Abstract
Measurements for determining the effect of chemically enhanced primary treatment (CEPT) on the efficiency of pollutant removal from wastewater were carried out using conventional inorganic coagulants PIX113 with polymer A110 (Kemipol, Police, Poland) and unconventional cationic organic coagulants Cofloc (Attana, Coalville, UK) C29510 (Kemipol, Police, Poland) and Sedifloc 575 (3F Chimica, Sandrigo, Italy). The average removal efficiency in the 2-h sedimentation process was 46%, 34%, 8%, 12% for the total suspended solids, organic matter (COD), total nitrogen, and total phosphorus, respectively. The use of organic coagulants contributed to 14–81% increase of pollutant removal efficiency. Substantial discrepancies in biological nutrient removal processes were not discovered in two-phase (anaerobic-anoxic) experiments without and with the addition of the organic coagulants. The increase in organic matter removal efficiency as a result of the CEPT process may contribute to a 65–80% increase in biogas production. The conducted research confirms the possibility of using organic coagulants in the primary precipitation process in wastewater treatment plants (WWTPs) in accordance with the principles of maximum energy recovery, thereby promoting renewable energy sources. Additionally, organic coagulants, as opposed to inorganic ones, do not cause a significant increase of chloride and sulfate ion concentrations, which facilitates the use of treated wastewater in the water reuse systems, such as irrigation of agricultural crops.
1. Introduction
The current paradigm regarding wastewater treatment plants requires not only stringent quality standards for treated wastewater, but also maximum energy recovery from wastewater, promoting renewable energy sources. In addition, in 2014, European Union legislation introduced a “zero waste” strategy to stimulate the development of new waste reuse technologies [1]. Conventional wastewater treatment systems were designed primarily for the removal of organic compounds, whereas the majority of large municipal wastewater treatment plants are equipped with primary clarifiers to reduce the load of particulate fraction directed to the bioreactor. According to the literature reports, wastewater after treatment in primary settling tanks is still characterized by a high content of slowly and readily biodegradable organic compounds (expressed as COD), in the range of 40–60% and 10–30% of total COD (tCOD), respectively [2]. However, studies carried out by Pagilla et al. [3] in four municipal wastewater treatment plants in northern Poland showed that organic compounds (in the colloidal and particulate fraction) can even constitute over 60% of tCOD. In order to increase the removal of organic compounds from wastewater in the particulate and colloidal fraction, chemically enhanced primary treatment (CEPT) is practiced [4,5,6,7,8]. For this purpose, inorganic coagulants are most often used, i.e., metal salts (e.g., aluminum sulfate, ferric chloride, ferric sulfate, polyaluminum chloride, sodium aluminate), although recently literature reports also indicate the use of organic coagulants (Ecofloc, Perrustol, Chitosan, HP-PACl, Musa sp. flower extract) [7,8,9,10,11].
The addition of coagulants also aims to reduce the load of pollutants inflowing to the biological part in facilities for which more stringent standards regarding the quality of treated wastewater are required [8]. Gori et al. [12] showed that intensified removal of organic matter in primary settling tanks can bring benefits such as reducing the energy demand for aeration in bioreactors and reducing CO2 emissions to the atmosphere during microbiological processes taking place in a wastewater treatment plant (WWTP).
According to Huang and Li [13], CEPT yields approximately 1.5–2.0 times higher primary sludge production than conventional sedimentation (i.e., without the use of coagulants). Larger production of primary sludge (after the application of CEPT) may contribute to a significant increase in the amount of biogas produced in the methane fermentation process [14]. Increased production of electricity from methane, combined with the removal of nitrogen from the liquid fraction of digestate in the deammonification process can lead to WWTP energy self-sufficiency [15]. Primary sludge separated in the CEPT process can also be used to produce bioelectricity in microbial fuel cells (MFCs). Wang et al. [16] have shown that the primary sludge obtained in the upflow dynamic membrane separation reactor enables greater electricity production compared to waste-activated sludge. According to the authors, the use of CEPT may lead to the removal of total suspended solids (TSS) (60–90%), organic matter expressed as BOD5 (40–80%) and COD (30–70%), phosphorus compounds (65–95%), and bacteria (80–90%) [6,9,13]. For comparison, in a conventional sedimentation process (i.e., without the use of chemical precipitation), 50–70% TSS, 25–40% BOD5, 5–15% phosphorus compounds, and 50–60% pathogens are removed in primary settlers [9,17]. The CEPT process with the use of organic coagulants is also applied to treat industrial wastewater, e.g., from the tannery industry, enabling high-efficiency removal of hazardous substances, such as total chromium (65.4%), hexavalent chromium (39.43%), and trivalent chromium (61.02%) [7].
Increased chemical precipitation of organic matter in primary settlers carries the risk of disturbing the proper nitrogen and phosphorus biological removal processes. The effectiveness of these processes is dependent on the appropriate availability of organic compounds, necessary for the proper metabolism of heterotrophic organisms. Despite the relatively significant number of publications on the use of CEPT for municipal and industrial wastewater treatment, information on the impact of this process on the biological removal rate of nutrients is very limited. Makinia et al. [18] drew attention to this problem in their research, showing that the removal of organic matter in particulate and colloidal form reduced (by up to 60%) the rate of biological processes. Drewnowski and Makinia [19] showed that the removal of colloidal and particulate fractions of organic compounds from municipal wastewater resulted in a 20–40% decrease in the rate of denitrification and a 40–70% decrease in anoxic phosphate uptake rate.
The aim of the study was to determine the impact of organic coagulant dosage in the CEPT process on the efficiency of biological nitrogen and phosphorus removal processes in the context of removing pollutants contained in raw municipal wastewater. This is a novelty of the work because this type of research is not available in the literature. In addition, the potential increase in biogas production as well as the salinity of treated wastewater will be assessed in comparison to the dosing of typical inorganic coagulant.
2. Materials and Methods
2.1. Material
2.1.1. Raw Wastewater and Activated Sludge
Raw wastewater and activated sludge samples were collected from municipal wastewater treatment plants in Gdansk, Gdynia, and Slupsk located in northern Poland. They are included in the largest facilities in the Polish coastal zone, with sizes of 565,000, 515,500, and 200,000 PE respectively. After the last modernization, the WWTP in Gdansk treats on average 81,000 m3/day of wastewater in the A2/O system. The configuration of the biological stage in the two remaining WWTPs is similar to that of Johannesburg (JHB), while in the WWTP in Gdynia (capacity 56,000 m3/day) the reactors are supplemented with circulation chambers with simultaneous denitrification, and in the WWTP in Slupsk (capacity 19,600 m3/day) the recirculated sludge pre-denitrification chamber is not fed with primary effluent.
The average daily samples of raw wastewater were collected in rainless periods from points located between the screens and grit chambers. Activated sludge was taken from the nitrification chambers of the treatment plant in which the tests were carried out. Wastewater and activated sludge samples were immediately transported to the laboratory in a thermostatic container at 4 °C.
2.1.2. Organic and Inorganic Coagulants
Three organic coagulants and one inorganic coagulant supplemented with polyelectrolyte dispensing were selected for the study. Organic coagulants were: Cofloc, C29510, and Sedifloc 575. In order to compare the effectiveness of organic coagulates with conventional reagents, dosing of commercial PIX113 coagulum with A110 anion polyelectrolyte from Kemipol was adopted.
Cofloc coagulant is a non-toxic, biodegradable, low-molecular-weight cationic polymer produced from renewable sources (cactus plants). The cationic coagulant C29510 has variable molecular weight and valence. It is available as an emulsion. Sedifloc 575 coagulant is a high-charge cationic polymer, completely soluble in water. The detailed characteristics of the organic coagulants used in the study are presented in Table S1. In contrast, PIX113 is a conventional iron reagent in the form of an approx. 12% solution of iron (III) sulfate, and A110 is an anionic polymer (anionic polyacrylamide) in the form of a powder to support the coagulation process in water and wastewater treatment, and to facilitate sludge dewatering. Based on the results of preliminary studies, the following doses of the analyzed reagents were adopted: 30 mg/L (Cofloc), 50 mg/L (C29510), 52 mg/L (Sedifloc 575), and 75 mg/L (PIX113) with 1 mg/L (A110) [15,20].
2.2. Research Methodology
2.2.1. Primary Precipitation
In order to determine the effectiveness of pollutant removal in the process of CEPT, laboratory tests were carried out with wastewater without primary precipitation (only after 2-h of sedimentation) and after coagulation with the addition of organic and inorganic coagulants (Figure 1).
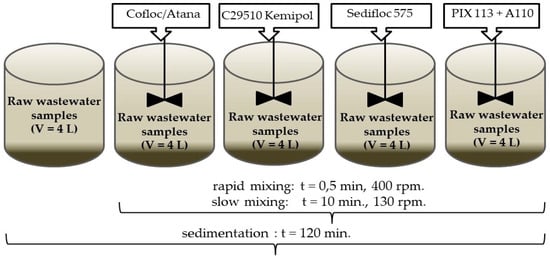
Figure 1.
Measuring the effectiveness of precipitation in primary settlers—overview diagram.
Wastewater after sedimentation (control sample). Raw wastewater samples with a volume of 4.0 L were poured into a beaker and then sedimented for two hours. After this time, the supernatant liquid from the precipitate was decanted.
Wastewater after coagulation. Raw wastewater samples (4 L each) were poured into 4 beakers and specified doses of coagulants were added. The volume changes due to the addition of coagulant stock solutions were minimal (from 0.1 to 0.2%). Then the wastewater was first subjected to rapid and then slow mixing. The rapid mixing time was 0.5 min at 400 rpm. The slow mixing lasted 10 min at a speed of 130 rpm. A110 anionic polymer was dosed into the beaker during the test with conventional reagents before the slow mixing phase. After completion of slow mixing, the wastewater was subjected to sedimentation for two hours, and then the supernatant liquid was decanted from the precipitate. The parameters of rapid and slow mixing were determined based on preliminary test results [20] while the 2-h sedimentation time is commonly used to determine the removal efficiency of easily sedimented suspended solids. The volume of the precipitated primary sludge was measured using Imhoff funnels, into which the sludge from the bottom of the beakers was overflown, after a 2-h sedimentation period.
In order to determine the maximum achievable removal efficiency of particulate and colloidal fraction from wastewater, the methodology described by Mamais et al. [21] was adopted. It is based on precipitation with zinc sulfate at a pH of 10.5. After 2-h of sedimentation in the decanted liquid, the original pH was restored by dispensing a 6 molar HCl solution.
2.2.2. Research on the Primary Precipitation Impact on Biological Processes
Experimental studies were carried out in laboratory conditions. In order to assess the effect of primary precipitation on the biological removal of nutrients, two-phase tests were performed, including phosphate release rate (PRR) under anaerobic conditions, nitrogen utilization rate (NUR), and anoxic phosphate uptake rate (PURANOX). The two-phase test used in the study facilitates the consideration of interactions between denitrification and enhanced biological P removal (EBPR) occurring in the combined N and P systems [22]. The tests were carried out in a specially designed device comprising two parallel 4L Plexiglas reactors (Plexikon, Gdansk, Poland). The contents of the reactors were mixed by means of mechanical stirrers at an intensity of 180 rpm. In the reactors, it was possible to maintain a constant, assumed temperature using a water jacket connected to the thermostat.
The tests were carried out on wastewater after a 2-h sedimentation period without dosing the reagents or preceded by dosing analyzed organic and inorganic coagulants (see Research Methodology, l Primary precipitation).
Two liters of activated sludge concentrated in the sedimentation process and 2 L of analyzed wastewater were fed into the reactors. The initial biomass concentration was about 4.0 g/L. The anaerobic phase lasted 2-h, followed by 4 to 5 h of anoxic phase. The length of the phases resulted from the time necessary to stabilize the final concentrations of phosphates (anaerobic phase) or nitrates (anoxic phase). At the beginning of the anoxic phase, potassium nitrate was added to the reactors to increase the nitrate nitrogen concentration to approx. 20 mg N/L.
2.2.3. Analytical Methods
In the raw wastewater and supernatant liquid samples (after sedimentation with and without addition of coagulants), the concentration of organic matter expressed in tCOD and dissolved chemical oxygen demand (dCOD) (after filtration of the sample through a 0.45 μm filter), nitrogen compounds (ammonium nitrogen (NH4-N) and total nitrogen (TN)), phosphorus compounds (phosphate phosphorus (PO4-P) and total phosphorus (TP)) as well as the total and organic suspension were determined.
During the tests to check the denitrification as well as the release and anoxic uptake of phosphates in the reactors, samples of a 100 mL sludge and wastewater mixture were collected, with a frequency of every 15–30 min. To separate activated sludge, the samples were filtered through 0.45 μm pore size Millipore nitrocellulose filters (MilliporeSigma, Billerica, MA, USA) using a vacuum set. The concentrations of COD, NH4-N, nitrite nitrogen (NO3-N), nitrate nitrogen (NO2-N), and TP, as well as PO4-P were determined in the filtrate. At the beginning and end of the test, the concentration of activated sludge in the reactors was also established, including the proportion of organic fraction.
TN concentrations were determined using a TOC/TN analyzer (SHIMADZU Corp., Kyoto, Japan). COD, NH4-N, NO3-N, NO2-N as well as PO4-P and TP concentrations were established using Hach Lange cuvette tests on the DR2000 spectrophotometer (Lange GmbH, Berlin, Germany). The concentrations of total suspended solids (TSS) and volatile suspended solids (VSS) were made in accordance with Standard Methods for Examination of Water and Wastewater [23].
2.2.4. Process Rate
The rate of phosphate release under anaerobic conditions (PRR) and the uptake of phosphate under anoxic conditions (PURANOX) were calculated according to Formula (1) and the rate of denitrification under anoxic conditions according to Formula (2):
where:
PRR or PUR = Δ(PO4 − P)i/(X·t)
NUR = Δ(NO3 − N)i/(X·t)
- Δ(PO4-P)i—concentration change of PO4-P in time ti, [mgP/L].
- X—concentration of activated sludge organic fraction, [gVSS/L]
- t—duration of the test, [h].
- Δ(NO3-N)i—concentration reduction of NO3-N in time ti, [mgN/L].
In order to determine the influence of coagulants on the biological nutrient removal processes (NUR, PPR, PUR) and the efficiency of pollutant removal, the analysis of one-way variance (ANOVA) was applied. Effects were considered significant at α = 0.05. Four study groups were identified (samples after 2-h sedimentation without and with the addition of a coagulant).
3. Results
3.1. Characteristics of Raw Wastewater
The concentrations of pollutants in all raw wastewater samples for the three tested WWTPs were within the ranges of variability determined during routine tests in these facilities (Table 1). On this basis, it can be concluded that the samples were reliable for raw wastewater inflowing to the analyzed municipal treatment plants. At the same time, they were within the range of “typical” values of raw wastewater quality parameters in Polish WWTPs given by Dymaczewski et al. [24]. Only the total suspension concentrations were clearly higher.

Table 1.
Characteristics of raw municipal wastewater, n—number of tests.
3.2. Efficiency of Pollutant Elimination from Raw Wastewater in the Primary Precipitation Process Using Organic and Inorganic Coagulants
Figure 2 (and Table S2) presents the effectiveness of pollutant elimination in the sedimentation process without the addition of coagulants (control sample) and after dosing the analyzed coagulants. The removal efficiency during the 2-h sedimentation process was on average 46% for TSS, 34% for COD, 8% for TN, and 12% for TP. These values are in the typical range of variability presented in the literature [9,17].
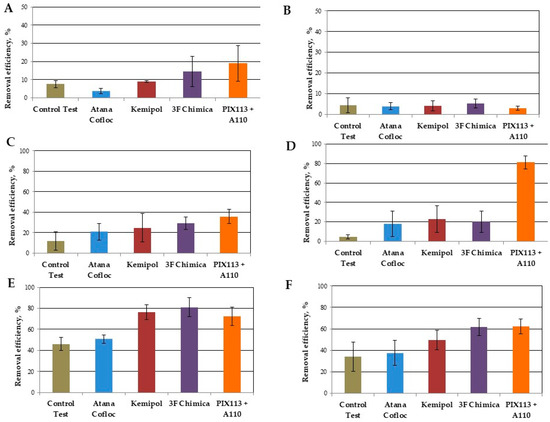
Figure 2.
Effectiveness of pollutant removal from wastewater during a 2-h sedimentation period with and without dosing coagulants, (A) TN, (B) NH4-N, (C) TP, (D) PO4-P, (E) TSS, (F) COD.
However, different removal efficiency was obtained after dosing of the analyzed coagulants. All coagulants used contributed to increasing the efficiency of TP removal, in the range of 21.0 to 36.0% (Figure 2C). However, these values are much lower than the data presented in the literature after the CEPT process (from 65.0 to 95.0%) [6,8,13]. This may be due to the formation of flocs with limited sedimentation properties. This thesis is confirmed by the values of phosphate phosphorus removal efficiency (Figure 2D), which exceed 80.0% when using PIX113 together with A110. At the same time, the use of organic coagulants resulted in a PO4-P removal efficiency of only 20.0%. Furthermore, the efficiency of removing the TSS with the use of inorganic coagulant was relatively low (72.4% on average) compared to the tests after dosing with Kemipol organic coagulates (76.0%) and 3F Chimica (81%). However, all these values were within the range of values presented in the literature for the CEPT process (from 60.0 to 90.0%). On the other hand, the use of Atana Cofloc coagulant did not contribute to a significant increase (ANOVA, p = 0.747) in the efficiency of removing TSS (Figure 2E). The efficiency of removing organic matter expressed as COD was different (Figure 2F). Moreover, in this case, the removal efficiency after adding the Atana Cofloc coagulant was close to that obtained in the control test (37.6% and 33.8%, respectively). Definitely the highest values, close to the upper limit of the range presented in the literature [8,13,25], were obtained for the organic coagulant 3F Chimica (average 61.6%) and inorganic PIX113 together with A110 (average 62.2%). These two coagulants allowed the removal of about 30% dCOD, which was three times higher compared to other coagulants. The efficiency of COD particulate fraction removal was about 40% (for wastewater without dosing of coagulants and after dosing of Atana Cofloc), and 70–80% (for other coagulants). According to Dong et al. [6] the use of inorganic coagulants (ferric chloride with polyaluminum chloride) enabled up to 76% tCOD and also up to 58% dCOD removal.
With respect to TN, only the use of 3F Chimica organic coagulant and PIX 113 coagulant together with the A110 polyelectrolyte contributed to a significant increase in its removal (14.5% and 19.0%, respectively) (ANOVA, p = 0.111) (Figure 2A). With respect to PIX113, it has been shown that a dose of 100 mg/L enabled the removal of dissolved (below 0.1 μm) and colloidal (from 0.1 to 1.2 μm) fractions of organic nitrogen contained in biologically treated wastewater with an efficiency exceeding 10% [26]. The same effect may also occur in the case of raw wastewater, as indicated by the greater efficiency of dissolved fraction of organic compounds removal (dCOD, above 0.45 μm) for both of these coagulants. The effect of dosing coagulants on the final concentration of ammonium nitrogen was negligible (Figure 2B).
3.3. The Effect of Initial Precipitation on the Efficiency of the Biological Nitrogen and Phosphorus Processes
Increased removal of organic matter in primary settling tanks can affect the efficiency of processes related to the biological removal of nutrients. In order to assess the impact of organic and inorganic coagulant dosing in the preliminary part of the WWTP, measurements of the process rates related to increased biological phosphorus removal and denitrification were carried out. Figure 3 shows an example of changes in phosphate phosphorus, nitrate nitrogen, and COD concentrations during the 2-phase test. Moreover, Table 2 summarizes the obtained values of the unit processes rates.
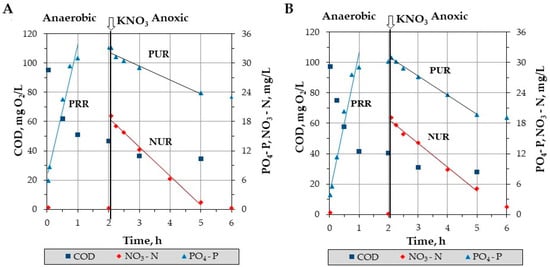
Figure 3.
Sample of concentration changes during the two-phase test (A) wastewater after 2-h sedimentation without dosing of coagulants, (B) wastewater after 2-h sedimentation preceded by 3F Chimica dosing.

Table 2.
Average nitrogen and phosphorus removal rates obtained using activated sludge samples from analyzed WWTPs and supernatant after 2-h sedimentation without and with addition of coagulant.
The CEPT process did not significantly affect the availability of volatile fatty acids used by phosphorus accumulating organisms (PAO) under anaerobic conditions. PRR values were similar for all tests performed and ranged from 9.2 to 9.6 mg P/gVSS h. These values are similar to those obtained in previous tests for primary effluent (7.9–11.2 mg P/gVSS h) [19]. No impact of the particulate and colloidal COD fraction removal on PRR values was observed (ANOVA, p = 0.994).
There was insignificant effect on the denitrification process rate during anoxic phosphate uptake (ANOVA, p = 0.992). Average NUR values for wastewater without dosing of reagents were 1.98 ± 0.52 mg N/gVSS h. These levels are similar to those obtained in previous tests for primary treated wastewater (1.8–2.3 mg N/gVSS h) [19] as well as presented in the literature for various sources of organic carbon (1.1–6.0 mg N/gVSS h) [27,28].
The rate of the anoxic phosphate uptake process (PURANOX) for preliminary treated wastewater was on average 1.35 ± 0.57 mg P/gVSS h. These values are definitely lower than those obtained in previous studies for preliminary treated municipal wastewater (2–6 mg P/gVSS h) [19] as well as those presented in the literature for various alternative organic carbon sources (2.4–4.3 mg P/gVSS h) [20]. Dosing the three analyzed organic coagulants in the CEPT process resulted in a slight (approx. 10%) decrease in the PURANOX value (ANOVA, p = 0.220). At the same time, full removal of the particulate and colloidal COD fraction resulted in a decrease of this rate by almost 30%. This also confirms the negligible effect of organic matter removal using organic coagulants in the CEPT process on the biological phosphorus removal processes rate.
4. Discussion
The new paradigm changes our approach to wastewater treatment. It should be considered as a source of valuable raw materials, such as water, energy, or nutrients, and not as a “stinky problem” that is difficult to solve. Wastewater treatment plants will want to achieve at least an energy neutral or even energy positive status, which is associated with better management of organic compounds contained in wastewater. The confirmed method is the use of the CEPT process. However, dosing of iron or aluminum salts is associated with an increase in salinity of wastewater, which worsens the possibility of using treated wastewater in water reuse systems. The solution to this problem could be the use of organic coagulants. Based on the presented research, it was shown that their use enables the achievement of a high removal efficiency of TSS (up to over 80%) and COD (up to over 60%). In practical terms, the increase in efficiency compared to the control sample has more importance (Figure 4A). Maximum values (approx. 30%) were obtained for two coagulants: 3F Chimica and PIX113 together with A110. For both of these reagents, the COD removal efficiency exceeded 80% of the maximum achievable value determined using the procedure presented by Mamais et al. [21] (Figure 4B). At the same time, the average content of particulate and colloidal COD fractions determined by this procedure (75%) was close to the values determined for these WWTPs in previous studies (69%) [16].
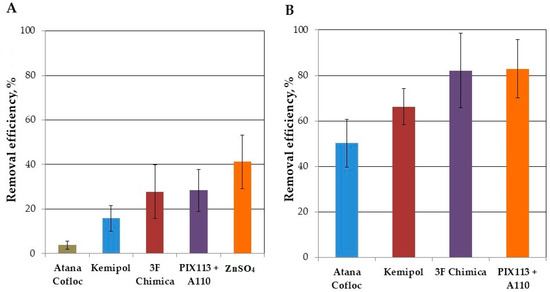
Figure 4.
Relative efficiency of organic matter (COD) removal from wastewater during their 2-h sedimentation with coagulant dosing (A) absolute increase compared to the control sample (B) share in the comparison of maximum efficiency with ZnSO4 dosing.
The analyzed organic coagulants and A110 polyelectrolyte contain trace concentrations of chlorides and sulfates (less than 1 mg/L). Thus, the effect of their dosing on the concentration of these ions is negligible. On the other hand, dosing 75 mg/L of PIX113 causes an increase in the concentration of sulfates by approx. 25 mg/L. This is a significant increase compared to typical values in biologically treated wastewater (e.g., approx. 100 mg SO43−/L for Slupsk WWTP). Thus, PIX113 dosing is associated with the use of approx. 15% limit value of the electrical conductivity of irrigation water for the medium level according to the salinity hazard classification (250 μS/cm) [29].
An increasing amount of primary sludge may contribute to an increase in biogas production in the methane fermentation process. Figure 5 presents (a) unit volumes of precipitated primary sludge with and without the addition of coagulants to the wastewater, and (b) organic matter content in the precipitated primary sludge. The conducted research indicates that with the use of organic coagulants it was possible to nearly double the volume of separated sludge with the content of organic compounds similar to the primary sludge (over 80%). However, the use of inorganic coagulant resulted in a significant (almost three-fold) increase in the volume of sludge, with a simultaneous decrease in the content of organic matter (up to 60%).
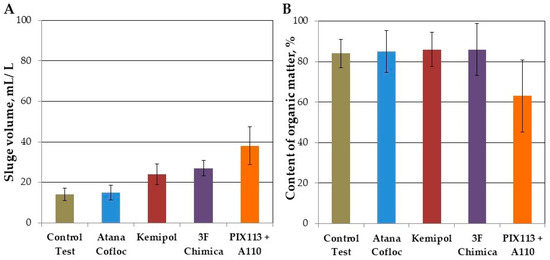
Figure 5.
Sludge separated in the chemically enhanced primary treatment (CEPT) process (A) volume after 2-h sedimentation, (B) organic matter content.
Assuming a unit volume of biogas production for primary sludge in Polish WWTPs at the level of 0.45 m3/kgVSS [15] and the efficiency of removing TSS in the CEPT process, taking into account the share of organic matter, the increase in biogas production after using organic coagulants can be estimated at 58–68 L biogas/m3 raw wastewater. This means a 65–80% increase compared to the sedimentation process in the primary settling tank without the CEPT process.
The conducted research confirmed the possibility of using organic coagulants in the primary precipitation process in WWTPs in accordance with the principles of maximum energy recovery from wastewater, thus promoting renewable energy sources. Precipitation of the suspension and colloidal fraction in the form of primary sludge increases the amount of substrate directed to the fermentation chambers, however, it can cause disturbances in the biological removal of nitrogen and phosphorus. It is therefore necessary to strike a balance between these two needs. The best method to verify the availability of organic compounds is to perform tests related to the release (under anaerobic conditions) and phosphate uptake (under anoxic or aerobic conditions), as well as the denitrification process. The results of the pollutant removal efficiency using selected organic coagulants are available in the literature, however, there is no information on the impact of their dosing on biological wastewater treatment in activated sludge chambers.
The application of the CEPT process with dosing the three analyzed organic coagulants had no effect on the NUR value (difference of 2–3.5%). However, the complete removal of the particulate and colloidal fraction of organic compounds (by ZnSO4 dosing) caused a significant decrease in the NUR value by 45%. Similar values of the NUR decrease (20–40%) were obtained by Drewnowski and Makinia [19] after removing the particulate and colloidal fraction from municipal wastewater. Moreover, full-scale tests in the Slupsk WWTP, with coagulant PIX113 and polymer A110 dosing, did not shown a significant effect on the nitrate nitrogen concentration in the outflow from biological reactors [15]. This confirms the negligible effect of organic matter removal using organic coagulants in the CEPT process on the biological nitrogen removal processes rate. It has been shown, that removal of a part of the particulate and colloidal fraction in the CEPT process using organic coagulants did not have a significant impact on the values of processes rates associated with the biological removal of phosphorus in the activated sludge process.
Currently, the barrier to the widespread use of organic coagulants is their price, which causes the cost balance to be negative, despite the positive energy balance [15]. With comparable removal efficiency, the cost of dosing a 3F Chimica organic coagulant (72.8 Euro/m3) is more than six times higher compared to the cost of dosing a conventional PIX113 coagulant with A110 polyelectrolyte (11.8 Euro/m3). However, this balance does not include restrictions related to the use of treated wastewater for irrigation, e.g., blue-green urban infrastructure, which in the era of global warming can be of great importance. This indicates the need to continue research on a comprehensive assessment of the impact of organic coagulant dosing on WWTPs, including the possibility of treated wastewater management.
5. Conclusions
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
Based on the analysis of the conducted research results, the following conclusions can be derived:
- The use of organic coagulants enables an almost two-fold increase in the removal efficiency of TSS (up to over 80%) and COD (up to over 60%) in relation to sedimentation without dosing of coagulants.
- The removal of a part of the particulate and colloidal fraction in the CEPT process using organic coagulants did not have a significant impact on the values of unit processes rates associated with the biological removal of nitrogen and phosphorus in the activated sludge process.
- The increase in the efficiency of organic matter removal in primary settling tanks as a result of the CEPT process may contribute to a 65–80% increase in biogas production in anaerobic digestion chambers.
- The conducted research confirmed the possibility of using organic coagulants in the primary precipitation process in WWTPs in accordance with the principles of maximum energy recovery from wastewater, promoting renewable energy sources.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4441/12/6/1650/s1, Table S1 Characteristics of the examined coagulants, Table S2 Effectiveness of pollutant removal from wastewater during a 2-h sedimentation period with and without dosing coagulants (average ± SD).
Author Contributions
Conceptualization, K.C. and A.T.; methodology, K.C.; formal analysis, K.C. and A.T.; investigation, K.C.; data curation, A.W.; writing—original draft preparation, A.W. and A.T.; writing—review and editing, K.C.; visualization, A.W.; supervision, K.C.; project administration, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was created within the scope of a project co-financed by the Norwegian Funds, under the Polish-Norwegian Research Cooperation program, Main Competition, implemented by the National Center for Research and Development, contract no. Pol-Nor/197025/37/2013.
Acknowledgments
Special thanks to Magdalena Szyszko for participating in research conducted under the auspices of the Baritech project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Towards a Circular Economy: A zero Waste Programme for Europe. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52014DC0398 (accessed on 1 June 2020).
- Drewnowski, J. The impact of slowly biodegradable organic compounds on the oxygen uptake rate in activated sludge systems. Water Sci. Technol. 2014, 69, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Pagilla, K.R.; Czerwionka, K.; Urgun-Demirtas, M.; Mąkinia, J. Nitrogen speciation in wastewater treatment plant influents and effluents–the US and Polish case studies. Water Sci. Technol. 2008, 57, 1511–1517. [Google Scholar] [CrossRef]
- Ayoub, M.; Afify, H.; Abdelfattah, A. Chemically enhanced primary treatment of sewage using the recovered alum from water treatment sludge in a model of hydraulic clari-flocculator. J. Water Process Eng. 2017, 19, 133–138. [Google Scholar] [CrossRef]
- Bezirgiannidis, A.; Plesia-Efstathopoulou, A.; Ntougias, S.; Melidis, P. Combined chemically enhanced primary sedimentation and biofiltration process for low cost municipal wastewater treatment. J. Environ. Sci. Health Part A 2019, 54. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Shewa, W.A.; Kyle, M.; Dagnew, M. Optimizing Chemically Enhanced Primary Treatment Processes for Simultaneous Carbon Redirection and Phosphorus Removal. Water 2019, 11, 547. [Google Scholar] [CrossRef]
- Pinto, M.B.; Samanamud, G.R.L.; Baston, E.P.; França, A.B.; Naves, L.L.; Loures, C.C.; Naves, F.L. Multivariate and multiobjective optimization of tannery industry effluent treatment using Musa sp. flower extract in the coagulation and flocculation process. J. Clean. Prod. 2019, 219, 655–666. [Google Scholar] [CrossRef]
- Sandino, J. Chemically Enhanced Primary Treatment (CEPT) and Its Applicability for Large Wastewater Treatments Plant; IWA Publishing: London, UK, 2004. [Google Scholar]
- De Feo, G.; De Gisi, S.; Galasso, M. Definition of a practical multi-criteria procedure for selecting the best coagulant in a chemically assisted primary sedimentation process for the treatment of urban wastewater. Desalination 2008, 230, 229–238. [Google Scholar] [CrossRef]
- Kos, L.; Michalska, K.; Żyłła, R. Removal of Pollutants from Textile Wastewater using Organic Coagulants. Fibres Text. East. Eur. 2006, 6, 218–224. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Li, F.; Keller, A.A.; Xu, R. Chemically enhanced primary treatment (CEPT) for removal of carbon and nutrients from municipal wastewater treatment plants: A case study of Shanghai. Water Sci. Technol. 2009, 60, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Gori, R.; Giaccherini, F.; Jiang, L.; Sobhani, R.; Rosso, D. Role of primary sedimentation on plant-wide energy recovery and carbon footprint. Water Sci. Technol. 2013, 68, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Li, L. An innovative approach to maximize primary treatment performance. Water Sci. Technol. 2000, 42, 209–222. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Zaborowska, E.; Czerwionka, K.; Mąkinia, J. Strategies for achieving energy neutrality in biological nutrient removal systems–a case study of the Slupsk WWTP (northern Poland). Water Sci. Technol. 2017, 73, 727–740. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Xu, Y.; Yu, H.; Wu, Z. Power production from different types of sewage sludge using microbial fuel cells: A comparative study with energetic and microbiological perspectives. J. Power Sources 2013, 235, 280–288. [Google Scholar] [CrossRef]
- Poon, C.S.; Chu, C.W. The use of ferric chloride and anionic polymer in the chemically assisted primary sedimentation process. Chemosphere 1999, 39, 1573–1582. [Google Scholar] [CrossRef]
- Makinia, J.; Drewnowski, J.; Swinarski, M.; Czerwionka, K.; Kaszubowska, M.; Majtacz, J. The impact of precipitation and external carbon source addition on biological nutrient removal in activated sludge systems–experimental investigation and mathematical modelling. Water Pract. Technol. 2012, 7. [Google Scholar] [CrossRef]
- Drewnowski, J.; Makinia, J. The role of biodegradable particulate and colloidal organic compounds in biological nutrient removal activated sludge systems. Int. J. Environ. Sci. Technol. 2014, 11, 1973–1988. [Google Scholar] [CrossRef]
- Czerwionka, K.; Szyszko, M.; Tuszynska, A.; Makinia, J. Impact of enhanced pretreatment on biological nutrient removal in municipal wastewater treatment plants. In Proceedings of the IWA Specialist Conference Nutrient Removal and Recovery: Moving Innovation into Practice, Gdansk, Poland, 18–21 May 2015; pp. 677–684. [Google Scholar]
- Mamais, D.; Jenkins, D.; Pitt, P. A rapid physical chemical method for the determination of readily biodegradable soluble COD in municipal wastewater. Water Res. 1993, 27, 195–197. [Google Scholar] [CrossRef]
- Tuszynska, A.; Kaszubowska, M.; Kowal, P.; Ciesielski, S.; Makinia, J. The metabolic activity of denitrifying microorganisms accumulating polyphosphate in response to addition of fusel oil. Bioprocess Biosyst. Eng. 2019, 42, 143–155. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Dymaczewski, Z. Sewage Treatment Plant Operator’s Guide, 3rd ed.; Polskie Zrzeszenie Inżynierów i Techników Sanitarnych: Poznań, Poland, 2012. (In Polish) [Google Scholar]
- Puig, S.; van Loosdrecht, M.C.M.; Flameling, A.G.; Colprim, J.; Meijer, S.C.F. The effect of primary sedimentation on full-scale WWTP nutrient removal performance. Water Res. 2010, 44, 3375–3384. [Google Scholar] [CrossRef]
- Czerwionka, K.; Makinia, J. Dissolved and colloidal organic nitrogen removal from wastewater treatment plants effluents and reject waters using physical–chemical processes. Water Sci. Technol. 2014, 70, 561–568. [Google Scholar] [CrossRef]
- Bo, F.; Hu, X.; Xie, L.; Zhou, Q. Cassava stillage and its anaerobic fermentation liquid as external carbon sources in biological nutrient removal. J. Zhejiang Univ. Sci. B 2015, 16, 304–316. [Google Scholar] [CrossRef]
- Czerwionka, K.; Makinia, J.; Kaszubowska, M.; Majtacz, J.; Angowski, M. Distillery wastes as external carbon sources for denitrification in municipal wastewater treatment plants. Water Sci. Technol. 2012, 65, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Shammi, M.; Rahman, R.M.; Rahman, M.M.; Moniruzzaman, M.; Bodrud-Doza, M.; Karmakar, B.; Uddin, M.K. Assessment of salinity hazard in existing water resources for irrigation and potentiality of conjunctive uses: A case report from Gopalganj District, Bangladesh, Sustain. Water Resour. Manag. 2016, 2, 369–378. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).