Analysis of the Microbiome (Bathing Biome) in Geothermal Waters from an Australian Balneotherapy Centre
Abstract
1. Introduction
2. Materials and Methods
2.1. Hot Spring Hydrology and Sampling
2.2. Physicochemical Data
2.3. Total and Faecal Coliforms Determination
2.4. Next Generation Sequencing (NGS) (16S rRNA) and Analysis
2.5. Statistical Analysis
3. Results and Discussion
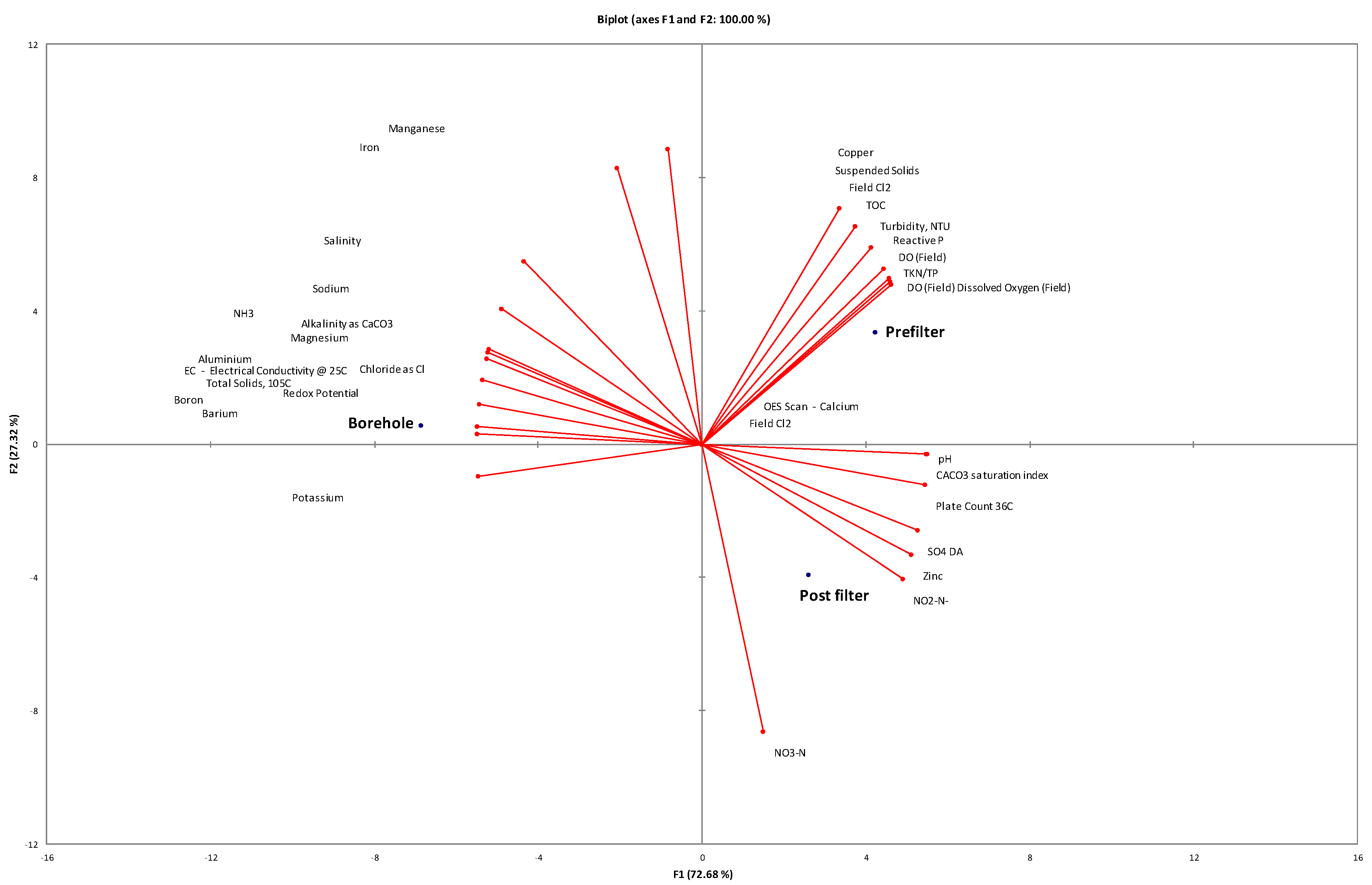
3.1. Physicochemical and Microbial Characteristics
3.2. Coliforms
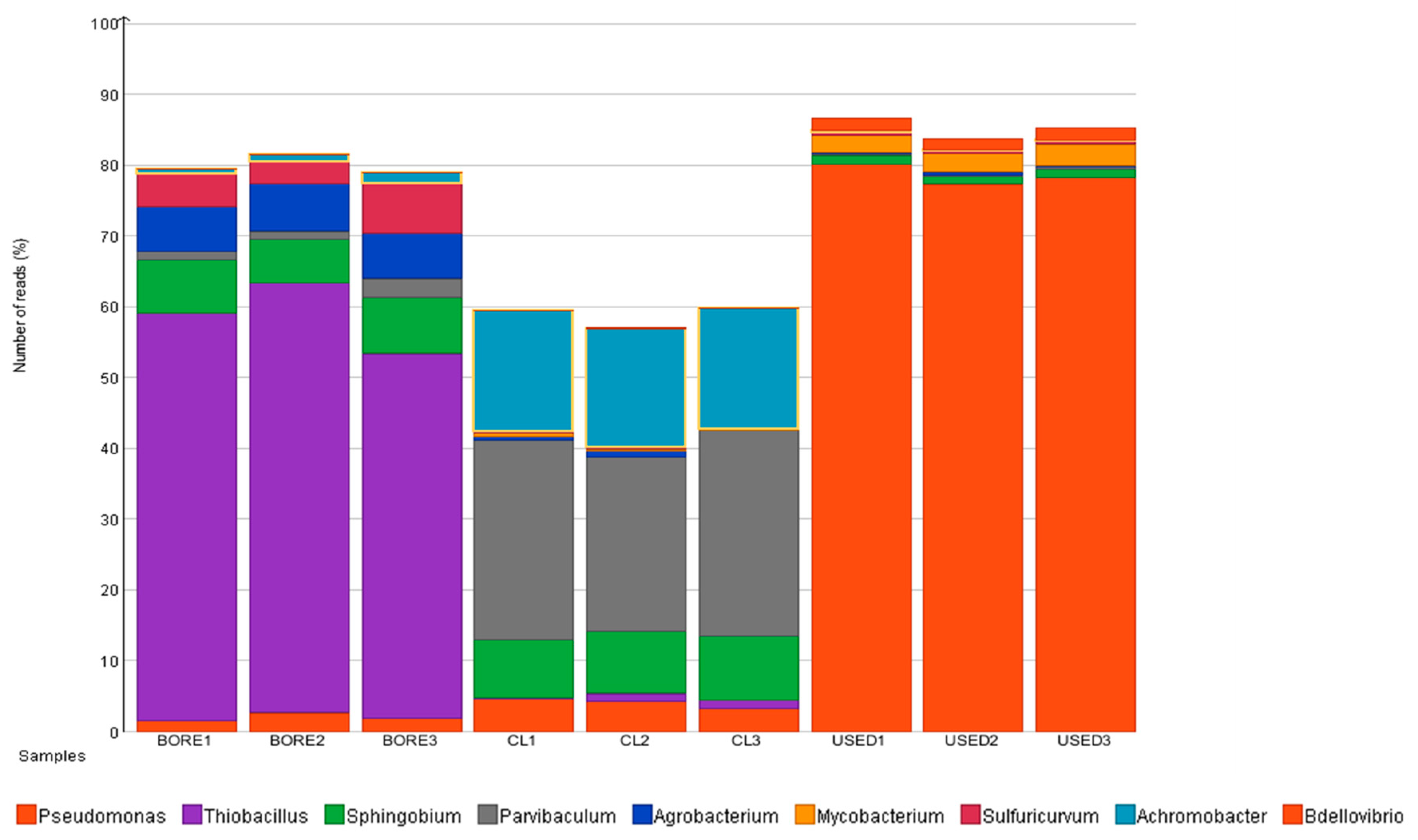
3.3. Microbial Diversity of the Hot Spring
3.4. Microbial Diversity Changes in the PHS Facilities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karagülle, M.; Karagülle, M.Z. Effectiveness of balneotherapy and spa therapy for the treatment of chronic low back pain: A review on latest evidence. Clin. Rheumatol. 2014, 34, 207–214. [Google Scholar] [CrossRef]
- Peroni, A.; Gisondi, P.; Zanoni, M.; Girolomoni, G. Balneotherapy for chronic plaque psoriasis at Comano spa in Trentino, Italy. Dermatol. Ther. 2008, 21, S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Miseta, R.; Palatinszky, M.; Makk, J.; Márialigeti, K.; Borsodi, A.K. Phylogenetic Diversity of Bacterial Communities Associated with Sulfurous Karstic Well Waters of a Hungarian Spa. Geomicrobiol. J. 2012, 29, 101–113. [Google Scholar] [CrossRef]
- Valeriani, F.; Crognale, S.; Protano, C.; Gianfranceschi, G.; Orsini, M.; Vitali, M.; Romano Spica, V.J. Metagenomic analysis of bacterial community in a travertine depositing hot spring. New Microbiol. 2018, 41, 126–135. [Google Scholar] [PubMed]
- Valeriani, F.; Margarucci, L.M.; Spica, V.R. Recreational Use of Spa Thermal Waters: Criticisms and Perspectives for Innovative Treatments. Int. J. Environ. Res. Public Health 2018, 15, 2675. [Google Scholar] [CrossRef]
- Valeriani, F.; Protano, C.; Gianfranceschi, G.; Leoni, E.; Galasso, V.; Mucci, N.; Vitali, M.; Romano-Spica, V. Microflora Thermarum Atlas project: Biodiversity in thermal spring waters and natural SPA pools. Water Supply 2017, 18, 1472–1483. [Google Scholar] [CrossRef]
- Adongo, C.A.; Amuquandoh, F.E.; Amenumey, E. Modelling spa-goers’ choices of therapeutic activities. J. Hosp. Tour. Manag. 2017, 31, 105–113. [Google Scholar] [CrossRef]
- Csirmaz, É.; Pető, K. International Trends in Recreational and Wellness Tourism. Procedia Econ. Financ. 2015, 32, 755–762. [Google Scholar] [CrossRef]
- Loureiro, S.M.C.; Almeida, M.; Rita, P. The effect of atmospheric cues and involvement on pleasure and relaxation: The spa hotel context. Int. J. Hosp. Manag. 2013, 35, 35–43. [Google Scholar] [CrossRef]
- McCarthy, J. Wellbeing. Global Spa & Wellness Trends. Psychology of Spas and Wellbeing. 2017. Available online: http://psychologyofwellbeing.com/201701/2017-global-spa-wellness-trends.html (accessed on 12 June 2020).
- Gutenbrunner, C.; Sukenik, S.; Bender, T.; Karagülle, Z.; Bálint, G.P.; Balint, P.V. Hydrotherapy, balneotherapy, and spa treatment in pain management. Rheumatol. Int. 2004, 25, 220–224. [Google Scholar] [CrossRef]
- Evcik, D.; Kızılay, B.; Gökçen, E. The effects of balneotherapy on fibromyalgia patients. Rheumatol. Int. 2002, 22, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Matz, H.; Orion, E.; Wolf, R. Balneotherapy in dermatology. Dermatol. Ther. 2003, 16, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Corral, M.M.; Galindo, E.; Ontiveros, C.; Muñoz, J.A.D. Hydrogeochemical areas as background for specific mineral and thermal waters of Spain. Environ. Earth Sci. 2014, 73, 2683–2697. [Google Scholar] [CrossRef]
- Bouvier, C. Balneotherapy in Europe—The current situation. Ann. Phys. Rehabil. Med. 2014, 57, e159. [Google Scholar] [CrossRef][Green Version]
- Baumgartner, M.; Yapi, A.; Stetter, K.O.; Gröbner-Ferreira, R. Cultivation and properties of Echinamoeba thermarum n. sp., an extremely thermophilic amoeba thriving in hot springs. Extremophiles 2003, 7, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, K.B.; Fagg, J.A.; Ferris, M.J.; Henson, J.M. PCR Detection and Analysis of the Free-Living Amoeba Naegleria in Hot Springs in Yellowstone and Grand Teton National Parks. Appl. Environ. Microbiol. 2003, 69, 5914–5918. [Google Scholar] [CrossRef] [PubMed]
- Badirzadeh, A.; Niyyati, M.; Babaei, Z.; Amini, H.; Badirzadeh, H.; Rezaeian, M. Isolation of Free-Living Amoebae from Sarein Hot Springs in Ardebil Province, Iran. Iran. J. Parasitol. 2011, 6, 1–8. [Google Scholar] [PubMed]
- Mohamed, Z.A. Toxic cyanobacteria and cyanotoxins in public hot springs in Saudi Arabia. Toxicon 2008, 51, 17–27. [Google Scholar] [CrossRef]
- Kanatani, J.-I.; Isobe, J.; Norimoto, S.; Kimata, K.; Mitsui, C.; Amemura-Maekawa, J.; Kura, F.; Sata, T.; Watahiki, M. Prevalence of Legionella species isolated from shower water in public bath facilities in Toyama Prefecture, Japan. J. Infect. Chemother. 2017, 23, 265–270. [Google Scholar] [CrossRef]
- Kuroki, T.; Amemura-Maekawa, J.; Ohya, H.; Furukawa, I.; Suzuki, M.; Masaoka, T.; Aikawa, K.; Hibi, K.; Morita, M.; Lee, K.-I.; et al. Outbreak of Legionnaire’s Disease Caused by Legionella pneumophila Serogroups 1 and 13. Emerg. Infect. Dis. 2017, 23, 349–351. [Google Scholar] [CrossRef]
- Sheehan, K.B.; Henson, J.M.; Ferris, M.J. Legionella Species Diversity in an Acidic Biofilm Community in Yellowstone National Park. Appl. Environ. Microbiol. 2005, 71, 507–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, W.-C.; Tsai, H.-C.; Tao, C.-W.; Chen, J.-S.; Shih, Y.-J.; Kao, P.-M.; Huang, T.-Y.; Hsu, B.-M. Approach to determine the diversity of Legionella species by nested PCR-DGGE in aquatic environments. PLoS ONE 2017, 12, e0170992. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.W.; Haas, C. Legionnaires’ disease: Evaluation of a quantitative microbial risk assessment model. J. Water Health 2008, 6, 149–166. [Google Scholar] [CrossRef]
- Żbikowska, E.; Walczak, M.; Krawiec, A. Distribution of Legionella pneumophila bacteria and Naegleria and Hartmannella amoebae in thermal saline baths used in balneotherapy. Parasitol. Res. 2012, 112, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Fukui, M. Molecular Characterization of Community Structures and Sulfur Metabolism within Microbial Streamers in Japanese Hot Springs. Appl. Environ. Microbiol. 2003, 69, 7044–7057. [Google Scholar] [CrossRef]
- Kublanov, I.; Perevalova, A.A.; Slobodkina, G.B.; Lebedinsky, A.V.; Bidzhieva, S.K.; Kolganova, T.V.; Kaliberda, E.N.; Rumsh, L.D.; Haertlï, T.; Bonch-Osmolovskaya, E.A. Biodiversity of Thermophilic Prokaryotes with Hydrolytic Activities in Hot Springs of Uzon Caldera, Kamchatka (Russia). Appl. Environ. Microbiol. 2008, 75, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ferris, M.J.; Ward, D.M. Seasonal distributions of dominant 16S rRNA-defined populations in a hot spring microbial mat examined by denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 1997, 63, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Ferris, M.J.; Muyzer, G.; Ward, D.M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl. Environ. Microbiol. 1996, 62, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Ferris, M.J.; Nold, S.C.; Bateson, M.M. A Natural View of Microbial Biodiversity within Hot Spring Cyanobacterial Mat Communities. Microbiol. Mol. Biol. Rev. 1998, 62, 1353–1370. [Google Scholar] [CrossRef] [PubMed]
- Colman, D.R.; Feyhl-Buska, J.; Robinson, K.J.; Fecteau, K.M.; Xu, H.; Shock, E.L.; Boyd, E.S. Ecological differentiation in planktonic and sediment-associated chemotrophic microbial populations in Yellowstone hot springs. FEMS Microbiol. Ecol. 2016, 92, 137. [Google Scholar] [CrossRef]
- Amin, A.; Ahmed, I.; Salam, N.; Kim, B.-Y.; Singh, D.; Zhi, X.-Y.; Xiao, M.; Li, W.-J. Diversity and Distribution of Thermophilic Bacteria in Hot Springs of Pakistan. Microb. Ecol. 2017, 74, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, X.; Zhang, L.; Jiang, L.; Chen, S. Linking microbial community structure to S, N and Fe biogeochemical cycling in the hot springs at the Tengchong geothermal fields, Southwest China. Geomicrobiol. J. 2015, 33, 135–150. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Gaur, M.; Das, A.; Singh, A.; Kumar, M.; Subudhi, E. Comparative analysis of 16S rRNA gene Illumina sequence for microbial community structure in diverse unexplored hot springs of Odisha, India. Geomicrobiol. J. 2016, 34, 567–576. [Google Scholar] [CrossRef]
- Lau, M.C.Y.; Aitchison, J.C.; Pointing, S.B. Bacterial community composition in thermophilic microbial mats from five hot springs in central Tibet. Extremophiles 2008, 13, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sugihara, M.; Yamamoto, H.; Patel, B.; Kato, K.; Hanada, S. Microbial community in a geothermal aquifer associated with the subsurface of the Great Artesian Basin, Australia. Extremophiles 2005, 9, 407–414. [Google Scholar] [CrossRef]
- Paduano, S.; Valeriani, F.; Spica, V.R.; Bargellini, A.; Borella, P.; Marchesi, I. Microbial biodiversity of thermal water and mud in an Italian spa by metagenomics: A pilot study. Water Supply 2017, 18, 1456–1465. [Google Scholar] [CrossRef]
- Valeriani, F.; Gianfranceschi, G.; Spica, V.R. The microbiota as a candidate biomarker for SPA pools and SPA thermal spring stability after seismic events. Environ. Int. 2020, 137, 105595. [Google Scholar] [CrossRef]
- Sharp, C.E.; Brady, A.L.; Sharp, G.H.; Grasby, S.; Stott, M.B.; Dunfield, P.F. Humboldt’s spa: Microbial diversity is controlled by temperature in geothermal environments. ISME J. 2014, 8, 1166–1174. [Google Scholar] [CrossRef]
- Birch, W.D. Geology of Victoria; Geological Society of Australia: Victoria, Australia, 2003. [Google Scholar]
- Edberg, S.C.; Allen, M.J.; Smith, D.B.; Kriz, N.J. Enumeration of total coliforms and Escherichia coli from source water by the defined substrate technology. Appl. Environ. Microbiol. 1990, 56, 366–369. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R.J.P. PRIMER v7: User Manual/Tutorial, 3rd ed.; Primer-E Ltd.: Devon, UK, 2015. [Google Scholar]
- World Health Organization. Guidelines for Safe Recreational Water Environments: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Le Roux, N.W.; Wakerley, D.S.; Hunt, S.D. Thermophilic Thiobacillus-type Bacteria from Icelandic Thermal Areas. J. Gen. Microbiol. 1977, 100, 197–201. [Google Scholar] [CrossRef]
- Skirnisdottir, S.; Hreggvidsson, G.O.; Hjörleifsdottir, S.; Marteinsson, V.; Petursdottir, S.K.; Holst, O.; Kristjansson, J.K.; Hjörleifsdottir, S. Influence of Sulfide and Temperature on Species Composition and Community Structure of Hot Spring Microbial Mats. Appl. Environ. Microbiol. 2000, 66, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Burton, N.P.; Norris, P.R. Microbiology of acidic, geothermal springs of Montserrat: Environmental rDNA analysis. Extremophiles 2000, 4, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Brito, E.M.S.; Villegas-Negrete, N.; Sotelo-González, I.A.; Caretta, C.A.; Goñi-Urriza, M.; Gassie, C.; Hakil, F.; Colin, Y.; Duran, R.; Gutierrez-Corona, F.; et al. Microbial diversity in Los Azufres geothermal field (Michoacán, Mexico) and isolation of representative sulfate and sulfur reducers. Extremophiles 2014, 18, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Arun, A.B.; Young, C.-C.; Busse, H.-J.; Kassmannhuber, J.; Rossello-Mora, R.; Geueke, B.; Rekha, P.; Chen, W.-M. Sphingomicrobium lutaoense gen. nov., sp. nov., isolated from a coastal hot spring. Int. J. Syst. Evol. Microbiol. 2012, 62, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Kanokratana, P.; Chanapan, S.; Pootanakit, K.; Eurwilaichitr, L. Diversity and abundance ofBacteria andArchaea in the Bor Khlueng Hot Spring in Thailand. J. Basic Microbiol. 2004, 44, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Cortez, Y.; Vera, N.; Villena, G.K.; Gutierrez-Correa, M. Metagenomic Analysis of Microbial Communities in the Soil-mousse Surrounding of an Amazonian Geothermal Spring in Peru. Br. Biotechnol. J. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Hedlund, B.P.; Dodsworth, J.A.; Cole, J.K.; Panosyan, H. An integrated study reveals diverse methanogens, Thaumarchaeota, and yet-uncultivated archaeal lineages in Armenian hot springs. Antonie Van Leeuwenh. 2013, 104, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, L.J.; Jørgensen, S.L.; Lauritzen, S.-E.; Schleper, C.; Urich, T. Sulfur-Oxidizing Chemolithotrophic Proteobacteria Dominate the Microbiota in High Arctic Thermal Springs on Svalbard. Astrobiology 2011, 11, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Rossmassler, K.; Engel, A.S.; Twing, K.I.; Hanson, T.E.; Campbell, B.J. Drivers of epsilonproteobacterial community composition in sulfidic caves and springs. FEMS Microbiol. Ecol. 2011, 79, 421–432. [Google Scholar] [CrossRef]
- NHMRC. The Guidelines for Managing Risks in Recreational Water; NHMRC publications: Canberra, Australia, 2008. Available online: http://www.nhmrc.gov.au/guidelines/publications/eh38 (accessed on 12 June 2020).
- Calderon, R.L.; Craun, G.F. Outbreaks associated with recreational water in the United States. Int. J. Environ. Health Res. 2005, 15, 243–262. [Google Scholar] [CrossRef]
- Dale, K.D.; Kirk, M.D.; Sinclair, M.; Hall, R.; Leder, K. Reported waterborne outbreaks of gastrointestinal disease in Australia are predominantly associated with recreational exposure. Aust. N. Z. J. Public Health 2010, 34, 527–530. [Google Scholar] [CrossRef]
- Margarucci, L.M.; Spica, V.R.; Gianfranceschi, G.; Valeriani, F. Untouchability of natural spa waters: Perspectives for treatments within a personalized water safety plan. Environ. Int. 2019, 133, 105095. [Google Scholar] [CrossRef] [PubMed]
- Water, S.; World Health Organization. Guidelines for Safe Recreational Water Environments; Volume 2, Swimming Pools and Similar Environments; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Fortney, N.W.; He, S.; Converse, B.J.; Boyd, E.S.; Roden, E.E. Investigating the Composition and Metabolic Potential of Microbial Communities in Chocolate Pots Hot Springs. Front. Microbiol. 2018, 9, 2075. [Google Scholar] [CrossRef] [PubMed]
- Giampaoli, S.; Valeriani, F.; Spica, V.R. Thermal water for recreational use: Overview of international standards. Ig. Sanita Pubb. 2013, 68, 863–873. [Google Scholar]
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Novel Zeaxanthin-Producing Bacteria Isolated from a Radioactive Hot Spring Water. In Advanced Structural Safety Studies; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2012; Volume 892, pp. 99–131. [Google Scholar]
- Sayeh, R.; Birrien, J.L.; Alain, K.; Barbier, G.; Hamdi, M.; Prieur, D. Microbial diversity in Tunisian geothermal springs as detected by molecular and culture-based approaches. Extremophiles 2010, 14, 501–514. [Google Scholar] [CrossRef]
- Takashima, C.; Okumura, T.; Nishida, S.; Shimamoto, T.; Koike, H.; Kano, A.; Reitner, J.; Quéric, N.-V.; Arp, G. Microbial Control on Lamina Formation in a Travertine of Crystal Geyser, Utah. In Earthquakes and Water; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2010; Volume 131, pp. 123–133. [Google Scholar]
- Schleheck, D.; Tindall, B.J.; Rossello-Mora, R.; Cook, A.M. Parvibaculum lavamentivorans gen. nov., sp. nov., a novel heterotroph that initiates catabolism of linear alkylbenzenesulfonate. Int. J. Syst. Evol. Microbiol. 2004, 54, 1489–1497. [Google Scholar] [CrossRef]
- Roeselers, G.; Norris, T.B.; Castenholz, R.W.; Rysgaard, S.; Glud, R.N.; Kühl, M.; Muyzer, G. Diversity of phototrophic bacteria in microbial mats from Arctic hot springs (Greenland). Environ. Microbiol. 2007, 9, 26–38. [Google Scholar] [CrossRef]
- Mori, K.; Suzuki, K.-I. Thiofaba tepidiphila gen. nov., sp. nov., a novel obligately chemolithoautotrophic, sulfur-oxidizing bacterium of the Gammaproteobacteria isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2008, 58, 1885–1891. [Google Scholar] [CrossRef]
- Naraoka, H.; Uehara, T.; Hanada, S.; Kakegawa, T. δ13C–δD distribution of lipid biomarkers in a bacterial mat from a hot spring in Miyagi Prefecture, NE Japan. Org. Geochem. 2010, 41, 398–403. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Senko, J.M.; Najar, F.Z.; Kenton, S.M.; Roe, B.A.; Dewers, T.A.; Spear, J.R.; Krumholz, L.R. Bacterial Diversity and Sulfur Cycling in a Mesophilic Sulfide-Rich Spring. Appl. Environ. Microbiol. 2003, 69, 5609–5621. [Google Scholar] [CrossRef]
- Tekere, M. Metagenomic analysis of bacterial diversity of Siloam hot water spring, Limpopo, South Africa. Afr. J. Biotechnol. 2011, 10, 18005–18012. [Google Scholar] [CrossRef]
- Ghalib, A.K.; Yasin, M.; Faisal, M. Characterization and Metal Detoxification Potential of Moderately Thermophilic Bacillus cereus from Geothermal Springs of Himalaya. Braz. Arch. Biol. Technol. 2014, 57, 554–560. [Google Scholar] [CrossRef]
- Maruyama, T.; Park, H.-D.; Ozawa, K.; Tanaka, Y.; Sumino, T.; Hamana, K.; Hiraishi, A.; Kato, K. Sphingosinicella microcystinivorans gen. nov., sp. nov., a microcystin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2006, 56, 85–89. [Google Scholar] [CrossRef]
- Szuróczki, S.; Kéki, Z.; Káli, S.; Lippai, A.; Márialigeti, K.; Tóth, E. Microbiological investigations on the water of a thermal bath at Budapest. Acta Microbiol. Immunol. Hung. 2016, 63, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, M.A. Otitis externa due to Pseudomonas in swimming pool bathers. J. R. Coll. Gen. Pr. 1977, 27, 359–360. [Google Scholar]


| Analyte | Units | Borehole | Pre-Filter (Chlorine, Cold and Post Bathing) | Post Filter |
|---|---|---|---|---|
| Total coliforms (n = 2) | cfu/mL | <1 | <1 (cold); 2.3 (post bathing) | - |
| Faecal coliforms (n = 2) | cfu/mL | <1 | <1 (cold); 2.3 (post bathing) | - |
| E. coli (n = 2) | cfu/mL | <1 | <1 | - |
| Temperature | °C | 44 | 24 | 24 |
| Dissolved Oxygen (Field) | mg/L | 0.9 | 7.0 | 3.0 |
| Dissolved Oxygen Calc (Field) | % | 14.4 | 90.8 | 39.0 |
| Free Chlorine (Field) | mg/L | 0.27 | <0.05 | |
| Total Chlorine (Field) | mg/L | 0.81 | <0.05 | |
| Field Monochloramine as Cl2 | mg/L | <0.05 | <0.05 | |
| pH | Units | 6.9 | 7.8 | 7.7 |
| Phosphorus, reactive as P | mg P/L | 0.14 | 0.21 | 0.16 |
| Phosphorus, total as P | mg P/L | 0.20 | 0.26 | 0.22 |
| Total Organic Carbon | mg/L | 1.6 | 2.6 | 1.7 |
| Suspended Solids | mg/L | <2 | 3 | <2 |
| Total Solids, 105 °C | mg/L | 3200 | 3000 | 3000 |
| Electrical Conductivity at 25 °C | uS/cm | 5600 | 5300 | 5300 |
| Salinity, calculated | mg/L | 3700 | 3600 | 3500 |
| Turbidity, NTU | NTU | <0.1 | 0.5 | 0.1 |
| Redox Potential against Calomel | mV | 200 | 180 | 180 |
| Chloride, as Cl | mg/L | 1500 | 1400 | 1400 |
| Sulphate, as SO4 | mg/L | 15 | 20 | 21 |
| Alkalinity Total Alkalinity as CaCO3 | mg CaCO3/L | 730 | 690 | 680 |
| Calcium carbonate saturation index (no units) | 0.12 | 1.0 | 0.90 | |
| Ammonia, as N | mg N/L | 2.0 | 0.7 | 0.4 |
| Nitrate, as N | mg N/L | 0.04 | <0.01 | 0.33 |
| Nitrite, as N | mg N/L | <0.01 | 0.02 | 0.03 |
| Aluminium | mg/L | 0.22 | 0.02 | <0.01 |
| Arsenic | mg/L | <0.001 | <0.001 | <0.001 |
| Barium | mg/L | 0.63 | 0.54 | 0.55 |
| Beryllium | mg/L | <0.001 | <0.001 | <0.001 |
| Boron | mg/L | 1.1 | 0.98 | 0.99 |
| Cadmium | mg/L | <0.0002 | <0.0002 | <0.0002 |
| Chromium | mg/L | <0.001 | <0.001 | <0.001 |
| Cobalt | mg/L | <0.001 | <0.001 | <0.001 |
| Copper | mg/L | <0.001 | 0.001 | <0.001 |
| Iron | mg/L | 0.03 | 0.03 | <0.01 |
| Lead | mg/L | <0.001 | <0.001 | <0.001 |
| Manganese | mg/L | 0.072 | 0.086 | 0.029 |
| Mercury | mg/L | <0.0001 | <0.0001 | <0.0001 |
| Nickel | mg/L | <0.001 | <0.001 | <0.001 |
| Vanadium | mg/L | <0.001 | <0.001 | <0.001 |
| Zinc | mg/L | 0.001 | 0.004 | 0.005 |
| Calcium | mg/L | 120 | 120 | 120 |
| Magnesium | mg/L | 97 | 92 | 91 |
| Potassium | mg/L | 68 | 64 | 65 |
| Sodium | mg/L | 880 | 840 | 820 |
| Plate Count 36 °C | orgs/mL | 1800 | >10,000 | >10,000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aburto-Medina, A.; Shahsavari, E.; Cohen, M.; Mantri, N.; Ball, A.S. Analysis of the Microbiome (Bathing Biome) in Geothermal Waters from an Australian Balneotherapy Centre. Water 2020, 12, 1705. https://doi.org/10.3390/w12061705
Aburto-Medina A, Shahsavari E, Cohen M, Mantri N, Ball AS. Analysis of the Microbiome (Bathing Biome) in Geothermal Waters from an Australian Balneotherapy Centre. Water. 2020; 12(6):1705. https://doi.org/10.3390/w12061705
Chicago/Turabian StyleAburto-Medina, Arturo, Esmaeil Shahsavari, Marc Cohen, Nitin Mantri, and Andrew S Ball. 2020. "Analysis of the Microbiome (Bathing Biome) in Geothermal Waters from an Australian Balneotherapy Centre" Water 12, no. 6: 1705. https://doi.org/10.3390/w12061705
APA StyleAburto-Medina, A., Shahsavari, E., Cohen, M., Mantri, N., & Ball, A. S. (2020). Analysis of the Microbiome (Bathing Biome) in Geothermal Waters from an Australian Balneotherapy Centre. Water, 12(6), 1705. https://doi.org/10.3390/w12061705






