuous and intermittent feeding.
The influence of antibiotics (enrofloxacin and ceftiofur) on removing metals by constructed wetlands was investigated in mesocosms planted with P. australis. More than 85% removal of Fe, Cu, and Zn was achieved. It was also noted that ceftiofur improved metal uptake by P. australis and showed no adverse impacts of antibiotics [171]. The research conducted by Verlicchi et al. [172] shows that P. australis was more effective than T. angustifolia in the removal of pharmaceutical compounds, including ibuprofen, diclofenac, and caffeine. The effectiveness of wastewater removal compounds such as salicylic acid, IBP, naproxen, diclofenac, and caffeine was respective for the VF-CW type deposit, 98%, 99%, 89%, and 73%, and for the HF-CW type, 96%, 71%, 85%, 15%, and 97%. Plants (Juncus, Typha, Berula, P. australis, and Iris) increase the process of microbial degradation, owing to oxygen availability. Similarly, higher hydraulic residence times and macrophyte covers were found to improve the removal efficiencies of androstenedione, carbamazepine, caffeine, diclofenac, estrone, IBP, paracetamol, propranolol, and triclosan in a CW treating hospital wastewater [173]. Kankılıç et al. [174] investigated the removal of methylene blue (MB) from aqueous solutions by using the reed species P. australis as an adsorbent. It was found that the adsorptive capacity of crude P. australis increased significantly by modification reaction. The results showed that both P. australis and its modified forms have the potential as an ecological adsorbent for removing MB from the volatile substance.
The increase in water pollution with nutrients affects the accelerated process of their eutrophication. Therefore, in the assessment of surface water quality, in addition to mineral forms of N and P, total nitrogen (TN) and total phosphorus (TP) are also determined. These parameters are also of key importance in assessing the efficiency of wastewater treatment. Total organic carbon (TOC) is one of the most important parameters for the knowledge of water and wastewater quality, because it concerns theoretically all organic compounds. Chemical oxygen demand (COD) and biological oxygen demand (BOD) are other parameters widely used in indicating organic pollution, with respect to both wastewater and surface water. Biological oxygen demand is defined as the oxygen requirement of microorganisms to carry out biological decomposition of dissolved solids or organic matter in wastewater, under standard temperature, after five days. Chemical oxygen demand is an indispensable parameter in the analysis of the quality of water, since it provides an index to assess the impact of discharge on the receiving water body. Another parameter of wastewater that is just as frequently monitored is total suspended solids (TSS).
There are also scientific reports that indicate a significant effect of microorganisms in the rhizosphere on increasing the capacity of common reed to remove organic compounds. Fifteen culturable bacterial species were grown on effluent-supplemented medium as a sole carbon source, resulting in the reduction of the levels of distillery effluent pollutants with heavy metals and their color by 75.5% [86]. Concomitantly, there was a reduction in, for example, phenol sulfate. The presence of certain microorganisms also depends on the chemicals released by the reed. Toyama et al. [85] found that P. australis root exudates containing phenolic compounds supported growth and degrading activity of the Mycobacterium gilvum strain. Mycobacterium-root exudate interactions can accelerate pyrene and benzo[a]pyrene degradation. The results by Dan et al. [87] also determine the effect of selected phenolic compounds (namely p-hydroxybenzoic acid, p-coumaric acid and ferulic acid) on enriching the composition of bacteria (including Luteolibacter, Reyranella, Asticcacaulis, Pseudomonas, Novosphingobium, and Rhodocus), degrading p-tert-butylphenol, as well as its chemical decomposition. The microbial degradation in the rhizosphere of P. australis ranged from 17 to 44%.
4. Common Reed in Constructed Wetlands
Macrophytes constitute the essential part of constructed wetland and manifest distinguished properties with respect to specific wastewater treatment processes. Among those are morphological adaptations to develop in water saturated soils, an extensive lacunar system facilitating substantial oxygen transport to the well-developed roots of the plant and rhizosphere, high growth rate, and the ability to incorporate biomass [24]. Currently, thousands of constructed wetlands are used for treatment purposes in polluted waters, since they constitute a low-cost alternative with respect to maintenance, operation, and construction. Additionally, constructed wetlands can be employed in different design and component combinations for different types of wastewater and concentrations of pollutants [175,176,177]. Through using constructed wetlands, the following objectives can be achieved: domestic wastewater treatment and agricultural runoff, industrial wastewater treatment, treatment of landfill leachate, flood treatment and urban runoff, post-treatment of wastewater, eutrophic lakes restoration, and treatment of water polluted by nutrients such as nitrate and phosphate [7,21,178,179,180]. The hydrophyte systems are used for wastewater treatment after a mechanical (preliminary) or biological first stage of wastewater treatment, often conducted in conventional wastewater treatment plants. For the purpose of effective treatment of wastewater in the artificially constructed wetlands horizontal flow (HF), vertical flow (VF), and hybrid constructed wetlands (Figure 7) are used worldwide [9,18,181,182,183]. Surface-flow wetlands are very similar to natural wetlands. The combined-technique approach is another innovation in recently developed constructed wetlands. It consists of employing two or more techniques (VF and HF systems) for the treatment of different wastewaters. This type of treatment shows higher efficiency and less infrastructure requirements, as well as low energy consumption [184].
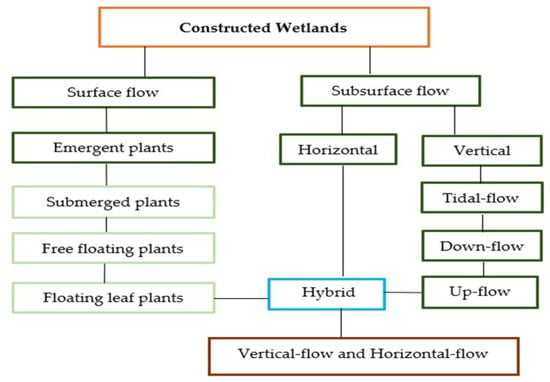
Figure 7.
Classification of constructed wetlands for wastewater treatment, sourced from References [185,186,187,188].
High efficiency in removing various types of contaminants, such as organic matter, detergents, pharmaceuticals, N and P compounds, heavy metals, suspended solids, and trace elements, e.g., Cu, Zn, Al, etc., in constructed wetlands in many continents (Europe, Canada, Australia, and most parts of Asia and Africa), confirmed the potential ability of common reed to undisturbed development in an environment with a high concentration of pollutions and sewage treatment [189]. The review of scientific articles published in the years 1995–2020 in the field of the use of P. australis in constructed wetlands (Figure 8) indicates that this species has been used to a greater extent in systems with subsurface flow of contaminated waters than with surface flow (1504 records from 2754 records). This is due to the photosynthetic properties of the CO2 pathway for this species. In a typically aquatic environment, as compared with a terrestrial environment, lower biomass growth and a smaller N removal effect are obtained.
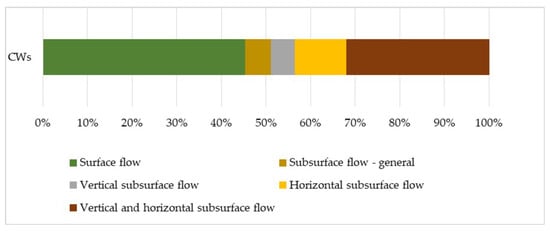
Figure 8.
Percentage rate of articles, from 1995 to 2020, about constructed wetlands with common reed.
The analysis of the functioning of constructed wetlands is associated with an assessment of the effectiveness of removing impurities (Figure 9). This is of great importance for ensuring the purity of natural aquatic ecosystems to which treated wastewater is discharged. Various elements of these systems are tested. In the literature, some of the most important issues that are considered and often described relate to the following: the impact of vegetation on the uptake of metals and biogenic compounds by common reeds and individual organs of this plant; the control of the purification process due to the introduction of other species of flora and fauna to the hydrophyte system; and, in recent years, a detailed description of the role of microorganisms inhabiting the rhizosphere on the efficiency of removing organic compounds. Genetic research is becoming increasingly more important to accurately describe the mechanisms of bacterial decontamination of contaminants and facilitative arbuscular microbial fungi. The examples of the effects of the most interesting scientific studies related to the above issues are presented below, based on the recent literature [13,87,190,191,192,193,194]. Mulkeen et al. [195] analyzed the seasonal variations of metals and nutrients in P. australis in a CWs treating municipal wastewater. Investigations of uptake and seasonal variations in storage capacities of nutrients in P. australis were also taken in CWs under Irish climatic conditions [196,197]. Vymazal and Březinová [198] assessed the amount of heavy metals absorbed in aboveground biomass P. australis and found that their amount in plant tissues in constructed wetlands is highly variable. The amount of heavy metals accumulated in the aboveground biomass of P. australis (aboveground standing stock) represents often only small fraction of the inflow annual load, but in some studies, this fraction was quite high, especially for Zn (up to 59%), and more rarely for Cd (55%) and Cr (38%).
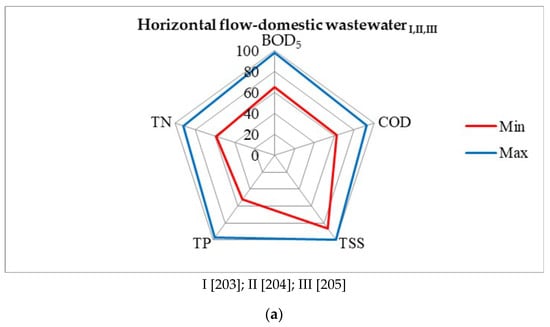
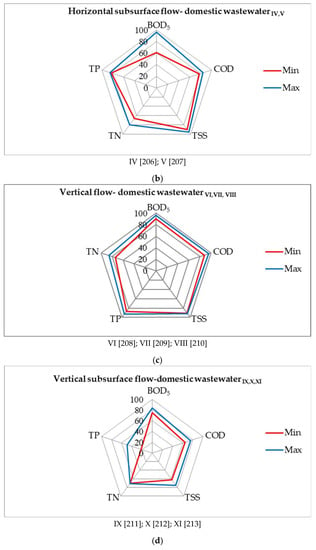
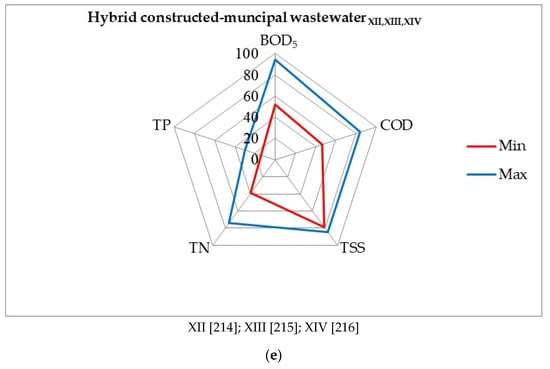
Figure 9.
Evaluation of the effectiveness of wastewater treatment systems using P. australis: (a) horizontal flow—domestic wastewater, (b) horizontal subsurface flow—domestic wastewater, (c) vertical flow—domestic wastewater, (d) vertical subsurface flow—domestic wastewater, (e) hybrid constructed—municipal wastewater [203,204,205,206,207,208,209,210,211,212,213,214,215,216].
The investigation of the growth dynamics and nutrient and heavy metal shoot accumulation of the two dominating macrophytes, P. australis and Bolboschoenus maritimus, was conducted on constructed wetland of the Venice lagoon watershed [199]. In order to assess the effects on vegetation, the research was carried out in a vegetative season in three locations, characterized by different distance to the inlet points. It was shown that the said distance had no effect on shoot biomass, nor on the nutrients (N, P, Potassium, and Sodium (Na)) or heavy metals (Cr, Cu, and Zn) shoot content. In comparison with B. maritimus, the concentrations of nutrient and heavy metals, however, with an exception of Na, was found to be higher in the shoots of P. australis. The obtained results confirm that P. australis shows a superior efficiency.
According to results by Toscano et al. [200], P. australis shows superior removal capacity in comparison with Vetiveria zizanoides, Miscanthus x giganteus, and Arundo donax. Furthermore, it confirms that this plant is a superior plant species to be used in constructed wetlands for wastewater treatment. Importantly, the vegetation growth positively affects NH4 growth. This particularly is the case with Phragmites, as 60% of the variable NH4 load is due to vegetation growth. This confirms that the changes in vegetation affect other processes in nutrients removal.
Massoudinejad et al. [201] evaluated the effectiveness of constructed wetland suburban flows (horizontal subsurface flow constructed wetlands) by the Gambusia fish and P. australis (sewage treatment plant) in municipal wastewater treatment. The presence of P. australis and Gambusia fish demonstrated the maximum removal efficiency. In the spring and summer season, the respective mean concentration of ammonium was 14.37 and 19.7 mg/L. Additionally, the presence of P. australis in wetlands resulted in the highest removal of ammonium. According to the results of this study, P. australis and Gambusia fish—when used simultaneously—show the superior properties in removal of COD and BOD5. As the results suggest, it could be a viable alternative to treatment of wastewater in small communities.
Tara et al. [202] presented the performance of a pilot-scale system, carrying P. australis in combination with three plant growth promoting and dye-degrading bacteria (Acinetobacter junii strain NT-15, Rhodococcus sp. strain NT-39, and Pseudomonas indoloxydans strain NT-38) for the purpose of treating textile industry wastewater. High removal capacity of organic and inorganic pollutants was determined for the vegetated tanks. The combined application of plants and bacteria showed a superior removal performance, i.e., COD reduction to 92%, BOD5 to 91%, color to 86%, and trace metals reduced to approximately 87% in the wastewater. The augmented bacteria displayed persistence in water, as well as in the roots and shoots of P. australis, suggesting a potential partnership with the host toward enhanced performance.
Phragmites australis stimulates bacteria-degrading hydrocarbons to do so in water. The results show that the floating treatment wetlands efficiently removed hydrocarbons from water, and that bacterial inoculation further enhanced its hydrocarbons degradation efficacy. The maximum reduction in hydrocarbons (95.8%), COD (98.6%), BOD5 (97.7%), TOC (95.2%), and phenol (98.9%), as well as toxicity, was analyzed in a combination of both plants and bacteria. The augmentation of hydrocarbons degrading bacteria in floating treatment wetlands was found to be a superior option for treating diesel polluted water [180]. Figure 9 shows the removal percentages of BOD5, COD, TSS, TP, and TN of wastewater treatment systems, using P. australis.
5. Conclusion and Future Outlook
Phragmites australis is a naturally robust and vigorous primary species in many wetland environments worldwide. This plant grows in different environmental conditions and can uptake, translocate, and accumulate a wide range of pollutants in both belowground and aboveground tissue. The ability of the plant to develop and grow in the waste sewage ecosystems allowed for the use of reeds in many types of sewage treatment plants. To increase the efficiency of phytoremediation of a polluted natural or artificial aquatic ecosystem and to estimate the required purification time and accelerate the rate of its reclamation, the interaction processes between common reeds and soil microbes, metal accumulation, and ionic homeostasis in the hydrophyte purification systems should be further tested. The researches of especially research carried out by interdisciplinary teams (plant physiologist, biochemist, geochemist, microbiologist, and agriculture and genetic engineer) in a short time can advance the efficiency of removing both metals and organic impurities.
Author Contributions
Conceptualization, M.G. and J.M.; investigation, J.M., M.G., and J.W.; writing—original draft preparation, J.M. and M.G.; writing—review and editing, J.M. and M.G.; supervision, M.G., J.M., and J.W. All authors have read and agreed to the sent version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Ikelle, L.T. Introduction to Earth Sciences: A Physics Approach, 2nd ed.; World Scientific Publishing Company: London, UK, 2020; p. 8. [Google Scholar]
- Sterner, R.W.; Keeler, B.; Polasky, S.; Poudel, R.; Rhude, K.; Rogers, M. Ecosystem services of Earth’s largest freshwater lakes. Ecosyst. Serv. 2020, 41, 101046. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division. Population facts No. 2019/6, December 2019: How Certain Are the United Nations Global Population Projections? United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Häder, D.P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosys-tems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. Eutrophication and recovery in experimental lakes: Implications for lake management. Science 1974, 174, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Niedzielski, P.; Klimaszyk, P.; Poniedziałek, B. Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ. Monit Assess. 2014, 186, 3199–3212. [Google Scholar] [CrossRef]
- Hadad, H.R.; Maine, M.A.; Bonetto, C.A. Macrophyte growth in a pilot-scale constructed wetland for industrial wastewater treatment. Chemosphere 2006, 63, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, N.; Wojciechowska, E.; Matej-Łukowicz, K.; Walkusz-Miotk, J.; Pazdro, K. Heavy metal accumulation and distribution in Phragmites australis seedlings tissues originating from natural and urban catchment. Environ. Sci. Pollut. Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef]
- González-Alcaraz, M.; Egea, C.; Jiménez-Cárceles, F.; Párraga, I.; Maria-Cervantes, A.; Delgado, M.; Álvarez-Rogel, J. Storage of organic carbon, nitrogen and phosphorus in the soil–plant system of Phragmites australis stands from a eutrophicated Mediterranean salt marsh. Geoderma 2012, 185, 61–72. [Google Scholar] [CrossRef]
- Iavniuk, A.A.; Shevtsova, N.L.; Gudkov, D.I. Disorders of the initial ontogenesis of seed progeny of the common reed (Phragmites australis) from water bodies within the Chernobyl Exclusion Zone. J. Environ. Radioact. 2020, 218, 106256. [Google Scholar] [CrossRef]
- Gałczyńska, M. Response of the Mare’s Tail (Hippuris vulgaris L.) and Frogbit (Hydrocharis morsus-ranae L.) to Water Pollution with Heavy Metals and a Possibility of Using These Plants for Water Phytoremediation’; Wydaw. Uczelniane ZUT: Szczecin, Poland, 2012; p. 85. [Google Scholar]
- Lv, T.; Carvalho, P.N.; Casas, M.E.; Bollmann, U.E.; Arias, C.A.; Brix, H.; Bester, K. Enantioselective uptake, translocation and degradation of the chiral pesticides tebuconazole and imazalil by Phragmites australis. Environ. Pollut. 2017, 229, 362–370. [Google Scholar] [CrossRef]
- Jie-Ting, Q.; Shao-Yong, L.; Xue-Yan, W.; Ke, L.; Wei, X.; Fang-Xin, C. Impact of hydraulic loading on removal of polycyclic aromatic hydrocarbons (PAHs) from vertical-flow wetland. Toxicol. Environ. Chem. 2015, 97, 388–401. [Google Scholar] [CrossRef]
- Dhir, B. Removal of pharmaceuticals and personal care products by aquatic plants. In Pharmaceuticals and Personal Care Products. Waste Manag. Treat. Techno. 2019, 2019, 321–340. [Google Scholar]
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H.; Burke, J. Water Pollution from Agriculture: A Global Review; Food and Agriculture Organization of the United Nations: Rome, Italy; International Water Management Institute on behalf of the Water Land and Ecosystems Research Program: Colombo, Sri Lanka, 2017. [Google Scholar]
- Pedescoll, A.; Sidrach-Cardona, R.; Hijosa-Valsero, M.; Bécares, E. Design parameters affecting metals removal in horizontal constructed wetlands for domestic wastewater treatment. Ecol. Eng. 2015, 80, 92–99. [Google Scholar] [CrossRef]
- Hernández-Crespo, C.; Gargallo, S.; Benedito-Durá, V.; Nácher-Rodríguez, B.; Rodrigo-Alacreu, M.A.; Martín, M. Performance of surface and subsurface flow constructed wetlands treating eutrophic waters. Sci. Total Environ. 2017, 595, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecol. Eng. 2005, 25, 478–490. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Akratos, C.S.; Tsihrintzis, V.A. Vertical Flow Constructed Wetlands: Eco-Engineering Systems for Wastewater and Sludge Treatment, 1st ed.; Elsevier Publishing: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Gajewska, M.; Obarska-Pempkowiak, H. 20 years of experience in the operation of wetlands in Poland. Rocz Ochr Sr. 2009, 11, 875–888. [Google Scholar]
- Gałczyńska, M.; Mańkowska, N.; Milke, J.; Buśko, M. Possibilities and limitations of using Lemna minor, Hydrocharis morsus-ranae and Ceratophyllum demersum in removing metals with contaminated water. J. Water Land Dev. 2019, 40, 161–173. [Google Scholar] [CrossRef][Green Version]
- Gałczyńska, M.; Milke, J.; Gamrat, R.; Stoltman, M. Common mare’s tail (Hippuris vulgaris L.) in the assessment of water status and their phytoremediation. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2019, 3481, 57–70. [Google Scholar] [CrossRef]
- Githuku, C.R.; Ndambuki, J.M.; Salim, R.W.B.; Adedayo, A. Treatment potential of Typha latifolia in removal of heavy metals from wastewater using constructed wetlands. In Trans-Formation towards Sustainable and Resilient Wash Services, Proceedings of the 41st WEDC International Conference, Nakuru, Kenya, 9–13 July 2018, WEDC; Shaw, R.J., Ed.; Loughborough University United Kingdom (UK): Loughborough, UK, 2018; pp. 9–13. [Google Scholar]
- Kumar, V.; Singh, J.; Saini, A.; Kumar, P. Phytoremediation of copper, iron and mercury from aqueous solution by water lettuce (Pistia stratiotes L.). Environ. Sustain. 2019, 2, 55–65. [Google Scholar] [CrossRef]
- Odjegba, J.; Fasidi, I.O. Phytoremediation of heavy metals by Eichhornia crassipes. Environmentalist 2007, 27, 349–355. [Google Scholar] [CrossRef]
- Guo, L.; Ott, D.W.; Cutright, T.J. Accumulation and histological location of heavy metals in Phragmites australis grown in acid mine drainage contaminated soil with or without citric acid. Env. Exp. Bot. 2014, 105, 46–54. [Google Scholar] [CrossRef]
- Bonanno, G. Trace element accumulation and distribution in the organs of Phragmites australis (common reed) and biomonitoring applications. Ecotoxicol. Environ. Saf. 2011, 74, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Park, J.; Rupani, P.F.; Darajeh, N.; Xu, X.; Shahrokhishahraki, R. Phytoremediation potential and control of Phragmites australis as a green phytomass: An overview. Environ. Sci. Pollut. Res. Int. 2019, 26, 7428–7441. [Google Scholar] [CrossRef]
- Eller, F.; Skálová, H.; Caplan, J.S.; Bhattarai, G.P.; Burger, M.K.; Cronin, J.T.; Guo, W.Y.; Guo, X.; Hazelton, E.L.G.; Kettenring, K.M.; et al. Cosmopolitan species as models for ecophysiological responses to global change: The Common Reed Phragmites australis. Front. Plant. Sci. 2017, 16, 1833. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Cronin, J.T.; Pyšek, P. Phragmites australis as a model organism for studying plant invasions. Biol. Invasions 2016, 18, 2421–2431. [Google Scholar] [CrossRef]
- Packer, J.G.; Meyerson, L.A.; Skalova, H.; Pyšek, P.; Kueffer, C. Biological flora of the British Isles: Phragmites australis. J. Ecol. 2017, 105, 1123–1162. [Google Scholar] [CrossRef]
- Lambertini, C.; Sorrell, B.K.; Riis, T.; Olesen, B.; Brix, H. Exploring the borders of European Phragmites within a cosmopolitan genus. Aob Plants 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Meadows, R.E.; Saltonstall, K. Distribution of native and introduced Phragmites australis in freshwater and oligohaline tidal marshes of the Delmarva Peninsula and southern New Jersey. J. Torrey Bot. Soc. 2007, 134, 99–107. [Google Scholar]
- Payne, R.E.; Blossey, B. Presence and abundance of native and introduced Phragmites australis (Poaceae) in Falmouth, Massachusetts. Rhodora 2007, 109, 96–100. [Google Scholar]
- Mal, T.K.; Narine, L. The biology of Canadian weeds. 129. Phragmites australis (Cav.) Trin. ex Steud. Can. J. Plant Sci. 2004, 84, 365–396. [Google Scholar] [CrossRef]
- Lessmann, J.M.; Brix, H.; Bauer, V.; Clevering, O.A.; Comin, F.A. Effect of climatic gradients on the photosynthetic responses of four Phragmites australis populations. Aquat. Bot. 2001, 69, 109–126. [Google Scholar] [CrossRef]
- Rooth, J.E.; Stevenson, J.C.; Cornwall, J.C. Increased sediment accretion rates following invasion by Phragmites australis: The role of litter. Estuaries 2003, 26, 475–483. [Google Scholar] [CrossRef]
- Saltonstall, K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc. Natl. Acad. Sci. USA 2002, 99, 2445–2449. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.K. Phragmites australis. In Wildland Shrubs of the United States and Its Territories: Thamnic Descriptions; Francis, J.K., Ed.; Gen. Tech. Rep. IITF-GTR-26; U.S. Department of Agriculture, Forest Service, International Institute of Tropical Forestry: San Juan, PR, USA; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2004; Volume 1, pp. 555–557. [Google Scholar]
- Saltonstall, K. Microsatellite variation within and among North American lineages of Phragmites australis. Mol. Ecol. 2003, 12, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Skálová, H.; Čuda, J.; Guo, W.Y.; Doležal, J.; Kauzál, O.; Meyerson, L.A. Physiology of a plant invasion: Biomass production, growth and tissue chemistry of invasive and native Phragmites australis populations. Preslia 2019, 91, 51–75. [Google Scholar] [CrossRef]
- Rutkowski, L. Key for the Determination of Lowland Poland Vascular Plants; Scientific Publisher PWN: Warsaw, Poland, 2006; p. 159. [Google Scholar]
- Brink, M.; Achigan-Dako, E.G. Plant Resources of Tropical Africa 16; Fibres PROTA Foundation: Wageningen, The Netherlands, 2012; pp. 152–154. [Google Scholar]
- Guo, W.Y.; Lambertini, C.; Li, X.Z.; Meyerson, L.M.; Brix, H. Invasion of Old World Phrag-mites australis in the New World: Precipitation and temperature patterns combined with human influences redesign the invasive niche. Glob. Chang. Biol. 2013, 19, 3406–3422. [Google Scholar]
- Haslam, S.M. Biological Flora of the British Isles. Phragmites communis Trin. J. Ecol. 1972, 60, 585–610. [Google Scholar] [CrossRef]
- Hansen, D.L.; Lambertini, C.; Jampeetong, A.; Brix, H. Clone-specific differences in Phragmites australis: Effects of ploidy level and geographic origin. Aquat. Bot. 2007, 86, 269–279. [Google Scholar] [CrossRef]
- Eller, F.; Brix, H. Different genotypes of Phragmites australis show distinct phenotypic plas-ticity in response to nutrient availability and temperature. Aquat. Bot. 2012, 103, 89–97. [Google Scholar] [CrossRef]
- Antonielli, M.; Pasqualini, S.; Batini, P.; Ederli, L.; Massacci, A.; Loreto, F.T.I. Physiological and anatomical characterisation of Phragmites australis leaves. Aquat. Bot. 2002, 72, 55–66. [Google Scholar] [CrossRef]
- Zheng, W.J.; Zheng, X.P.; Zhang, C.L. A survey of photosynthetic carbon metabolism in 4 ecotypes of Phragmites australis in northwest China: Leaf anatomy, ultra-structure, and activities of ribulose 1,5-biphosphate carboxylase. Physiol. Plant. 2000, 110, 201–208. [Google Scholar] [CrossRef]
- Golet, F.C.; Myshrall, D.H.; Oliver, L.R.; Paton, P.W.; Tefft, B.C. Role of Science and Partnerships in Salt Marsh Restoration at the Galilee Bird Sanctuary, Narragansett, Rhode Island. In Tidal Marsh Restoration; Island Press: Washington, DC, USA, 2012; pp. 333–353. [Google Scholar]
- Ge, Z.M.; Zhang, L.Q.; Yuan, L.; Zhang, C. Effects of salinity on temperature-dependent photosynthetic parameters of a native C3 and a non-native C4 marsh grass in the Yangtze estuary, China. Photosynthetica 2014, 52, 484–492. [Google Scholar] [CrossRef]
- Eller, F.; Lambertini, C.; Nielsen, M.W.; Radutoiu, S.; Brix, H. Expression of major photosynthetic and salt-resistance genes in invasive reed lineages grown under elevated CO2 and temperature. Ecol. Evol. 2014, 4, 4161–4172. [Google Scholar] [CrossRef] [PubMed]
- Nada, R.M.; Khedr, A.H.A.; Serag, M.S.; El-Nagar, N.A. Growth, photosynthesis and stress-inducible genes of Phragmites australis (Cav.) Trin. Ex Steudel from different habitats. Aquat. Bot. 2015, 124, 54–62. [Google Scholar] [CrossRef]
- Schöb, C.; Armas, C.; Guler, M.; Prieto, I.; Pugnaire, F.I. Variability in functional traits mediates plant interactions along stress gradients. J. Ecol. 2013, 101, 753–762. [Google Scholar] [CrossRef]
- Bhattarai, G.P.; Meyerson, L.A.; Cronin, J.T. Geographical variation in apparent competition between native and invasive Phragmites australis. Ecology 2017, 98, 349–358. [Google Scholar] [CrossRef]
- Al-Garni, S.M.S. Increasing NaCl—Salt tolerance of a halophytic plant Phragmites australis by mycorrhizal symbiosis. Am. Eurasian, J. Agric. Environ. Sci. 2006, 1, 119–126. [Google Scholar]
- Saltonstall, K.; Castillo, H.E.; Blossey, B. Confirmed field hybridization of native and introduced Phragmites australis (Poaceae) in North America. J. Am. Bot. 2014, 101, 211–215. [Google Scholar] [CrossRef]
- Douhovnikoff, V.; Hazelton, E.L. Clonal growth: Invasion or stability? A comparative study of clonal architecture and diversity in native and introduced lineages of Phragmites australis (Poaceae). Am. J. Bot. 2014, 101, 1577–1584. [Google Scholar] [CrossRef]
- Haslam, S.M. A book of reed: (Phragmites australis (Cav.) Trin. ex Steudel, Phragmites communis Trin.). Forrest 2010, 18, 34. [Google Scholar]
- Silliman, B.R.; Bertness, M.D. Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conserv. Biol. 2004, 18, 1424–1434. [Google Scholar] [CrossRef]
- Bart, D.; Burdick, D.; Chambers, R.; Hartman, J.M. Human facilitation of Phragmites australis invasions in tidal marshes: A review and synthesis. Wetl. Ecol. Manag. 2006, 14, 53–65. [Google Scholar] [CrossRef]
- Vasquez, E.A.; Glenn, E.P.; Guntenspergen, G.R.; Brown, J.J.; Nelson, S.G. Salt tolerance and osmotic adjustment of Spartina alterniflora (Poaceae) and the invasive M haplotype of Phragmites australis (Poaceae) along a salinity gradient. Am. J. Bot. 2006, 93, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Engloner, A.I.; Szego, D. Genetic diversity of riverine reed stands indicating the water regime of the habitat. Ecol. Indic. 2016, 61, 846–849. [Google Scholar] [CrossRef][Green Version]
- Lelong, B.; Lavoie, C.; Jodoin, Y.; Belzile, F. Expansion pathways of the exotic common reed (Phragmites australis) a historical and genetic analysis. Divers. Distrib. 2007, 13, 430–437. [Google Scholar] [CrossRef]
- Tulbure, M.G.; Johnston, C.A. Environmental conditions promoting non-native Phragmites australis expansion in Great Lakes coastal wetlands. Wetlands 2010, 30, 577–587. [Google Scholar] [CrossRef]
- Kettenring, K.M.; Mock, K.E. Genetic diversity, reproductive mode, and dispersal differ between the cryptic invader, Phragmites australis, and its native conspecific. Biol. Invasions 2012, 14, 2489–2504. [Google Scholar] [CrossRef]
- Srivastava, J.; Swinder Kalra, S.J.S.; Naraian, R. Environmental perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Appl. Water Sci. 2014, 4, 193–202. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Saltonstall, K.; Windham, L.; Kiviat, E.; Findlay, S. A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetl. Ecol. Manag 2000, 8, 89–103. [Google Scholar] [CrossRef]
- Sarma, H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef]
- Obinna, B.I.; Enyoh, E.C. A review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants. J. Anal. Methods Chem. 2019, 2, 5–38. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B.D. Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Bioresour. Technol. 2008, 99, 7091–7097. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef]
- Morkunas, I.; Woźniak, A.; Mai, V.; Rucińska-Sobkowiak, R.; Jeandet, P. The role of heavy metals in plant response to biotic stress. Molecules 2018, 3, 2320. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Dodd, J.C.; Castro, P.M.L. The mycorrhizal status of Phragmites australis in several polluted soils and sediments of an industrialized region of Northern Portugal. Mycorrhiza 2001, 10, 241–247. [Google Scholar] [CrossRef]
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Gautam, A. Heavy metal detoxification and tolerance mechanisms in plants: Implications for phytoremediation. Environ. Rev. 2015, 24, 39–51. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Swędrzyńska, D. Analysis of the interactions of microorganisms in soil environment. Kosmos 2016, 65, 49–55. [Google Scholar]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Hao, D.; Ya, X.U. Effects of reed roots on rhizosphere microbes in constructed wetland. Syst. Sci. Compr. Stud. Agric. 2008, 24, 222–241. [Google Scholar]
- Toyama, T.; Furukawa, T.; Maeda, N.; Inoue, D.; Sei, K.; Mori, K.; Kikuchi, S.; Ike, M. Accelerated biodegradation of pyrene and benzo[a]pyrene in the Phragmites australis rhizosphere by bacteria-root exudate interactions. Water Res. 2011, 45, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Chandra, R.; Rai, V. Isolation and characterization of Phragmites australis L. rhizosphere bacteria from contaminated site for bioremediation of colored distillery effluent. Ecol. Eng. 2006, 27, 202–207. [Google Scholar] [CrossRef]
- Dan, A.; Zhang, N.; Qiu, R.; Li, C.; Wang, S.; Ni, Z. Accelerated biodegradation of p-tert-butylphenol in the Phragmites australis rhizosphere by phenolic root exudates. Environ. Exp. Bot. 2020, 169, 103891. [Google Scholar]
- Xu, J.; Zheng, L.; Xu, L.; Wang, X. Uptake and allocation of selected metals by dominant vegetation in Poyang Lake wetland: From rhizosphere to plant tissues. Catena 2020, 189, 104477. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, W.; Pang, L.; Li, R.; Li, S. Influence of Phragmites communis and Zizania aquatica on rhizosphere soil enzyme activity and bacterial community structure in a surface flow constructed wetland treating secondary domestic effluent in China. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Piotrowska-Seget, Z.; Chmura, D. Arbuscular mycorrhizal and microbial profiles of an aged phenol-polynuclear aromatic hydrocarbon-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 192, 110299. [Google Scholar] [CrossRef]
- Wu, J.T.; Wang, L.; Zhao, L.; Huang, X.C.; Ma, F. Arbuscular mycorrhizal fungi effect growth and photosynthesis of Phragmites australis (Cav.) Trin ex. Steudel under copper stress. Plant Biol. 2020, 22, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of plant associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Saunders, A.M.; Schramm, A. Effect of lake trophic status and rooted macrophytes on community composition and abundance of ammonia-oxidizing prokaryotes in freshwater sediments. Appl. Environ. Microbiol. 2009, 75, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zeng, J.; Zhao, D.; Huang, R.; Yu, Z.; Wu, Q.L. Contrasting patterns in diversity and community assembly of Phragmites australis root-associated bacterial communities from different seasons. Appl. Environ. Microbiol. 2020. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Fang, M. Arbuscular mycorrhizal fungus modulates the phytotoxicity of Cd via combined responses of enzymes, thiolic compounds, and essential elements in the roots of Phragmites australis. Chemosphere 2017, 187, 221–229. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef]
- Bonanno, G.; Borg, J.A.; Martino, V.D. Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: A comparative assessment. Sci. Total Environ. 2017, 576, 796–806. [Google Scholar] [CrossRef]
- Baranowska-Morek, A. Mechanisms of plants tolerance to toxic influence of heavy metals. Kosmos 2003, 52, 283–298. [Google Scholar]
- Arazi, T.; Kaplan, B.; Fromm, H. A high-affinity calmodulin-binding site in tobacco plasma-membrane channel protein coincides with a characteristic element of cyclic nucleotide-binding domains. Plant Mol. Biol. 2000, 42, 591–601. [Google Scholar] [CrossRef]
- Geebelen, W.; Vangrosfeld, J.; Adriano, D.C.; Van Poucke, L.C.; Clijsters, H. Effects of Pb-EDTA and EDTA on oxidative stress reactions and mineral uptake in Phaseolus vulgaris. Physiol. Plant 2002, 115, 377–384. [Google Scholar] [CrossRef]
- Ali, Z.; Mohammad, A.; Riaz, Y.; Quraishi, U.M.; Malik, R.N. Treatment efficiency of a hybrid constructed wetland system for municipal wastewater and its suitability for crop irrigation. Int. J. Phytoremediat. 2018, 20, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Wani, R.A.; Ganai, B.A.; Shah, M.A.; Uqab, B. Heavy metal uptake potential of aquatic plants through phytoremediation technique—A review. J. Bioremediat. Biodegrad. 2017, 8, 404. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–775. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.J.; Weis, J.S.; Weis, P. Release of metals by the leaves of the salt marsh grasses Spartina alterniflora and Phragmites australis. Estuar Coast. Shelf Sci. 2000, 51, 153–159. [Google Scholar] [CrossRef]
- Aksoy, A.; Demirezen, D.; Duman, F. Bioaccumulation, detection and analysis of heavy metal pollution in Sultan Marsh and its environment. Water Air Soil Pollut. 2005, 164, 241–255. [Google Scholar] [CrossRef]
- Peltier, E.E.; Webb, S.M.; Gaillard, J. Zinc and lead sequestration in an impacted wetland system. Adv. Environ. Res. 2003, 8, 103–112. [Google Scholar] [CrossRef]
- Kastratović, V.; Krivokapić, S.; Durović, D.; Blagojević, N. Seasonal changes in metal accumulation and distribution in the organs of Phragmites australis (common reed) from Lake Skadar, Montenegro. J. Serb. Chem. Soc. 2013, 78, 1241–1258. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Reshi, Z.A.; Shah, M.A.; Rashid, I.; Ara, R.; Andrabi, S.M. Phytoremediation potential of Phragmites australis in Hokersar wetland-a Ramsar site of Kashmir Himalaya. Int. J. Phytoremediat. 2014, 16, 1183–1191. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Karbassi, A.; Moattar, F. Heavy metals in sediments and their bioaccumulation in Phragmites australis in the Anzali wetland of Iran. Chin. J. Oceanol Limn. 2016, 34, 810–820. [Google Scholar] [CrossRef]
- Bonanno, G. Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Al-Homaidan, A.A.; Al-Otaibi, T.G.; El-Sheikh, M.A.; Al-Ghanayem, A.A.; Ameen, F. Accumulation of heavy metals in a macrophyte Phragmites australis: Implications to phytoremediation in the Arabian Peninsula wadis. Environ. Monit. Assess. 2020, 192, 1–10. [Google Scholar] [CrossRef]
- Prica, M.; Andrejic, G.; Šinžar-Sekulić, J.; Rakić, T.; Dželetović, Ž. Bioaccumulation of heavy metals in common reed (Phragmites australis) growing spontaneously on highly contaminated mine tailing ponds in Serbia and potential use of this species in phytoremediation. Bot. Serb. 2019, 43, 85–95. [Google Scholar] [CrossRef]
- Šíma, J.; Svoboda, L.; Šeda, M.; Krejsa, J.; Jahodová, J. The fate of selected heavy metals and arsenic in a constructed wetland. J. Environ. Sci. Health Part A 2019, 54, 56–64. [Google Scholar] [CrossRef]
- Bonanno, G.; Vymazal, J.; Cirelli, G.L. Translocation, accumulation and bioindication of trace elements in wetland plants. Sci. Total Environ. 2018, 631, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; Shaltout, K.H.; Al-Sodany, Y.M.; Haroun, S.A.; Galal, T.M.; Ayed, H.; Jensen, K. Common reed (Phragmites australis (Cav.) Trin. ex Steudel) as a candidate for predicting heavy metal contamination in Lake Burullus, Egypt: A biomonitoring approach. Ecol. Eng. 2020, 148, 105787. [Google Scholar] [CrossRef]
- Štrbac, S.; Šajnović, A.; Grubin, K.M.; Vasić, N.; Dojčinović, B.P.; Simonović, P.; Jovančićević, B. Metals in sediment and Phragmites australis (common reed) from Tisza River, Serbia. Appl Ecol Env Res. 2014, 12, 105–122. [Google Scholar] [CrossRef]
- Bonanno, G.; Lo Giudice, R. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Anjum, N.A.; Ahmad, I.; Válega, M.; Pacheco, M.; Figueira, E.; Duarte, A.C.; Pereira, E. Salt marsh macrophyte Phragmites australis strategies assessment for its dominance in mercury-contaminated coastal lagoon (Ria de Aveiro, Portugal). Environ. Sci. Pollut. Res. 2012, 19, 2879–2888. [Google Scholar] [CrossRef]
- Eid, E.M.; Galal, T.M.; Sewelam, N.A.; Talha, N.I.; Abdallah, S.M. Phytoremediation of heavy metals by four aquatic macrophytes and their potential use as contamination indicators: A comparative assessment. Environ. Sci. Pollut. Res. 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Chernykh, N.A.; Chan, H.K.; Baeva, Y.I.; Grachev, V.A. The regularities of heavy metals and arsenic accumulation in the vegetation of riverside depending on the level of technogenic load. Int. J. Pharm. Sci. Res. 2018, 10, 800–804. [Google Scholar]
- Klink, A. A comparison of trace metal bioaccumulation and distribution in Typha latifolia and Phragmites australis: Implication for phytoremediation. Environ. Sci. Pollut. Res. 2017, 24, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Tayebi, L.; Atabati, H.; Mortazavi, S. Phragmites australis as a heavy metal bioindicator in the Anzali wetland of Iran. Toxicol. Environ. Chem. 2014, 96, 1428–1434. [Google Scholar] [CrossRef]
- Morari, F.; Dal Ferro, N.; Cocco, E. Municipal wastewater treatment with Phragmites australis L. and Typha latifolia L. for irrigation reuse. Boron and heavy metals. Water Air Soil Pollut. 2015, 226, 56. [Google Scholar] [CrossRef]
- Jiang, B.; Xing, Y.; Zhang, B.; Cai, R.; Zhang, D.; Sin, G. Effective phytoremediation of low-level heavy metals by native macrophytes in a vanadium mining area, China. Environ. Sci. Pollut. Res. 2018, 25, 31272. [Google Scholar] [CrossRef]
- Kastratović, V.; Krivokapić, S.; Đurović, D. Vanadium uptake, translocation and bioaccumulation in ecosystem of Skadar Lake, Montenegro. Zaštita Materijala 2020, 61, 31–40. [Google Scholar] [CrossRef]
- Catling, P.M.; Mitrow, G.; Haber, E.; Posluszny, U.; Charlton, W.A. The biology of Canadian weeds. 124. Hydrocharis morsus-ranae L. Can. J. Plant Sci. 2003, 83, 1001–1016. [Google Scholar] [CrossRef]
- Ramprasad, C.; Philip, L. Greywater treatment using horizontal, vertical and hybrid flow constructed wetlands. Curr. Sci. 2018, 114, 155–165. [Google Scholar] [CrossRef]
- Zena, F.A.; Qusay, A.A.A.; Rana, F.A.; Saad, H.K. Knowing of accumulation capacity of [Ceratophyllum demersum L. and Hydrilla verticillata plant] when one plant is used to remove the copper element in a laboratory-contaminated water-polluting ecosystem. Curr. Res. Microbiol. Biotechnol. 2018, 6, 1501–1505. [Google Scholar]
- Sasmaz, A.; Dogan, I.M.; Sasmaz, M. Removal of Cr, Ni, and Co in the water of chromium mining areas by using Lemna gibba L. and Lemn. Minor L. Water Environ. J. 2016, 30, 235–242. [Google Scholar] [CrossRef]
- Polechońska, L.; Samecka-Cymerman, A. Cobalt and nickel content in Hydrocharis morsus-ranae and their bioremoval from single- and binary solutions. Environ. Sci. Pollut. Res. 2018, 25, 32044. [Google Scholar] [CrossRef]
- Aurangzeb, N.; Nisa, S.; Bibi, Y.; Javed, F.; Hussain, F. Phytoremediation potential of aquatic herbs from steel foundry effluent. Braz. J. Chem. Eng. 2014, 31, 881–886. [Google Scholar] [CrossRef]
- Lizama-Allende, K.; Jaque, I.; Ayala, J.; Montes-Atenas, G.; Leiva, E. Arsenic removal using horizontal subsurface flow Constructed Wetlands: A sustainable alternative for Arsenic-Rich Acidic Waters. Water 2018, 10, 1447. [Google Scholar] [CrossRef]
- Johnson, U.E.; Adeogun, B.K.; Ugya, A.Y. Efficacy of aquatic plants in industrial effluent treatment using vertical subsurface flow constructed wetland: Studies on Ceratophyllum demersum, Ludwigia abyssinica and Hydrolea glabra. Int. J. Eng. 2019, XVII, 213–217. [Google Scholar]
- Daud, M.; Ali, S.; Abbas, Z.; Zaheer, I.E.; Riaz, M.A.; Malik, A.; Zhu, S.J. Potential of duckweed (Lemna minor) for the phytoremediation of landfill leachate. J. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Abbas, Z.; Arooj, F.; Ali, S.; Zaheer, I.E.; Rizwan, M.; Riaz, M.A. Phytoremediation of landfill leachate waste contaminants through floating bed technique using water hyacinth and water lettuce. Int. J. Phytoremediat. 2019, 31, 1–12. [Google Scholar] [CrossRef]
- Bello, A.O.; Tawabini, S.; Khalil, A.B.; Boland, C.R.; Saleh, T.A. Phytoremediation of cadmium, lead and nickel contaminated water by Phragmites australis in hydroponic systems. Ecol Eng. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Parnian, A.; Chorom, M.; Jaafarzadeh, N.; Dinarvand, M. Use of two aquatic macrophytes for the removal of heavy metals from synthetic medium. Ecohydrol. Hydrobiol. 2016, 163, 194–200. [Google Scholar] [CrossRef]
- Al-Khafaji, M.S.; Al-Ani, F.H.; Ibrahim, A.F. Removal of some heavy metals from industrial wastewater by Lemna minor. KSCE J. Civ. Eng. 2018, 22, 1077–1082. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Jayawardana, C.K. Potential of aquatic macrophytes Eichhornia crassipes, Pistia stratiotes and Salvinia molesta in phytoremediation of textile wastewater. J. Water Secur. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Shirinpur-Valadi, A.; Hatamzadeh, A.; Sedaghathoor, S. Study of the accumulation of contami-nants by Cyperus alternifolius, Lemna minor, Eichhornia crassipes, and Canna × generalis in some contaminated aquatic environments. Environ. Sci. Pollut. Res. 2019, 26, 21340. [Google Scholar] [CrossRef] [PubMed]
- Amare, E.; Kebede, F.; Mulat, W. Wastewater treatment by Lemna minor and Azolla filiculoides in tropical semiarid regions of Ethiopia. Ecol. Eng. 2018, 120, 464–473. [Google Scholar] [CrossRef]
- Tabinda, A.B.; Irfan, R.; Yasar, A.; Iqbal, A.; Mahmood, A. Phytoremediation potential of Pistia stratiotes and Eichhornia crassipes to remove chromium and copper. Environ. Technol. 2018, 41, 1514–1519. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Whitby, P.; Rogerson, M. Constructed wetlands for steel slag leachate management: Partitioning of arsenic, chromium, and vanadium in waters, sediments, and plants. J. Environ. Manag. 2019, 243, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Jenkins, R.O.; Haris, P.I. Extending the geographic reach of the water hyacinth plant in removal of heavy metals from a temperate Northern Hemisphere river. Sci. Rep. 2018, 8, 11071. [Google Scholar] [CrossRef] [PubMed]
- Úsuga, F.A.; Patiño, A.F.; Rodríguez, D.C.; Peñuela, G.A. Kinetic study and removal of contaminants in the leachate treatment using subsurface wetlands at pilot scale. Rev. Ion 2017, 30, 55–63. [Google Scholar]
- Anning, A.K.; Korsah, P.E.; Addo-Fordjour, P. Phytoremediation of wastewater with Limnocharis flava, Thalia geniculata and Typha latifolia in constructed wetlands. Int. J. Phytoremediat. 2013, 15, 452–464. [Google Scholar] [CrossRef]
- Tufaner, F. Post-treatment of effluents from UASB reactor treating industrial wastewater sediment by constructed wetland. Environ. Technol. 2018, 41, 912–920. [Google Scholar] [CrossRef]
- Goswami, C.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Arsenic uptake by Lemna minor in hydroponic system. Int. J. Phytoremediat. 2014, 16, 1221–1227. [Google Scholar] [CrossRef]
- Chorom, M.; Parnian, A.; Jaafarzadeh, N. Nickel removal by the aquatic plant (Ceratophyllum demersum L.). Int. J. Environ. Sci. Dev. 2012, 3, 372–375. [Google Scholar] [CrossRef]
- Rai, U.; Upadhyay, A.; Singh, N.; Dwivedi, S.; Tripathi, R. Seasonal applicability of horizontal sub-surface flow constructed wetland for trace elements and nutrient removal from urban wastes to conserve Ganga River water quality at Haridwar, India. Ecol. Eng. 2015, 81, 115–122. [Google Scholar] [CrossRef]
- Rana, V.; Maiti, S.K. Municipal wastewater treatment potential and metal accumulation strategies of Colocasia esculenta (L.) Schott and Typha latifolia L. in a constructed wetland. Environ. Monit Assess. 2018, 190, 328. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Muradov, N.; Gujar, A.; Stevenson, T.; Nugegoda, D.; Ball, A.; Mouradov, A. Application of Aquatic Plants for the Treatment of Selenium-Rich Mining Wastewater and Production of Renewable Fuels and Petrochemicals. J. Sustain. Bioenergy Syst. 2014, 4, 97–112. [Google Scholar] [CrossRef][Green Version]
- Nguyen, H.T.H.; Nguyen, B.Q.; Duong, T.T.; Bui, A.T.K.; Nguyen, H.T.A.; Cao, H.T.; Mai, N.T.; Nguyen, K.M.; Pham, T.T.; Kim, K.W. Pilot-scale removal of arsenic and heavy metals from mining wastewater using adsorption combined with constructed wetland. Minerals 2019, 9, 379. [Google Scholar] [CrossRef]
- Ugya, A. The efficiency of Lemna minor L. In the phytoremediation of Rani Stream: A case study of Kaduna Refinery and Petrochemical Company polluted stream. J. Appl. Biol. Biotech. 2015, 3, 11–14. [Google Scholar]
- Foroughi, M.; Najafi, P.; Toghiani, S. Trace elements removal from waster water by Ceratophyllum demersum. JASEM 2011, 15, 197–201. [Google Scholar] [CrossRef]
- Mahmoud, K.M.A.; Mahmoud, H.A.; Sayed, S.S.M. Potential role of Ceratophyllum demersum in bioaccumulation and tolerance of some heavy metals. EJABF 2018, 22, 1–12. [Google Scholar] [CrossRef][Green Version]
- Türker, O.C.; Yakar, A.; Türe, C.; Saz, Ç. Boron (B) removal and bioelectricity captured from irrigation water using engineered duckweed-microbial fuel cell: Effect of plant species and vegetation structure. Environ. Sci. Pollut. Res. 2019, 26, 31522–31536. [Google Scholar] [CrossRef]
- Kumari, M.; Tripathi, B. Effect of Phragmites australis and Typha latifolia on biofiltration of heavy metals from secondary treated effluent. Int. J. Environ. Sci. Technol. 2015, 12, 1029–1038. [Google Scholar] [CrossRef]
- Kumari, M.; Tripathi, B.D. Efficiency of Phragmites australis and Typha latifolia for heavy metal removal from wastewater. Ecotoxicol. Environ. Saf. 2015, 112, 80–86. [Google Scholar] [CrossRef]
- Ranieri, E.; Fratino, U.; Petruzzelli, D.; Borges, A.C. A comparison between Phragmites australis and Helianthus annuus in chromium phytoextraction. Water Air Soil Pollut. 2013, 224, 1465. [Google Scholar] [CrossRef]
- Yeh, T.Y.; Chou, C.C.; Pan, C.T. Heavy metal removal within pilot-scale constructed wetlands receiving river water contaminated by confined swine operations. Desalination 2009, 249, 368–373. [Google Scholar] [CrossRef]
- Marchand, L.; Nsanganwimana, F.; Oustrière, N.; Grebenshchykova, Z.; Lizama-Allende, K.; Mench, M. Copper removal from water using a bio-rack system either unplanted or planted with Phragmites australis, Juncus articulatus and Phalaris arundinacea. Ecol. Eng. 2014, 64, 291–300. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Chopra, A.K. Assessment of plant growth attributes bioaccumulation, enrichment, and translocation of heavy metals in water lettuce (Pistia stratiotes L.) grown in sugar mill effluent. Int. J. Phytoremediat. 2018, 20, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Dan, A.; Fujii, D.; Soda, S.; Machimura, T.; Ike, M. Removal of phenol, bisphenol A, and 4-tert-butylphenol from synthetic landfill leachate by vertical flow constructed wetlands. Sci. Total Environ. 2017, 578, 566–576. [Google Scholar]
- Cui, H.; Hense, B.A.; Müller, J.; Schröder, P. Short term uptake and transport process for metformin in roots of Phragmites australis and Typha latifolia. Chemosphere 2015, 134, 307–312. [Google Scholar] [CrossRef]
- Petrie, B.; Smith, B.D.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-residue determination of micropollutants in Phragmites australis from constructed wetlands using microwave assisted extraction and ultra-high-performance liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2017, 959, 91–101. [Google Scholar] [CrossRef]
- He, Y.; Langenhoff, A.A.; Sutton, N.B.; Rijnaarts, H.H.; Blokland, M.H.; Chen, F.; Huber, C.; Schröder, P. Metabolism of ibuprofen by Phragmites australis: Uptake and phytodegradation. Environ. Sci Technol. 2017, 51, 4576–4584. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Basto, M.C.; Almeida, C.M. Potential of Phragmites australis for the removal of veterinary pharmaceuticals from aquatic media. Bioresour Technol. 2012, 116, 497–501. [Google Scholar] [CrossRef]
- Lv, T.; Carvalho, P.N.; Zhang, L.; Zhang, Y.; Button, M.; Arias, C.A.; Weber, K.P.; Brix, H. Functionality of microbial communities in constructed wetlands used for pesticide remediation: Influence of system design and sampling strategy. Water Res. 2017, 110, 241–251. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Santos, F.; Ferreira, A.C.F.; Gomes, C.R.; Basto, M.C.P.; Mucha, A.P. Constructed wetlands for the removal of metals from livestock wastewater–Can the presence of veterinary antibiotics affect removals? Ecotoxicol. Environ. Saf. 2017, 137, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Zambello, E. How efficient are constructed wetlands in removing pharmaceuticals from untreated and treated urban wastewaters? A review. Sci. Total Environ. 2014, 470–471, 1281–1306. [Google Scholar] [CrossRef] [PubMed]
- Vystavna, Y.; Frkova, Z.; Marchand, L.; Vergeles, Y.; Stolberg, F. Removal efficiency of pharmaceuticals in a full scale constructed wetland in East Ukraine. Ecol. Eng. 2017, 108, 50–58. [Google Scholar] [CrossRef]
- Kankılıç, G.B.; Metin, A.Ü.; Tüzün, İ. Phragmites australis: An alternative biosorbent for basic dye removal. Ecol. Eng. 2016, 86, 85–94. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Bai, J.; Liu, Z.; Song, X.; Yan, D.; Abiyu, A.; Zhao, Z.; Yan, D. High efficiency of inorganic nitrogen removal by integrating biofilmelectrode with constructed wetland: Autotrophic denitrifying bacteria analysis. Bioresour. Technol. 2017, 227, 7–14. [Google Scholar] [CrossRef]
- Liang, Y.; Zhua, H.; Banuelos, G.; Yan, B.; Zhou, Q.; Yu, X.; Cheng, X. Constructed wetlands for saline wastewater treatment: A review. Ecol. Eng. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands, 2nd ed.; CRC Press: New York, NY, USA, 2008; pp. 6–10. [Google Scholar]
- Moore, M.T.; Rodgers, J.H., Jr.; Cooper, C.M.; Smith, S., Jr. Constructed wetlands for mitigation of atrazine-associated agricultural runoff. Environ. Pollut. 2000, 110, 393–399. [Google Scholar] [CrossRef]
- Kivaisi, A.K. The potential for constructed wetlands for wastewater treatment and reuse in developing countries: A review. Ecol. Eng. 2001, 16, 545–560. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed wetlands for wastewater treatment: Five decades of experience. Environ. Sci. Technol. 2010, 45, 61–69. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, R. Performance evaluation of semi continuous vertical flow constructed wetlands (SC-VF-CWs) for municipal wastewater treatment. Bioresour. Technol. 2017, 232, 321–330. [Google Scholar] [CrossRef]
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed wetlands for treatment of industrial wastewaters: A review. Ecol. Eng. 2014, 73, 724–751. [Google Scholar] [CrossRef]
- Halalsheh, M.M.; Rumman, M.Z.A.; Field, J.A. Anaerobic wastewater treatment of concentrated sewage using a two-stage upflow anaerobic sludge blanket-anaerobic filter system. J. Environ. Sci. Health 2010, 45, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Almuktar, S.A.; Abed, S.N.; Scholz, M. Wetlands for wastewater treatment and subsequent recycling of treated effluent: A review. Environ. Sci. Pollut. Res. 2018, 25, 23595–23623. [Google Scholar] [CrossRef]
- Nivala, J.; Knowles, P.; Dotro, G.; García, J.; Wallace, S. Clogging in subsurface-flow treatment wetlands: Measurement, modeling and management. Water Res. 2012, 46, 1625–1640. [Google Scholar] [CrossRef] [PubMed]
- Fonder, N.; Headley, T. Systematic classification, nomenclature and reporting for constructed treatment wetlands. In Water and Nutrient Management in Natural and Constructed Wetlands; Springer: Berlin/Heidelberg, Germany, 2010; pp. 191–219. [Google Scholar]
- Vymazal, J.; Kröpfelová, L. Wastewater Treatment in Constructed Wetlands with Horizontal Sub-Surface Flow; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 14, pp. 328–451. [Google Scholar]
- Chung, A.K.C.; Wu, Y.; Tam, N.F.Y.; Wong, M.H. Nitrogen and phosphate mass balance in a subsurface flow constructed wetland for treating municipal wastewater. Ecol. Eng. 2008, 32, 81–89. [Google Scholar] [CrossRef]
- Rehman, F.; Pervez, A.; Mahmood, Q.; Nawab, B. Wastewater remediation by optimum dissolve oxygen enhanced by macrophytes in constructed wetlands. Ecol. Eng. 2017, 102, 112–126. [Google Scholar] [CrossRef]
- Al-Isawi, R.; Ray, S.; Scholz, M. Comparative study of domestic wastewater treatment by mature vertical-flow constructed wetlands and artificial ponds. Ecol. Eng. 2017, 100, 8–18. [Google Scholar] [CrossRef]
- Vystavna, Y.; Yakovlev, V.; Diadin, D.; Vergeles, Y.; Stolberg, F. Hydrochemical characteristics and water quality assessment of surface and ground waters in the transboundary (Russia/Ukraine) Seversky Donets basin. Environ. Earth Sci. 2015, 74, 585–596. [Google Scholar] [CrossRef]
- Chow, K.L.; Man, Y.B.; Tam, N.F.Y.; Liang, Y.; Wong, M.H. Removal of decabromodiphenyl ether (BDE-209) using a combined system involving TiO2 photocatalysis and wetland plants. J. Hazard. Mater. 2017, 322, 263–269. [Google Scholar] [CrossRef]
- Du Laing, G.; Tack, F.M.G.; Verloo, M.G. Performance of selected destruction methods for the determination of heavy metals in reed plants (Phragmites australis). Anal. Chim. Acta 2003, 497, 191–198. [Google Scholar] [CrossRef]
- Mulkeen, C.; Williams, C.; Gormally, M.; Healy, M. Seasonal patterns of metals and nutrients in Phragmites australis (Cav.) Trin. ex Steudel in a constructed wetland in the west of Ireland. Ecol. Eng. 2017, 107, 192–197. [Google Scholar] [CrossRef]
- Healy, M.G.; Newell, J.; Rodgers, M. Harvesting effects on biomass and nutrient retention in Phragmites australis in a free-water surface constructed wetland in western Ireland. Biol. Environ. Proc. R. Ir. Acad. 2007, 107, 139–145. [Google Scholar] [CrossRef]
- Mustafa, A.; Scholz, M. Nutrient accumulation in Typha latifolia L. and sediment of a representative integrated constructed wetland. Water Air Soil Pollut. 2011, 219, 329–341. [Google Scholar] [CrossRef]
- Vymazal, J.; Březinová, T. Accumulation of heavy metals in aboveground biomass of Phragmites australis in horizontal flow constructed wetlands for wastewater treatment: A review. Chem. Eng. Sci. 2016, 290, 232–242. [Google Scholar] [CrossRef]
- Bragato, C.; Brix, H.; Malagoli, M. Accumulation of nutrients and heavy metals in Phragmites australis (cav.) Trin. ex Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environ. Pollut. 2006, 144, 967–975. [Google Scholar] [CrossRef]
- Toscano, A.; Marzo, A.; Milani, M.; Cirelli, G.L.; Barbagallo, S. Comparison of removal efficiencies in Mediterranean pilot constructed wetlands vegetated with different plant species. Ecol. Eng. 2015, 75, 155–160. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Alavi, N.; Ghaderpoori, M.; Musave, F.; Massoudinejad, S. Feasibility removal of BOD5, COD, and ammonium by using Gambusia fish and Phragmites australis in HSSF wetland. Int. J. Environ. Sci. Technol. 2019, 16, 5891–5900. [Google Scholar] [CrossRef]
- Tara, N.; Arslan, M.; Hussain, Z.; Iqbal, M.; Khan, Q.; Afzal, M. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J. Clean. Prod. 2019, 217, 541–548. [Google Scholar] [CrossRef]
- Dębska, A.; Jóźwiakowski, K.; Gizińska-Górna, M.; Pytka, A.; Marzec, M.; Sosnowska, B.; Pieńko, A. The efficiency of pollution removal from domestic wastewater in constructed wetland systems with vertical flow with common reed and Glyceria maxima. J. Ecol. Eng. 2015, 16, 110–118. [Google Scholar] [CrossRef][Green Version]
- Andreo-Martínez, P.; García-Martínez, N.; Quesada-Medina, J.; Almela, L. Domestic wastewaters reuse reclaimed by an improved horizontal subsurface-flow constructed wetland: A case study in the southeast of Spain. Bioresour Technol. 2017, 233, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Çakir, R.; Gidirislioglu, A.; Çebi, U. A study on the effects of different hydraulic loading rates (HLR) on pollutant removal efficiency of subsurface horizontal flow constructed wetlands used for treatment of domestic wastewaters. J. Environ. Manag. 2015, 164, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kalipci, E. Investigation of decontamination effect of Phragmites australis for Konya domestic wastewater treatment. J. Appl. Res. Med. Aromat. Plants 2011, 5, 6571–6577. [Google Scholar] [CrossRef]
- Lopez, D.; Sepúlveda, M.; Vidal, G. Phragmites australis and Schoenoplectus californicusin constructed wetlands: Development and nutrient uptake. J. Soil Sci. Plant. Nutr. 2016, 16, 763–777. [Google Scholar]
- Shahamat, Y.D.; Asgharnia, H.; Kalankesh, L.R. Data on wastewater treatment plant by using wetland method, Babol, Iran. Data Brief 2018, 16, 1056–1061. [Google Scholar] [CrossRef]
- Abou-Elela, S.I.; Hellal, M.S. Municipal wastewater treatment using vertical flow constructed wetlands planted with Canna, Phragmites and Cyprus. Ecol. Eng. 2012, 47, 209–213. [Google Scholar] [CrossRef]
- Fan, J.; Liang, S.; Zhang, B.; Zhang, J. Enhanced organics and nitrogen removal in batch-operated vertical flow constructed wetlands by combination of intermittent aeration and step feeding strategy. Environ. Sci. Pollut. Res. 2013, 20, 2448. [Google Scholar] [CrossRef]
- García-Ávila, F.; Patiño-Chávez, J.; Zhinín-Chimbo, F.; Donoso-Moscoso, S.; del Pino, L.F.; Avilés-Añazco, L. Performance of Phragmites Australis and Cyperus Papyrus in the treatment of municipal wastewater by vertical flow subsurface constructed wetlands. Int. Soil Water Conserv. Res. 2019, 7, 286–296. [Google Scholar] [CrossRef]
- Mietto, A.; Borin, M. Performance of two small subsurface flow constructed wetlands treating domestic wastewaters in Italy. Environ. Technol. 2013, 34, 1085–1095. [Google Scholar] [CrossRef]
- Abdelhakeem, S.G.; Aboulroos, S.A.; Kamel, M.M. Performance of a vertical subsurface flow constructed wetland under different operational conditions. J. Adv. Res. 2016, 7, 803–814. [Google Scholar] [CrossRef]
- Barco, A.; Borin, M. Treatment performance and macrophytes growth in a restored hybrid constructed wetland for municipal wastewater treatment. Ecol. Eng. 2017, 107, 160–171. [Google Scholar] [CrossRef]
- Rivas, A.; Barceló-Quintal, I.; Moeller, G.E. Pollutant removal in a multistage municipal wastewater treatment comprised of constructed wetlands and a maturation pond, in a temperate climate. Water Sci. Technol. 2011, 64, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J.; Greenway, M.; Tonderski, K.; Brix, H.; Mander, Ü. Constructed Wetlands for Wastewater Treatment. In Wetlands and Natural Resource Management. Ecological Studies (Analysis and Synthesis); Verhoeven, J.T.A., Beltman, B., Bobbink, R., Whigham, D.F., Eds.; Springer: Berlin, Germany, 2006; Volume 190, pp. 69–96. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).