Modeling Soil Nitrate Accumulation and Leaching in Conventional and Conservation Agriculture Cropping Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Location and Characterization
2.2. Flow and Transport Modelling Approach
2.3. Flow and Transport Boundary Conditions
2.4. Model Calibration and Validation Procedure
3. Results and Discussion
3.1. Flow Model Results
3.2. Nonreactive Transport Model Results
3.3. Reactive Transport Model Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Exner, M.E.; Hirsh, A.J.; Spalding, R.F. Nebraska’s groundwater legacy: Nitrate contamination beneath irrigated cropland. Water Resour. Res. 2014, 50, 4474–4489. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, A.J.; Duff, J.H.; Saad, D.A.; Spahr, N.E.; Wolock, D.M. Vulnerability of streams to legacy nitrate sources. Environ. Sci. Technol. 2013, 47, 3623–3629. [Google Scholar] [CrossRef]
- Jessen, S.; Postma, D.; Thorling, L.; Müller, S.; Leskelä, J.; Engesgaard, P. Decadal variations in groundwater quality: A legacy from nitrate leaching and denitrification by pyrite in a sandy aquifer. Water Resour. Res. 2017, 53, 184–198. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293. [Google Scholar] [CrossRef]
- Ascott, M.J.; Gooddy, D.C.; Wang, L.; Stuart, M.E.; Lewis, M.A.; Ward, R.S.; Binley, A.M. Global patterns of nitrate storage in the vadose zone. Nat. Commun. 2017, 8, 1416. [Google Scholar] [CrossRef]
- Gerber, C.; Purtschert, R.; Hunkeler, D.; Hug, R.; Sültenfuss, J. Using environmental tracers to determine the relative importance of travel times in the unsaturated and saturated zones for the delay of nitrate reduction measures. J. Hydrol. 2018, 561, 250–266. [Google Scholar] [CrossRef]
- Colombani, N.; Mastrocicco, M.; Castaldelli, G.; Aravena, R. Contrasting biogeochemical processes revealed by stable isotopes of H2O, N, C and S in shallow aquifers underlying agricultural lowlands. Sci. Total Environ. 2019, 691, 1282–1296. [Google Scholar] [CrossRef]
- Rodríguez, S.B.; Alonso-Gaite, A.; Álvarez-Benedí, J. Characterization of nitrogen transformations, sorption and volatilization processes in urea fertilized soils. Vadose Zone J. 2005, 4, 329–336. [Google Scholar] [CrossRef]
- Hanson, B.R.; Šimůnek, J.; Hopmans, J.W. Evaluation of urea—Ammonium—Nitrate fertigation with drip irrigation using numerical modeling. Agric. Water Manag. 2006, 86, 102–113. [Google Scholar] [CrossRef]
- Li, Y.; Šimůnek, J.; Zhang, Z.; Jing, L.; Ni, L. Evaluation of nitrogen balance in a direct-seeded-rice field experiment using Hydrus-1D. Agric. Water Manag. 2015, 148, 213–222. [Google Scholar] [CrossRef]
- Castaldelli, G.; Colombani, N.; Tamburini, E.; Vincenzi, F.; Mastrocicco, M. Soil type and microclimatic conditions as drivers of urea transformation kinetics in maize plots. Catena 2018, 166, 200–208. [Google Scholar] [CrossRef]
- Šimunek, J.; Šejna, M.; Saito, H.; Sakai, M.; van Genuchten, M.T. The HYDRUS-1D software package for simulating the movement of water, heat, and multiple solutes in variably saturated media, Version 4.0. In HYDRUS Software Series 3; Department of Environmental Sciences, University of California Riverside: Riverside, CA, USA, 2008; Volume 3, p. 315. [Google Scholar]
- Hansen, S.; Abrahamsen, P.; Petersen, C.T.; Styczen, M. Daisy: Model use, calibration, and validation. Trans. ASABE 2012, 55, 1317–1333. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Karlberg, L. Coupled Heat and Mass Transfer Model for Soil–Plant–Atmosphere Systems; Department of Civil and Environmental Engineering, Royal Institute of Technology: Stockholm, Sweden, 2001; p. 321. [Google Scholar]
- Izaurralde, R.C.; Williams, J.R.; McGill, W.B.; Rosenberg, N.J.; Jakas, M.Q. Simulating soil C dynamics with EPIC: Model description and testing against long-term data. Ecol. Model. 2006, 192, 362–384. [Google Scholar] [CrossRef]
- Zhu, Q.; Castellano, M.J.; Yang, G. Coupling soil water processes and nitrogen cycle across spatial scales: Potentials, bottlenecks and solutions. Earth-Sci. Rev. 2018, 187, 248–258. [Google Scholar] [CrossRef]
- Liang, H.; Qi, Z.; Hu, K.; Li, B.; Prasher, S.O. Modelling subsurface drainage and nitrogen losses from artificially drained cropland using coupled DRAINMOD and WHCNS models. Agric. Water Manag. 2018, 195, 201–210. [Google Scholar] [CrossRef]
- Banger, K.; Nafziger, E.D.; Wang, J.; Pittelkow, C.M. Modeling Inorganic Soil Nitrogen Status in Maize Agroecosystems. Soil Sci. Soc. Am. J. 2019, 83, 1564–1574. [Google Scholar] [CrossRef]
- Marjerison, R.D.; Melkonian, J.; Hutson, J.L.; van Es, H.M.; Sela, S.; Geohring, L.D.; Vetsch, J. Drainage and nitrate leaching from artificially drained maize fields simulated by the Precision Nitrogen Management model. J. Environ. Qual. 2016, 45, 2044–2052. [Google Scholar] [CrossRef]
- Knowler, D.; Bradshaw, B. Farmers’ adoption of conservation agriculture: A review and synthesis of recent research. Food Policy 2007, 32, 25–48. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Sayre, K.; Gupta, R. The role of conservation agriculture in sustainable agriculture. Philos. Trans. R. Soc. B 2007, 363, 543–555. [Google Scholar] [CrossRef]
- Gerke, H.H.; Arning, M.; Stöppler-Zimmer, H. Modeling long-term compost application effects on nitrate leaching. Plant Soil 1999, 213, 75–92. [Google Scholar] [CrossRef]
- Iqbal, S.; Guber, A.K.; Khan, H.Z. Estimating nitrogen leaching losses after compost application in furrow irrigated soils of Pakistan using HYDRUS-2D software. Agric. Water Manag. 2016, 168, 85–95. [Google Scholar] [CrossRef]
- Asada, K.; Eguchi, S.; Tsunekawa, A.; Tsuji, M.; Itahashi, S.; Katou, H. Predicting nitrogen leaching with the modified LEACHM model: Validation in soils receiving long-term application of animal manure composts. Nutr. Cycl. Agroecosyst. 2015, 102, 209–225. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration. Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; p. 15. [Google Scholar]
- Mastrocicco, M.; Colombani, N.; Soana, E.; Vincenzi, F.; Castaldelli, G. Intense rainfalls trigger nitrite leaching in agricultural soils depleted in organic matter. Sci. Total Environ. 2019, 665, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, D.; Tessari, U.; Faccini, B.; Coltorti, M. The use of particle density in sedimentary provenance studies: The superficial sediment of Po Plain (Italy) case study. Geosci. J. 2014, 18, 449–458. [Google Scholar] [CrossRef]
- Durner, W. Hydraulic conductivity estimation for soils with heterogeneous pore structure. Water Resour. Res. 1994, 30, 211–223. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. Convective-dispersive transport of solutes involved in sequential first-order decay reactions. Comput. Geosci. 1985, 11, 129–147. [Google Scholar] [CrossRef]
- Rogora, M.; Arisci, S.; Marchetto, A. The role of nitrogen deposition in the recent nitrate decline in lakes and rivers in Northern Italy. Sci. Total Environ. 2012, 417, 214–223. [Google Scholar] [CrossRef]
- Castaldelli, G.; Soana, E.; Racchetti, E.; Pierobon, E.; Mastrocicco, M.; Tesini, E.; Fano, E.A.; Bartoli, M. Nitrogen budget in a lowland coastal area within the Po river basin (Northern Italy): Multiple evidences of equilibrium between sources and internal sinks. Environ. Manag. 2012, 52, 567–580. [Google Scholar] [CrossRef]
- Gumiero, B.; Candoni, F.; Boz, B.; Da Borso, F.; Colombani, N. Nitrogen Budget of Short Rotation Forests Amended with Digestate in Highly Permeable Soils. Appl. Sci. 2019, 9, 4326. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Nachabe, M.H. Estimating hydraulic conductivity for models of soils with macropores. J. Irrig. Drain. Eng. 1995, 121, 95–102. [Google Scholar] [CrossRef]
- Høgh Jensen, K.; Refsgaard, J.C. Spatial Variability of Physical Parameters and Processes in Two Field Soils Part II: Water Flow at Field Scale. Hydrol. Res. 1991, 22, 303–326. [Google Scholar] [CrossRef]
- Ismail, I.; Blevins, R.L.; Frye, W.W. Long-term no-tillage effects on soil properties and continuous corn yields. Soil Sci. Soc. Am. J. 1994, 58, 193–198. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Bainard, L.D.; LaForge, K.; Schellenberg, M.; Houston, B.; Hamel, C. Long-term agricultural land use affects chemical and physical properties of soils from southwest Saskatchewan. Can. J. Soil Sci. 2017, 97, 650–666. [Google Scholar] [CrossRef]
- Hill, M.C. The practical use of simplicity in developing ground water models. Groundwater 2006, 44, 775–781. [Google Scholar] [CrossRef]
- Šimůnek, J.; Jarvis, N.J.; Van Genuchten, M.T.; Gärdenäs, A. Review and comparison of models for describing non-equilibrium and preferential flow and transport in the vadose zone. J. Hydrol. 2003, 272, 14–35. [Google Scholar] [CrossRef]
- Tilahun, K.; Botha, J.F.; Bennie, A.T.P. Bromide movement and uptake under bare and cropped soil conditions at Dire Dawa, Ethiopia. S. Afr. J. Plant Soil 2006, 23, 1–6. [Google Scholar] [CrossRef]
- Iragavarapu, T.K.; Posner, J.L.; Bubenzer, G.D. The effect of various crops on bromide leaching to shallow groundwater under natural rainfall conditions. J. Soil Water Conserv. 1998, 53, 146–151. [Google Scholar]
- Jemison, J.M., Jr.; Fox, R.H. Corn uptake of bromide under greenhouse and field conditions. Commun. Soil Sci. Plan. 1991, 22, 283–297. [Google Scholar] [CrossRef]
- Mastrocicco, M.; Colombani, N.; Castaldelli, G. Direct measurement of dissolved dinitrogen to refine reactive modelling of denitrification in agricultural soils. Sci. Total Environ. 2019, 647, 134–140. [Google Scholar] [CrossRef] [PubMed]





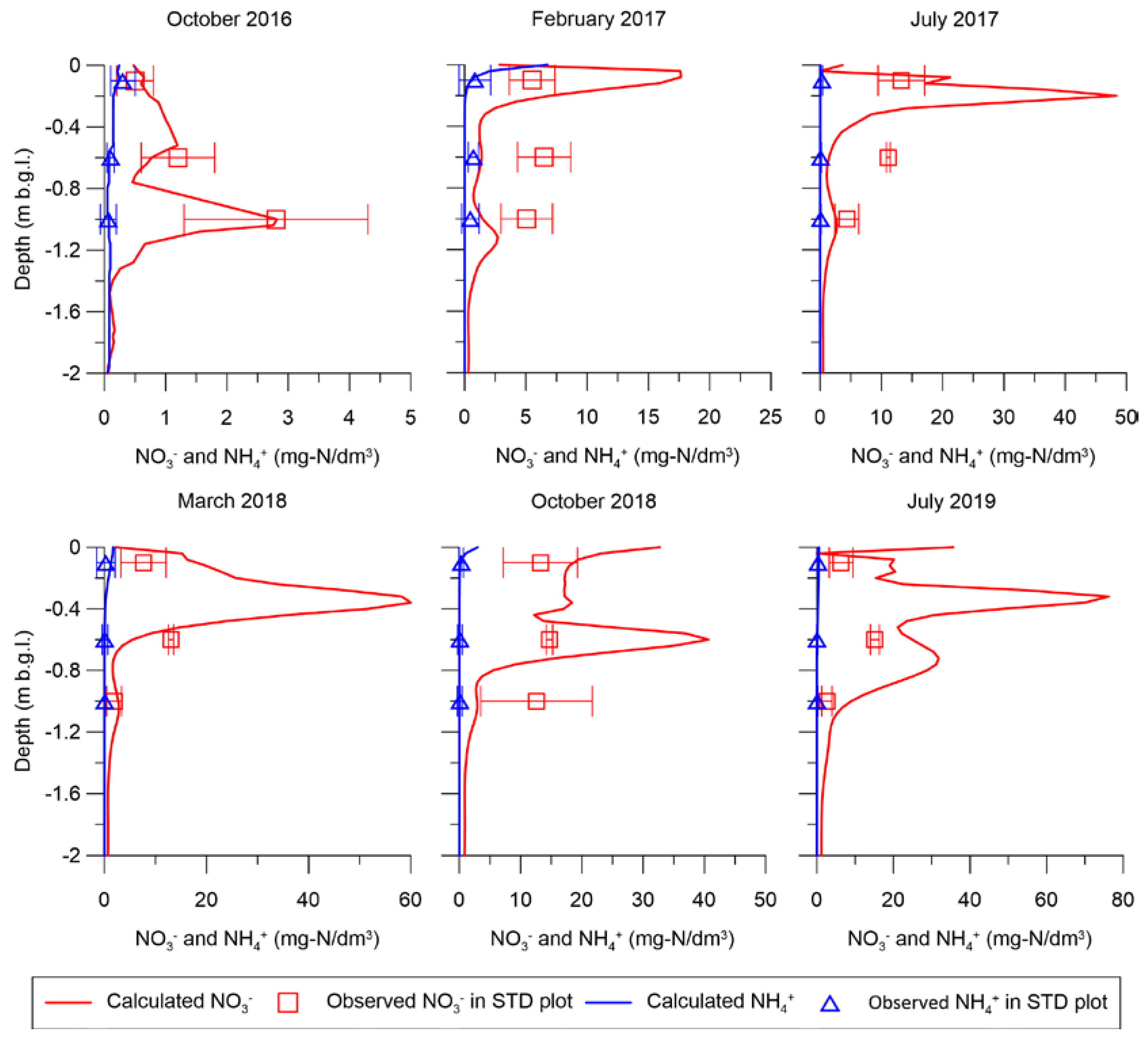
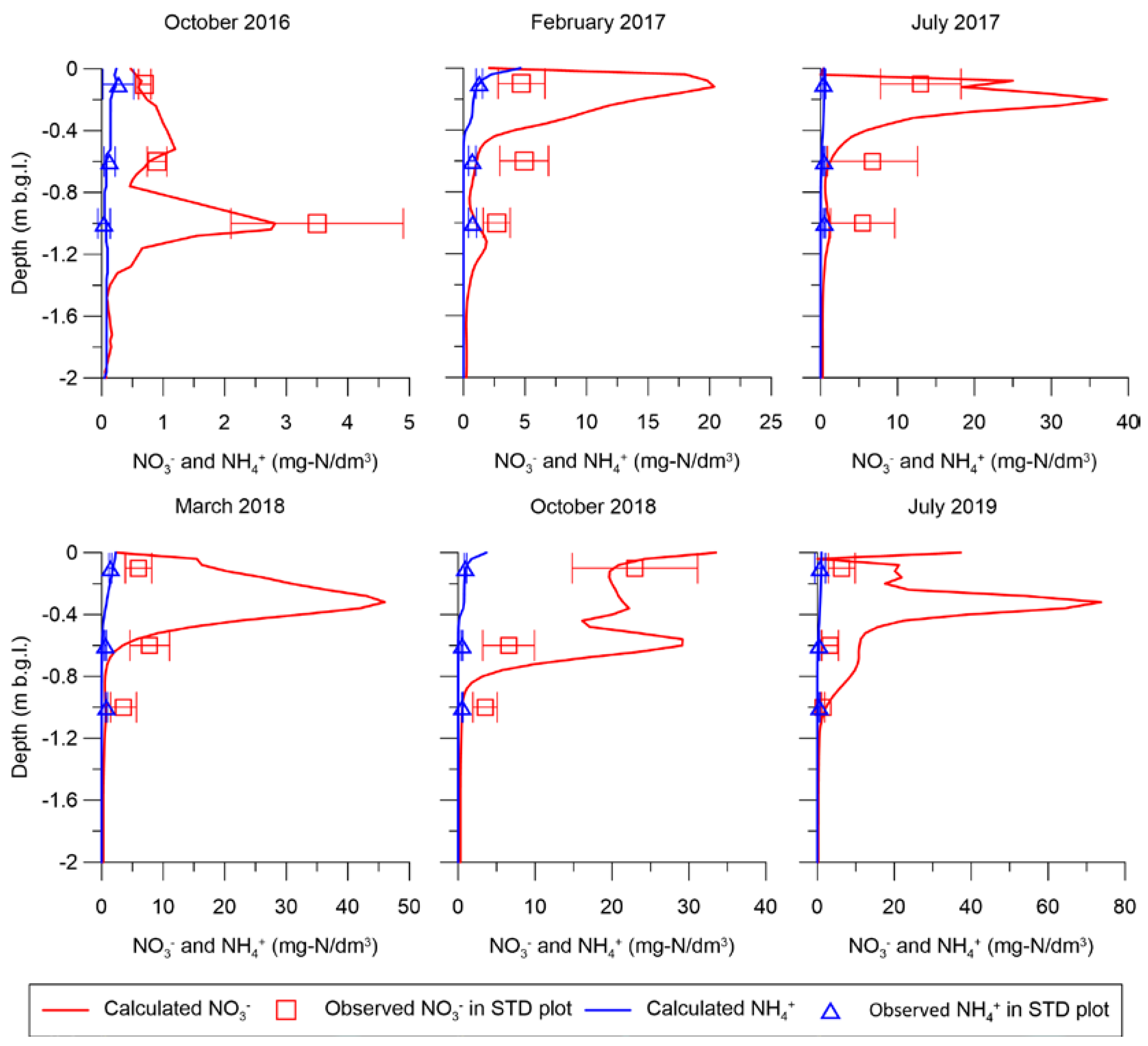
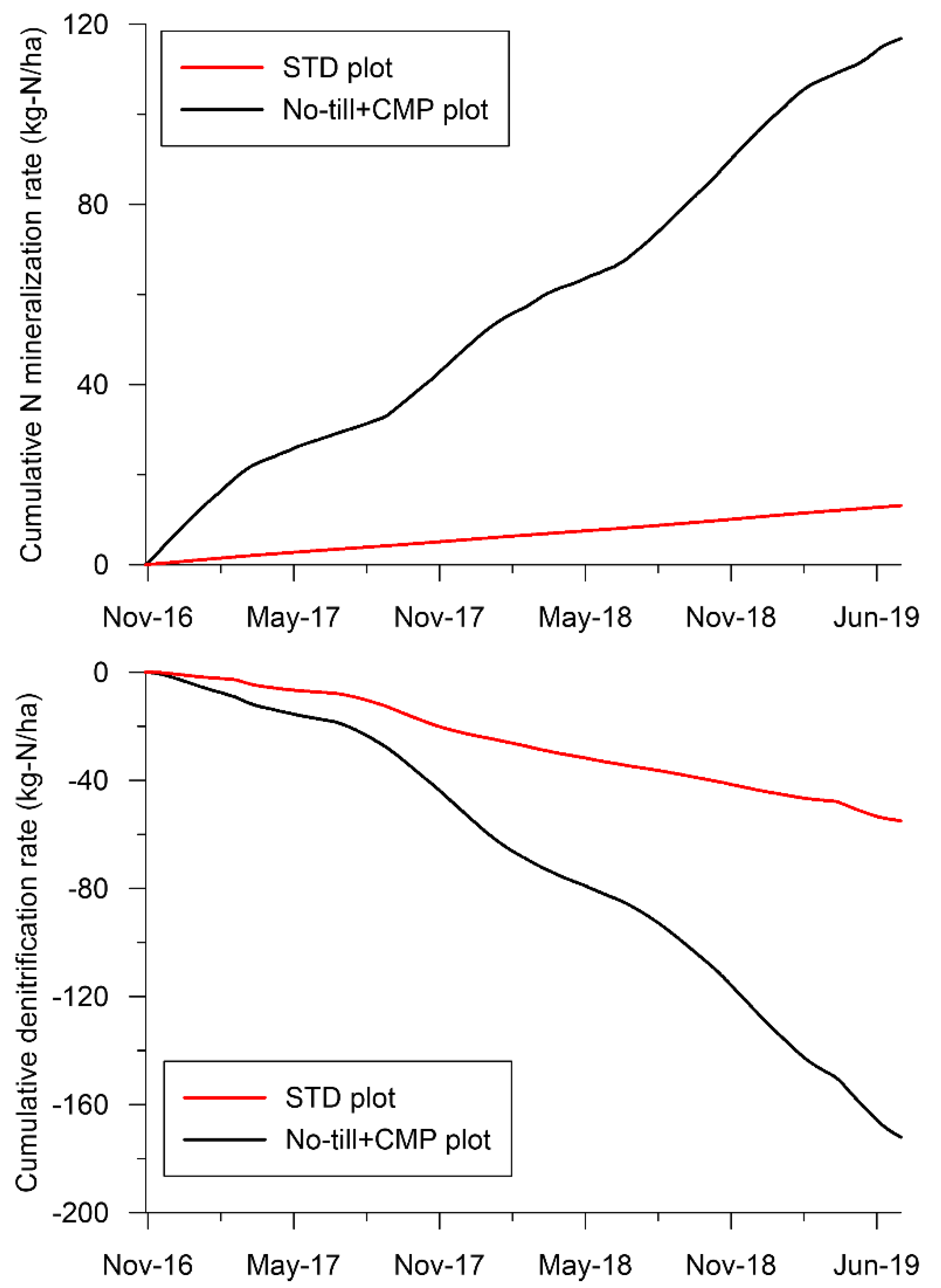
| Crops | Date | Fertilizer | STD Plot (kg-N/ha) | No-till+CMP Plot (kg-N/ha) |
|---|---|---|---|---|
| Winter wheat | 13 October 2016 | NH4NO3 | 40 | 92 * |
| 23 February 2017 | NH4NO3 | 85 | 40 | |
| 03 April 2017 | Urea | 46 | 46 | |
| Maize | 29 March 2018 | (NH4)3PO4 | 36 | 36 |
| 07 April 2018 | NH4H2PO4 | 3 | 3 | |
| 30 April 2018 | Urea | 115 | 115 | |
| 11 May 2018 | Urea | 115 | 115 | |
| Winter wheat | 10 October 2018 | (NH4)3PO4 | 36 | 36 |
| 27 February 2019 | NH4NO3 | 54 | 54 | |
| 04 April 2019 | Urea | 64 | 64 |
| Parameter | 0–20 (cm b.g.l.) | 50–70 (cm b.g.l.) | 90–110 (cm b.g.l.) |
|---|---|---|---|
| Grain size (%) | |||
| Fine Sand (0.63–2 mm) | 10.1 ± 1.8 | 8.1 ± 0.8 | 9.0 ± 1.3 |
| Silt (2–63 μm) | 55.8 ± 2.5 | 56.9 ± 3.5 | 47.5 ± 4.1 |
| Clay (<2 μm) | 34.1 ± 3.0 | 35.0 ± 2.4 | 43.5 ± 3.1 |
| Dry bulk density (kg/dm3) | 1.01 ± 0.05 | 1.31 ± 0.11 | 1.57 ± 0.20 |
| Soil pH (−) | 7.7 ± 0.1 | 7.8 ± 0.1 | 7.9 ± 0.1 |
| SOM (%) | 1.8 ± 0.4 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Carbonates (%) | 9.1 ± 0.6 | 11.5 ± 0.4 | 4.0 ± 0.3 |
| Parameter | Layer 1 | Layer 2 | Layer 3 | Layer 4 |
|---|---|---|---|---|
| Qr | 0.03 | 0.04 | 0.05 | 0.05 |
| Qs | 0.44 | 0.54 | 0.53 | 0.35 |
| n (-) | 1.71 1 | 1.71 1 | 1.71 1 | 2.80 |
| α (1/m) | 0.51 1 | 0.52 1 | 0. 52 1 | 0.05 |
| Ks (m/day) | 0.08 | 0.07 | 0.03 2 | 0.90 2 |
| w (-) | 0.5 1 | 0.5 1 | 0.5 1 | 0.5 |
| n−2 (-) | 1.5 1 | 1.5 1 | 1.5 1 | 2.80 |
| α−2 (1/m) | 0.20 1 | 0.20 1 | 0.20 1 | 0.05 |
| Parameter Name | Optimized Value |
|---|---|
| λv layer 1 (mm) | 11.9 |
| λv layer 2 (mm) | 6.1 |
| λv layer 3 (mm) | 3.0 |
| λv layer 4 (mm) | 3.0 |
| STD plot (kg-N/ha/year) | |||
| N input | N output | ||
| Fertilizer N input | 223 | Crop N uptake | 139 |
| N deposition | 25 | Denitrification | 20 |
| N mineralized | 5 | N volatilized | 2 |
| Sum of N input | 254 | Sum of N output | 161 |
| In-out difference | 93 | ||
| No-till+CMP plot (kg-N/ha/year) | |||
| N input | N output | ||
| Fertilizer N input | 191 | Crop N uptake | 141 |
| N deposition | 25 | Denitrification | 67 |
| N mineralized | 45 | N volatilized | 2 |
| Sum of N input | 262 | Sum of N output | 210 |
| In-out difference | 52 |
| Parameter Name | Optimized Value STD | Optimized Value No-till+CMP |
|---|---|---|
| Organic N→ NO3− Layer 1 | 1.03 × 10−3 | 0.11 |
| Organic N→ NO3− Layer 2 | 1.00 × 10−3 | 0.10 |
| Organic N→ NO3− Layer 3 | 1.00 × 10−3 | 1.00 × 10−3 |
| Organic N→ NO3− Layer 4 | 0.0 | 0.0 |
| NO3−→ N2 Layer 1 | 1.93 × 10−3 | 3.36 × 10−3 |
| NO3−→ N2 Layer 2 | 1.93 × 10−3 | 3.36 × 10−3 |
| NO3−→ N2 Layer 3 | 0.0 | 3.36 × 10−3 |
| NO3−→ N2 Layer 4 | 0.0 | 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombani, N.; Mastrocicco, M.; Vincenzi, F.; Castaldelli, G. Modeling Soil Nitrate Accumulation and Leaching in Conventional and Conservation Agriculture Cropping Systems. Water 2020, 12, 1571. https://doi.org/10.3390/w12061571
Colombani N, Mastrocicco M, Vincenzi F, Castaldelli G. Modeling Soil Nitrate Accumulation and Leaching in Conventional and Conservation Agriculture Cropping Systems. Water. 2020; 12(6):1571. https://doi.org/10.3390/w12061571
Chicago/Turabian StyleColombani, Nicolò, Micòl Mastrocicco, Fabio Vincenzi, and Giuseppe Castaldelli. 2020. "Modeling Soil Nitrate Accumulation and Leaching in Conventional and Conservation Agriculture Cropping Systems" Water 12, no. 6: 1571. https://doi.org/10.3390/w12061571
APA StyleColombani, N., Mastrocicco, M., Vincenzi, F., & Castaldelli, G. (2020). Modeling Soil Nitrate Accumulation and Leaching in Conventional and Conservation Agriculture Cropping Systems. Water, 12(6), 1571. https://doi.org/10.3390/w12061571








