Removal of Herbicides from Landfill Leachate in Biofilters Stimulated by Ammonium Acetate
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot Site and Water Supply
2.2. Construction of Biofilters
2.3. Experimental Design
2.4. Oxygen and Total Organic Carbon and Nitrogen (TOC/TN)
2.5. Chemicals
2.6. Quantification of Phenoxyacids
3. Results and Discussion
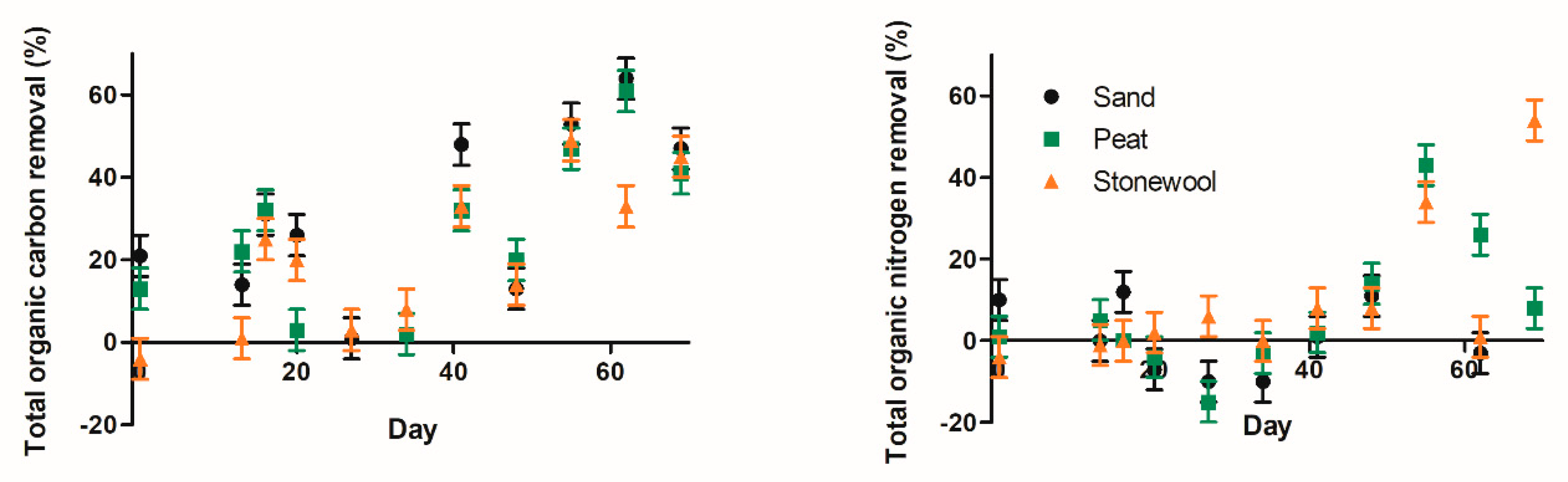
3.1. Oxygen, TOC, and TN
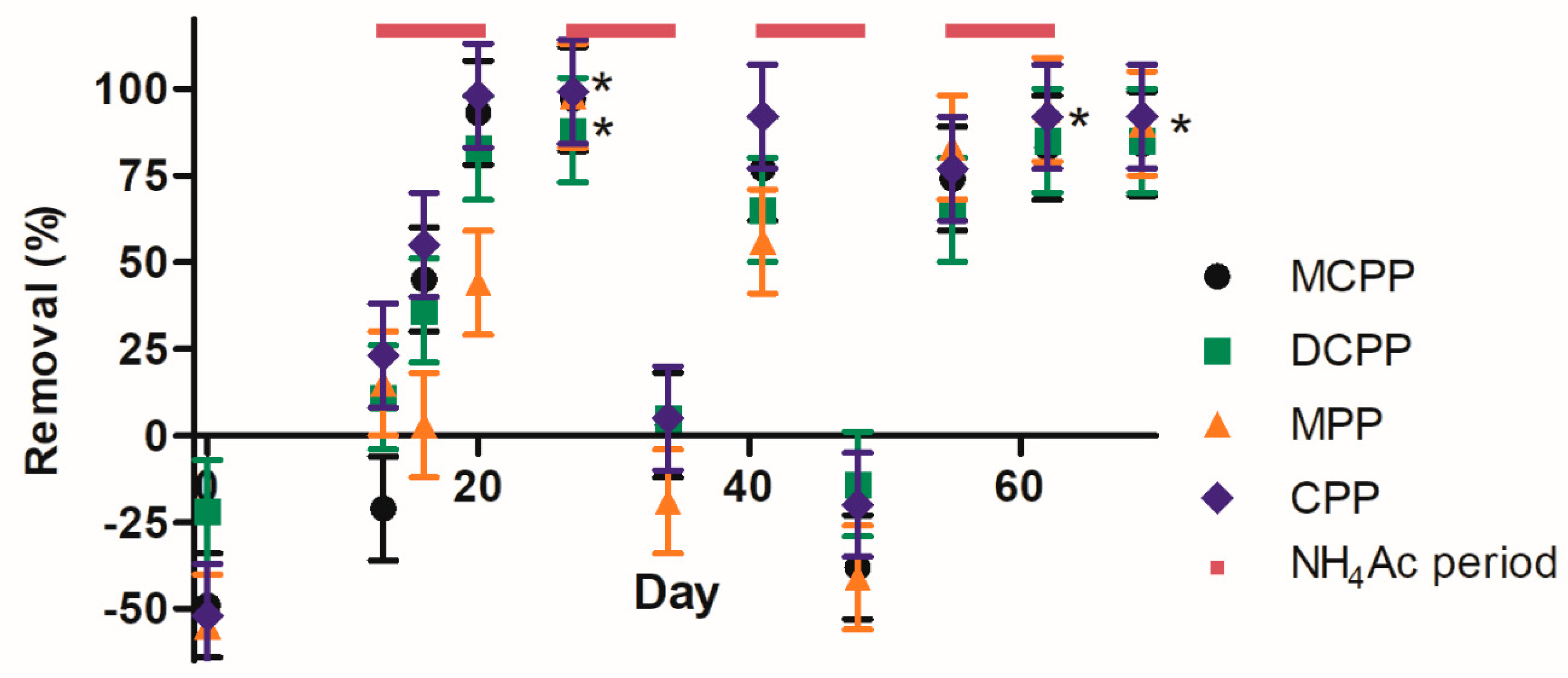
3.2. Phenoxyacids and Metabolites
3.2.1. Sand Biofilter
3.2.2. Peat and Stonewool Biofilters
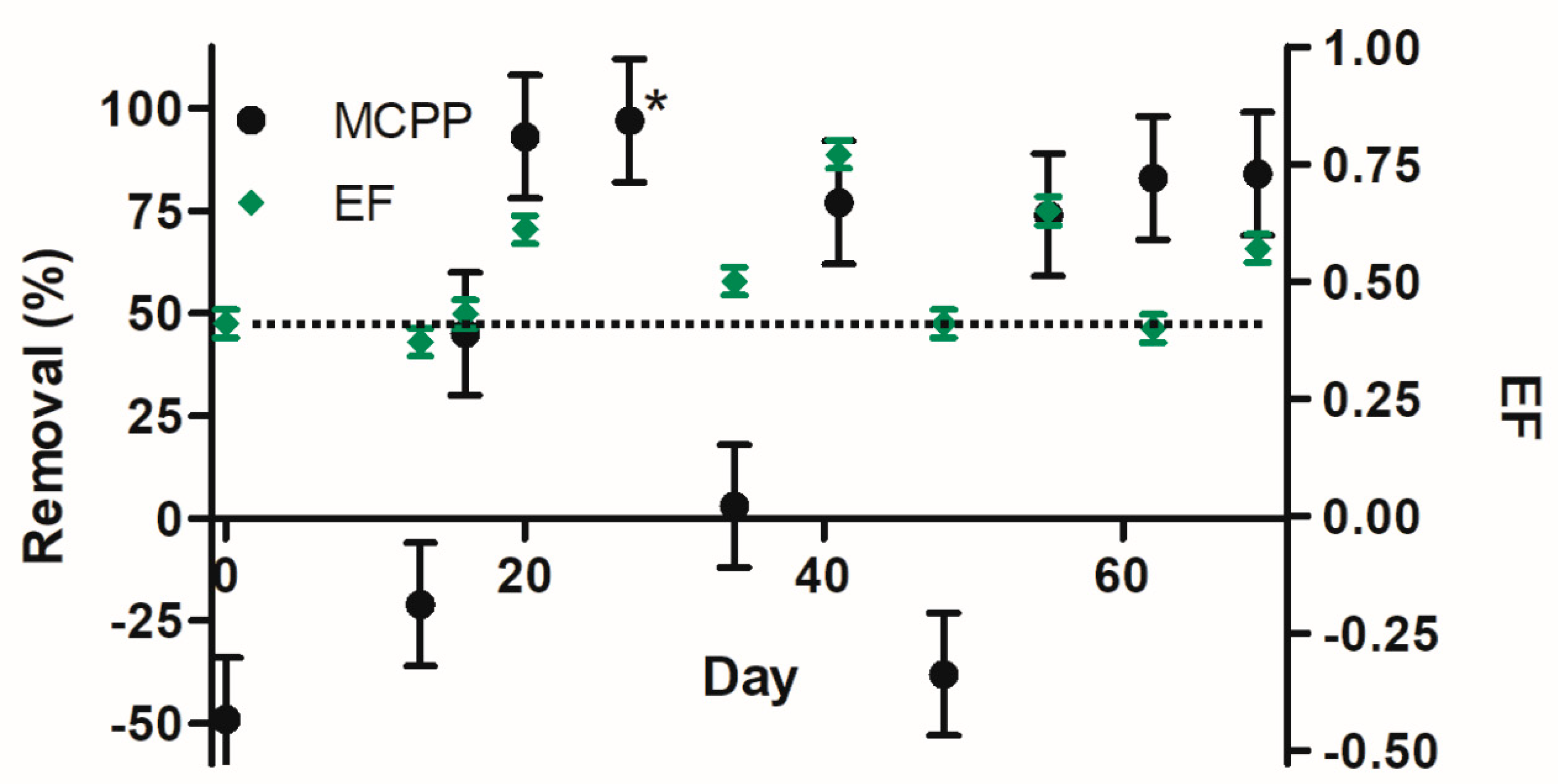
3.3. Enantioselectivity
3.3.1. Mecoprop
3.3.2. Dichlorprop
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miljøstyrelsen Sådan Fremstilles Drikkevand. Available online: https://mst.dk/natur-vand/vand-i-hverdagen/drikkevand/saadan-fremstilles-drikkevand/ (accessed on 31 January 2020).
- Danish Environmental Protection Agency. Guidelines on Remediation of Contaminated Sites; Danish Environmental Protection Agency: Copenhagen, Denmark, 2002; Volume 7, p. 290. [Google Scholar]
- Thorling, L.; Ditlefsen, C.; Ernstsen, V.; Hansen, B.; Johnsen, A.R.; Troldborg, L. Grundvandsovervågning. Status og Udvikling 1989–2018; GEUS: Copenhagen, Denmark, 2019. [Google Scholar]
- Overheu, N.D.; Møller, M.G.; Jepsen, T.S.; Larsen, L.C.; Thomsen, J.; Tuxen, N. Tilvejebringelse af Beslutningsgrundlag for den Fremtidige Afværge på Stengårdens Losseplads; Orbicon: Roskilde, Denmark, 2015. [Google Scholar]
- Paszko, T.; Muszyński, P.; Materska, M.; Bojanowska, M.; Kostecka, M.; Jackowska, I. Adsorption and degradation of phenoxyalkanoic acid herbicides in soils: A review. Environ. Toxicol. Chem. 2016, 35, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cruz, S.; Jones, J.E.; Bending, G.D. Field-scale study of the variability in pesticide biodegradation with soil depth and its relationship with soil characteristics. Soil Biol. Biochem. 2006, 38, 2910–2918. [Google Scholar] [CrossRef]
- Buss, S.; Thrasher, J.; Morgan, J.; Smith, J.W. A review of mecoprop attenuation in the subsurface. Q. J. Eng. Geol. Hydrogeol. 2006, 39, 283–292. [Google Scholar] [CrossRef]
- Milosevic, N.; Qiu, S.; Elsner, M.; Einsiedl, F.; Maier, M.P.; Bensch, H.K.V.; Albrechtsen, H.J.; Bjerg, P.L. Combined isotope and enantiomer analysis to assess the fate of phenoxy acids in a heterogeneous geologic setting at an old landfill. Water Res. 2013, 47, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Williams, A. Opportunities for chiral agrochemicals. Pest Manag. Sci. 1996, 46, 3–9. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual: A World Compendium; Tomlin, C.D.S., Ed.; British Crop Production Council: Hampshire, UK, 1997; ISBN 9781901396188. [Google Scholar]
- Zipper, C.; Nickel, K.; Angst, W.; Kohler, H.P.E. Complete microbial degradation of both enantiomers of the chiral herbicide mecoprop [(RS)-2-(4-chloro-2-methylphenoxy)propionic acid] in an enantioselective manner by Sphingomonas herbicidovorans sp. nov. Appl. Environ. Microbiol. 1996, 62, 4318–4322. [Google Scholar] [CrossRef]
- Zipper, C.; Bunk, M.; Zehnder, A.J.B.; Kohler, H.P.E. Enantioselective uptake and degradation of the chiral herbicide dichlorprop [(RS)-2-(2,4-dichlorophenox)propanoic acid] by Sphingomonas herbicidovorans MH. J. Bacteriol. 1998, 180, 3368–3374. [Google Scholar] [CrossRef]
- Müller, T.A.; Kohler, H.P.E. Chirality of pollutants - Effects on metabolism and fate. Appl. Microbiol. Biotechnol. 2004, 64, 300–316. [Google Scholar] [CrossRef]
- Escolà Casas, M.; Nielsen, T.K.; Kot, W.; Hansen, L.H.; Johansen, A.; Bester, K. Degradation of mecoprop in polluted landfill leachate and waste water in a moving bed biofilm reactor. Water Res. 2017, 121, 213–220. [Google Scholar] [CrossRef]
- Frková, Z.; Johansen, A.; de Jonge, L.W.; Olsen, P.; Gosewinkel, U.; Bester, K. Degradation and enantiomeric fractionation of mecoprop in soil previously exposed to phenoxy acid herbicides—New insights for bioremediation. Sci. Total Environ. 2016, 569–570, 1457–1465. [Google Scholar] [CrossRef]
- Williams, G.M.; Harrison, I.; Carlick, C.A.; Crowley, O. Changes in enantiomeric fraction as evidence of natural attenuation of mecoprop in a limestone aquifer. J. Contam. Hydrol. 2003. [Google Scholar] [CrossRef]
- Wong, C.S. Environmental fate processes and biochemical transformations of chiral emerging organic pollutants. Anal. Bioanal. Chem. 2006, 386, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Tsitnonaki, K.; Roost, S.; Larsen, L.C.; Andersen, K.; Johansen, A.; Gosewinkel, U.; Nielsen, T.K. Pilotprojekt—In Situ Test af Stimuleret Aerob Nedbrydning til Oprensning af Pesticidpunktkilder; Danish Environmental Protection Agency: Copenhagen, Denmark, 2017. [Google Scholar]
- Tuxen, N.; Reitzel, L.A.; Albrechtsen, H.J.; Bjerg, P.L. Oxygen-enhanced biodegradation of phenoxy acids in ground water at contaminated sites. Ground Water 2006, 44, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Heijman, S.G.J.; Siegers, W.; Sterk, R.; Hopman, R. Prediction of breakthrough of pesticides in GAC-filters and breakthrough of colour in ion-exchange-filters. Water Sci. Technol. Water Supply 2002, 2, 103–108. [Google Scholar] [CrossRef]
- Feld, L.; Nielsen, T.K.; Hansen, L.H.; Aamand, J.; Albers, C.N. Establishment of bacterial herbicide degraders in a rapid sand filter for bioremediation of phenoxypropionate-polluted groundwater. Appl. Environ. Microbiol. 2015, 82, 878–887. [Google Scholar] [CrossRef]
- Bester, K.; Banzhaf, S.; Burkhardt, M.; Janzen, N.; Niederstrasser, B.; Scheytt, T. Activated soil filters for removal of biocides from contaminated run-off and waste-waters. Chemosphere 2011, 85, 1233–1240. [Google Scholar] [CrossRef]
- Carpenter, C.M.G.; Helbling, D.E. Removal of micropollutants in biofilters: Hydrodynamic effects on biofilm assembly and functioning. Water Res. 2017, 120, 211–221. [Google Scholar] [CrossRef]
- Casas, M.E.; Bester, K. Can those organic micro-pollutants that are recalcitrant in activated sludge treatment be removed from wastewater by biofilm reactors (slow sand filters)? Sci. Total Environ. 2015, 506–507, 315–322. [Google Scholar] [CrossRef]
- Hermes, N.; Jewell, K.S.; Schulz, M.; Müller, J.; Hübner, U.; Wick, A.; Drewes, J.E.; Ternes, T.A. Elucidation of removal processes in sequential biofiltration (SBF) and soil aquifer treatment (SAT) by analysis of a broad range of trace organic chemicals (TOrCs) and their transformation products (TPs). Water Res. 2019, 114857. [Google Scholar] [CrossRef]
- Kunc, F.; Rybářová, J. Effect of glucose on the amount of bacteria mineralizing 2,4-dichlorophenoxyacetic acid in soil. Folia Microbiol. (Praha) 1983, 28, 54–56. [Google Scholar] [CrossRef]
- Fischer, K.; Majewsky, M. Cometabolic degradation of organic wastewater micropollutants by activated sludge and sludge-inherent microorganisms. Appl. Microbiol. Biotechnol. 2014, 98, 6583–6597. [Google Scholar] [CrossRef]
- Zhang, L.; Carvalho, P.N.; Bollmann, U.E.; EI-taliawy, H.; Brix, H.; Bester, K. Enhanced removal of pharmaceuticals in a biofilter: Effects of manipulating co-degradation by carbon feeding. Chemosphere 2019, 236, 124303. [Google Scholar] [CrossRef]
- de Lipthay, J.R.; Sørensen, S.R.; Aamand, J. Effect of herbicide concentration and organic and inorganic nutrient amendment on the mineralization of mecoprop, 2,4-D and 2,4,5-T in soil and aquifer samples. Environ. Pollut. 2007, 148, 83–93. [Google Scholar] [CrossRef]
- Frková, Z.; Johansen, A.; Karlson, U.G. Mecoprop mineralization potential at oxygen-reduced conditions in subsoil with phenoxy acid contamination history. Soil Biol. Biochem. 2015, 84, 189–198. [Google Scholar] [CrossRef]
- Nord, N.B.; Bester, K. Can the removal of pharmaceuticals in biofilters be influenced by short pulses of carbon? Sci. Total Environ. 2020, 707, 135901. [Google Scholar] [CrossRef]
- Harner, T.; Wiberg, K.; Norstrom, R. Enantiomer fractions are preferred to enantiomer ratios for describing chiral signatures in environmental analysis. Environ. Sci. Technol. 2000, 34, 218–220. [Google Scholar] [CrossRef]
- Snowden, R.E.D.; Wheeler, B.D. Chemical changes in selected wetland plant species with increasing Fe supply, with specific reference to root precipitates and Fe tolerance. New Phytol. 1995, 131, 503–520. [Google Scholar] [CrossRef]
- Reitzel, L.A.; Tuxen, N.; Ledin, A.; Bjerg, P.L. Can Degradation Products Be Used as Documentation for Natural Attenuation of Phenoxy Acids in Groundwater? Environ. Sci. Technol. 2004, 38, 457–467. [Google Scholar] [CrossRef]
- Gao, J.; Ellis, L.B.M.; Wackett, L.P. The University of Minnesota Biocatalysis/Biodegradation Database: Improving public access. Nucleic Acids Res. 2009, 38, 488–491. [Google Scholar] [CrossRef]
- Levi, S.; Hybel, A.M.; Bjerg, P.L.; Albrechtsen, H.J. Stimulation of aerobic degradation of bentazone, mecoprop and dichlorprop by oxygen addition to aquifer sediment. Sci. Total Environ. 2014, 473–474, 667–675. [Google Scholar] [CrossRef]
- Mohn, W.W.; Tiedje, J.M. Microbial reductive dehalogenation. Microbiol. Rev. 1992, 56, 482–507. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, M.J.; Arvin, E.; Corfitzen, C.B.; Albrechtsen, H.J. Mecoprop (MCPP) removal in full-scale rapid sand filters at a groundwater-based waterworks. Sci. Total Environ. 2014, 499, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, S.; El-taliawy, H.; Bester, K. Concentration dependent degradation of pharmaceuticals in MBBR from wastewater treatment plant effluent. Water Res. submitted in June 2020.
- Rügge, K.; Juhler, R.K.; Broholm, M.M.; Bjerg, P.L. Degradation of the (R)- and (S)-enantiomers of the herbicides MCPP and dichlorprop in a continuous field-injection experiment. Water Res. 2002, 36, 4160–4164. [Google Scholar] [CrossRef]
- Buser, H.-R.; Müller, M.D. Conversion Reactions of Various Phenoxyalkanoic Acid Herbicides in Soil. 2. Elucidation of the Enantiomerization Process of Chiral Phenoxy Acids from Incubation in a D2O/Soil System. Environ. Sci. Technol. 1997, 31, 1960–1967. [Google Scholar] [CrossRef]
- Matamoros, V.; Franco, J. Assessing the use of sand, peat soil, and pine bark for the attenuation of polar pesticides from agricultural run-off: A bench-scale column experiment. Environ. Sci. Pollut. Res. 2018, 25, 20640–20647. [Google Scholar] [CrossRef]
- Hylling, O.; Nikbakht Fini, M.; Ellegaard-Jensen, L.; Muff, J.; Madsen, H.T.; Aamand, J.; Hansen, L.H. A novel hybrid concept for implementation in drinking water treatment targets micropollutant removal by combining membrane filtration with biodegradation. Sci. Total Environ. 2019, 694, 133710. [Google Scholar] [CrossRef]
- Pinilla-Redondo, R.; Olesen, A.K.; Russel, J.; de Vries, L.E.; Christensen, L.D.; Musovic, S.; Nesme, J.; Sørensen, S.J. Conjugative dissemination of plasmids in rapid sand filters: A trojan horse strategy to enhance pesticide degradation in groundwater treatment. bioRxiv 2020. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nord, N.B.; Berthelsen, N.M.S.; Milter, H.; Bester, K. Removal of Herbicides from Landfill Leachate in Biofilters Stimulated by Ammonium Acetate. Water 2020, 12, 1649. https://doi.org/10.3390/w12061649
Nord NB, Berthelsen NMS, Milter H, Bester K. Removal of Herbicides from Landfill Leachate in Biofilters Stimulated by Ammonium Acetate. Water. 2020; 12(6):1649. https://doi.org/10.3390/w12061649
Chicago/Turabian StyleNord, Nadia Brogård, Nils M. Sevelsted Berthelsen, Hasse Milter, and Kai Bester. 2020. "Removal of Herbicides from Landfill Leachate in Biofilters Stimulated by Ammonium Acetate" Water 12, no. 6: 1649. https://doi.org/10.3390/w12061649
APA StyleNord, N. B., Berthelsen, N. M. S., Milter, H., & Bester, K. (2020). Removal of Herbicides from Landfill Leachate in Biofilters Stimulated by Ammonium Acetate. Water, 12(6), 1649. https://doi.org/10.3390/w12061649





