Hydrochemical Characteristic of Groundwater and Its Impact on Crop Yields in the Baojixia Irrigation Area, China
Abstract
1. Introduction
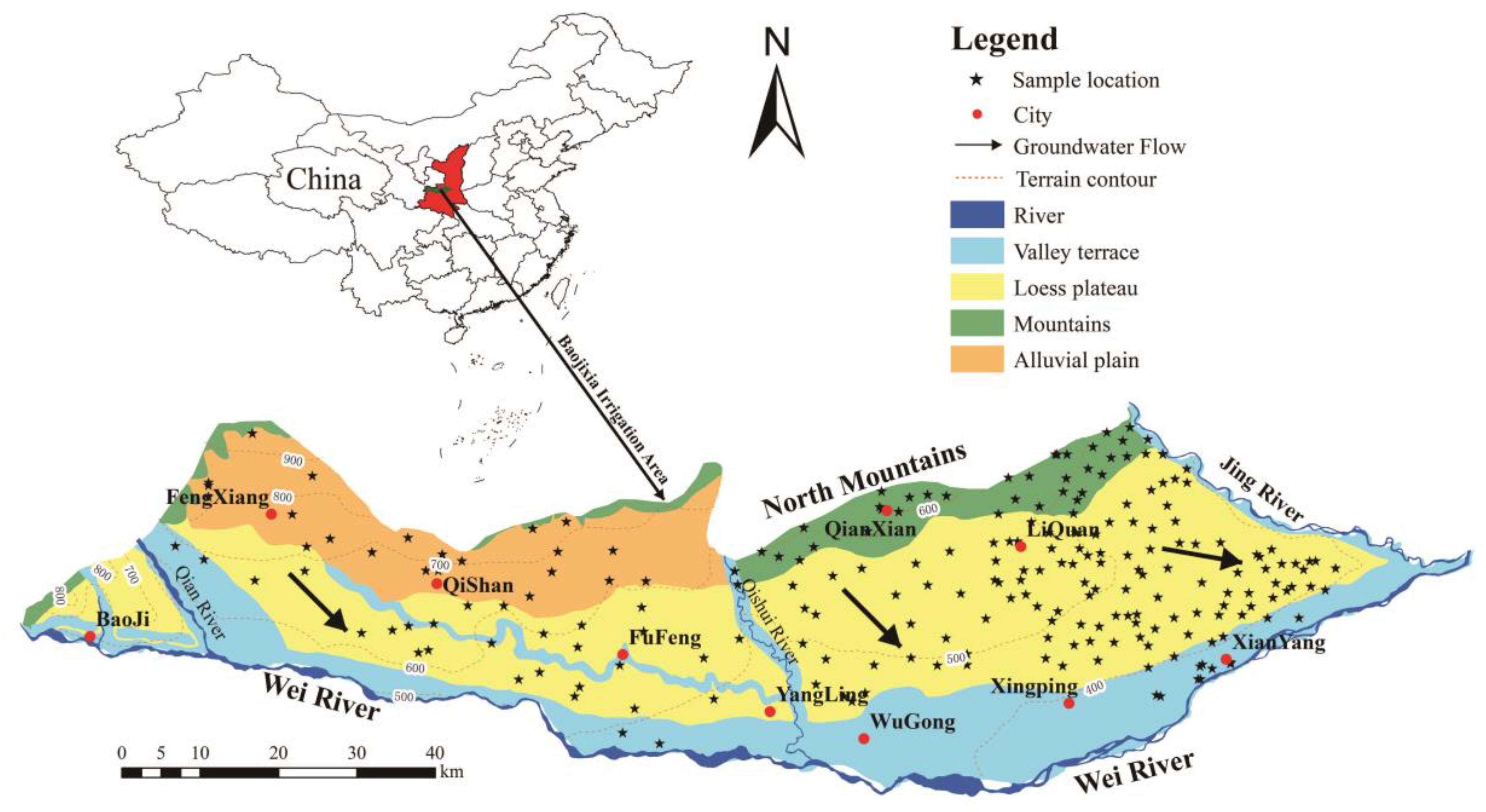
2. Study Area
2.1. Location and Climate
2.2. Hydrogeology
3. Materials and Methods
3.1. Sample Collection and Analysis
3.2. Irrigation Quality Assessment
3.3. Relative Production Calculation
4. Results
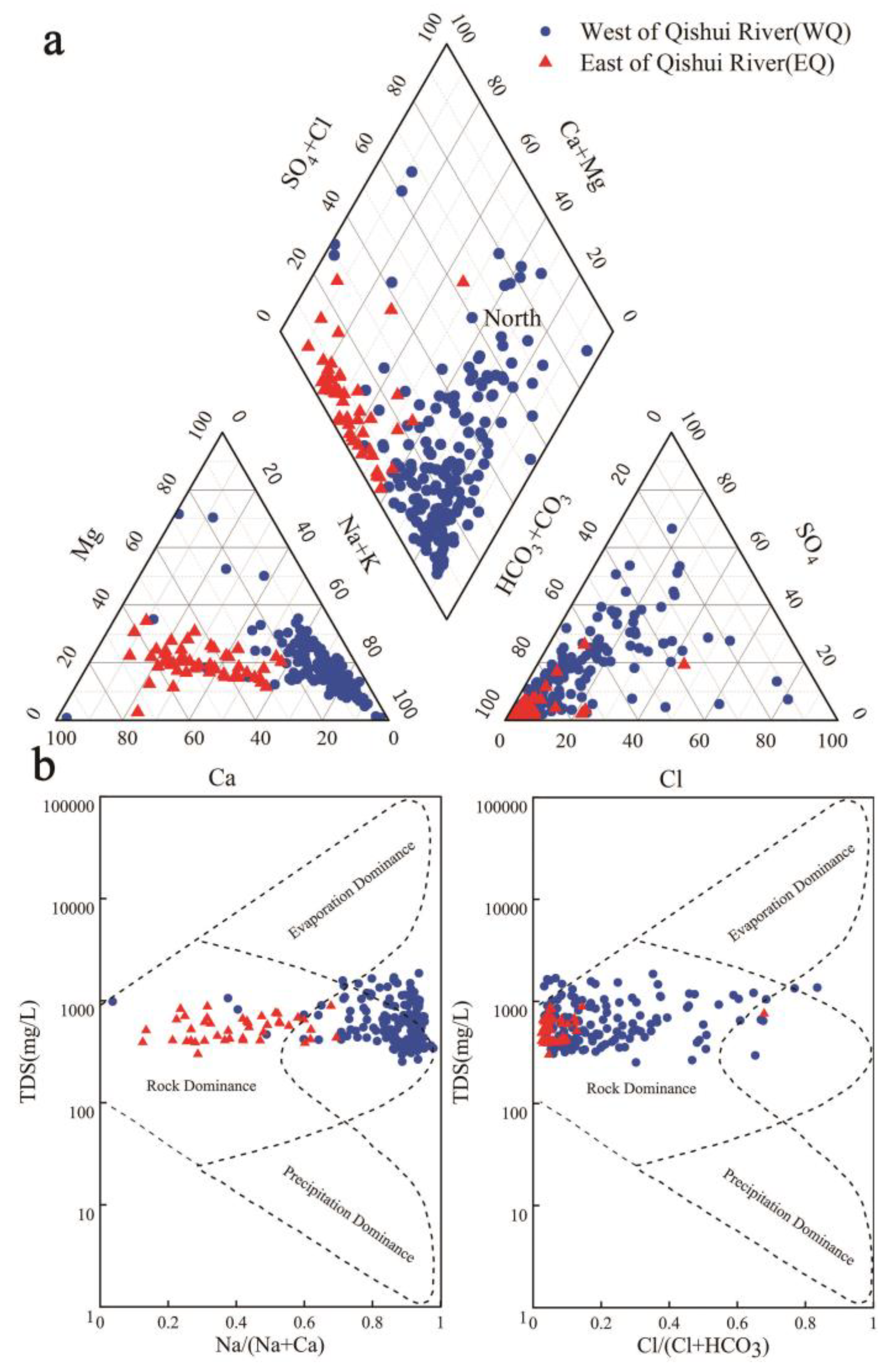
4.1. Groundwater Chemistry
4.2. Hydrogeochemical Facies
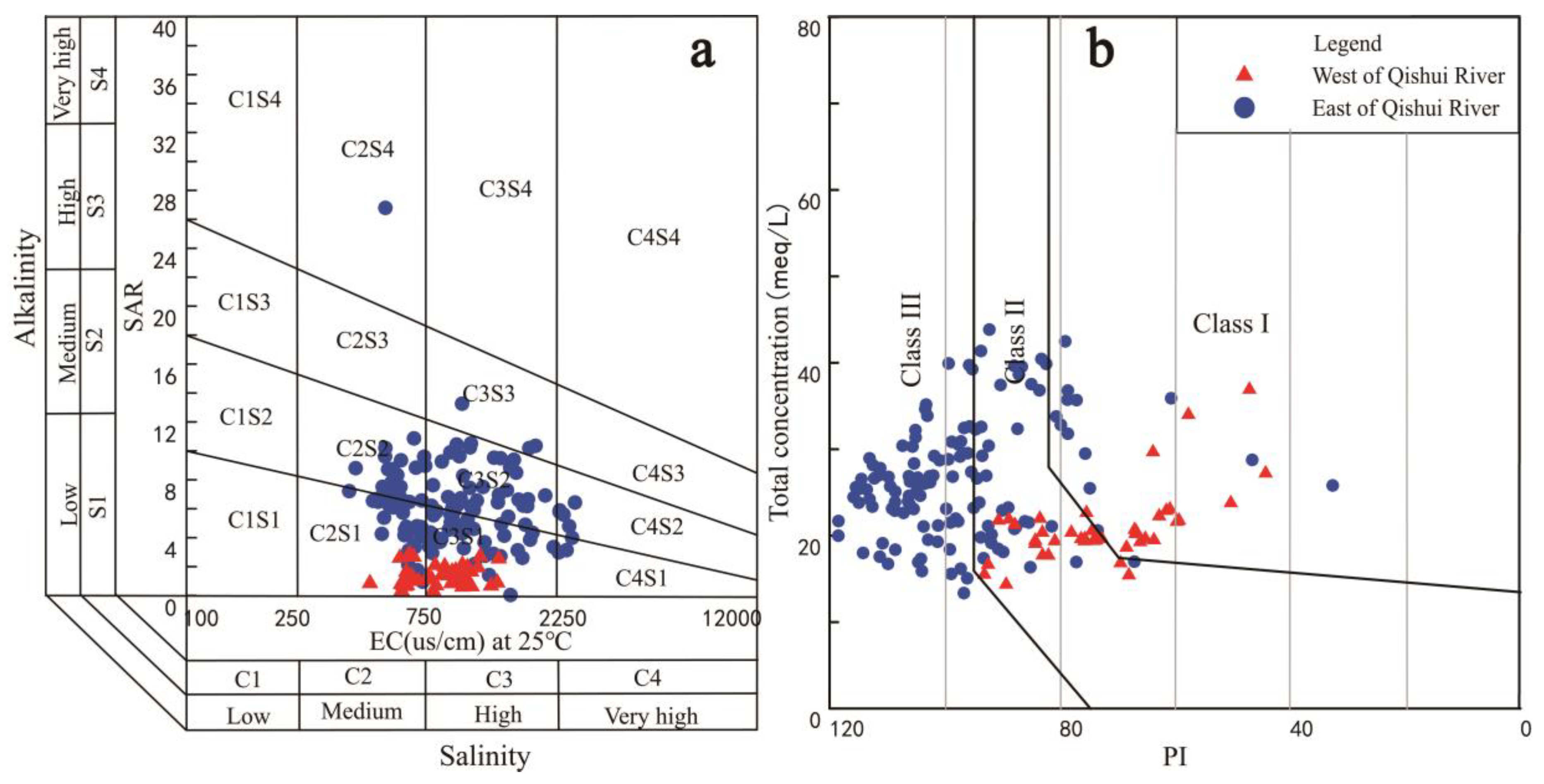
4.3. Suitability for Irrigation Purpose
5. Discussion
5.1. Hydrogeochemical Characteristics
5.1.1. Correlation Matrix
5.1.2. Factor Analysis
5.2. Major Factors Influencing Groundwater Chemistry
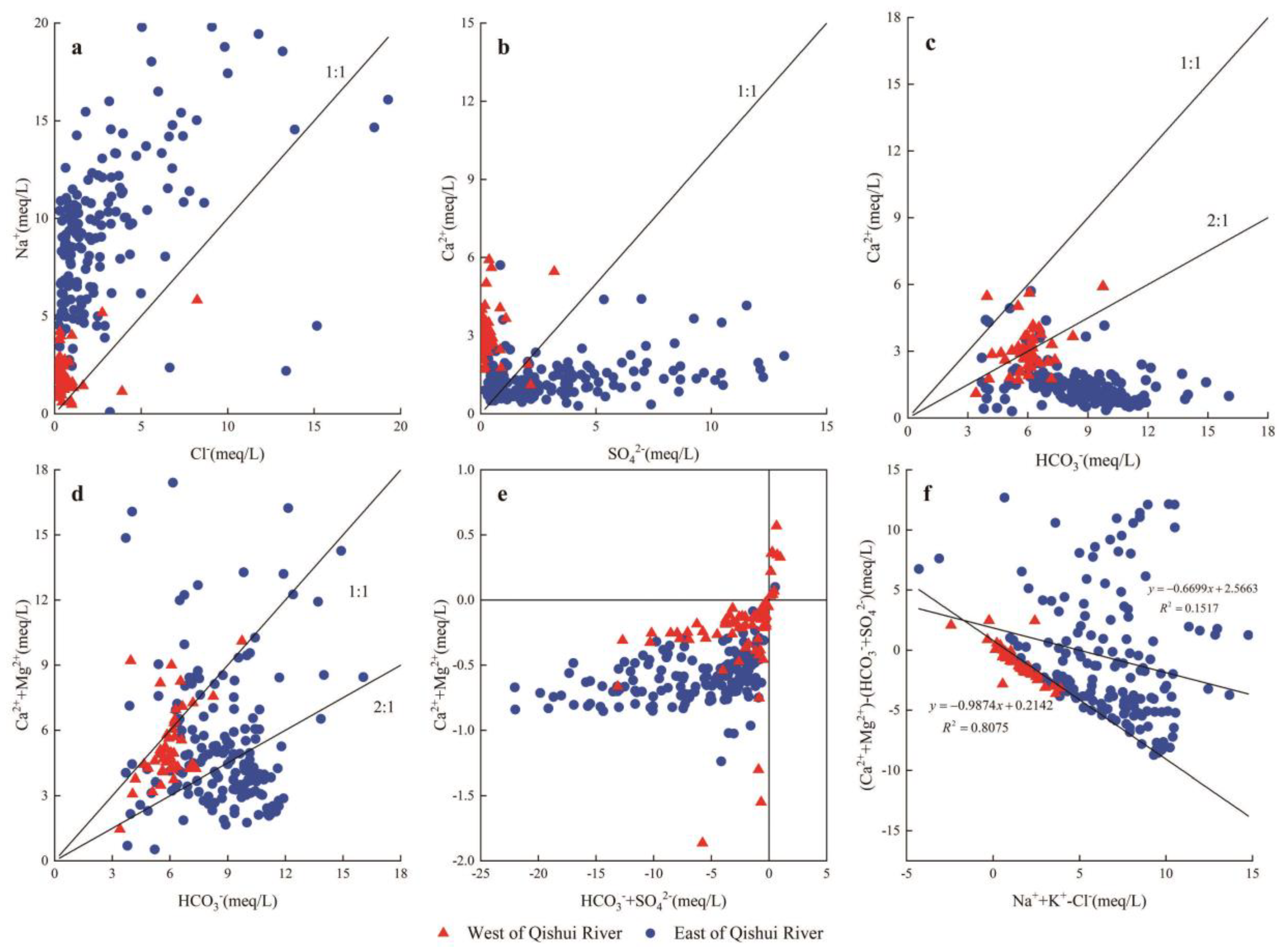
5.2.1. Water-Rock Interaction
5.2.2. Cation Exchange
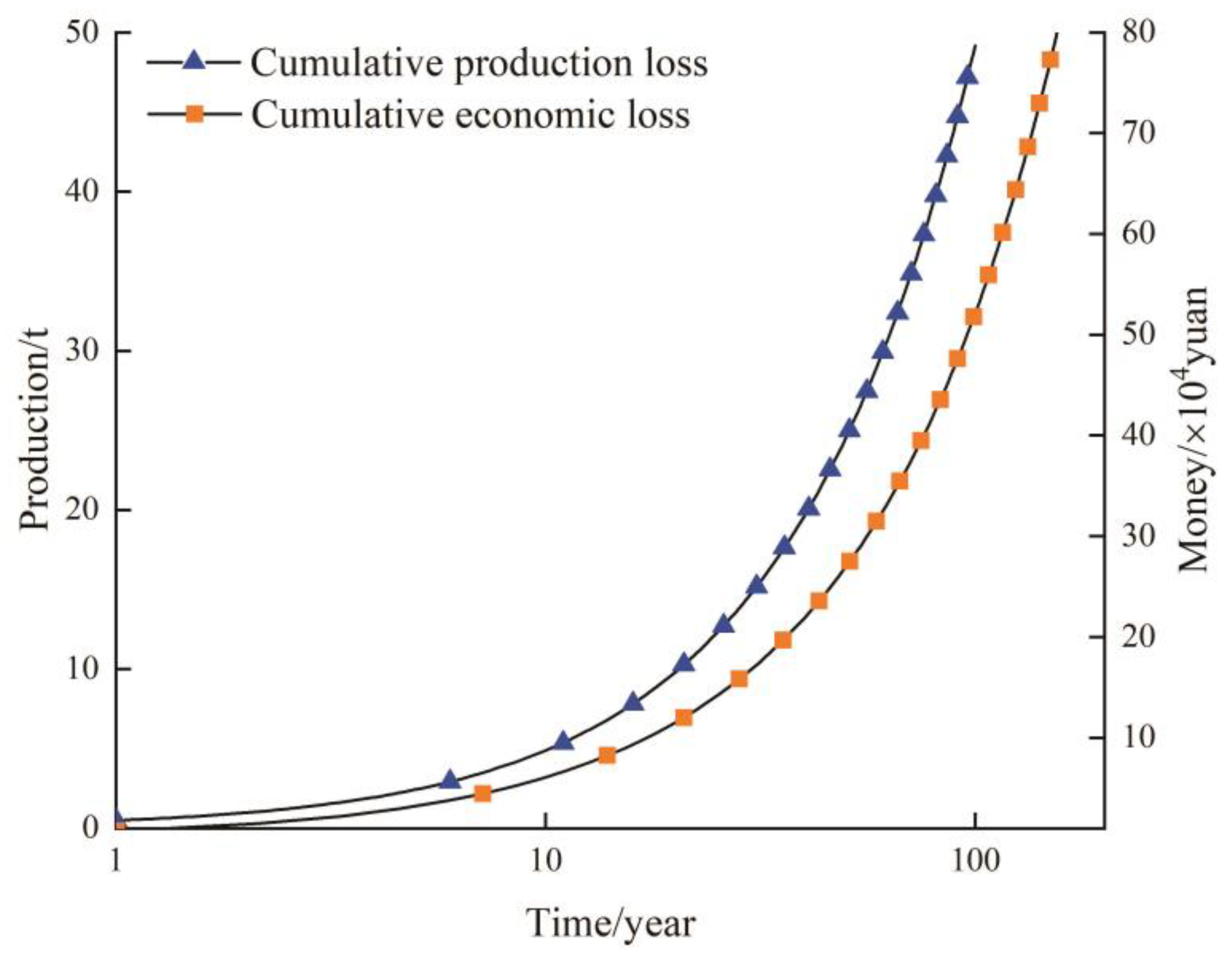
5.3. Water Quality Influence on Crop Yields
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anapalli, S.S.; Fisher, D.K.; Reddy, K.N.; Rajan, N.; Pinnamaneni, S.R. Modeling evapotranspiration for irrigation water management in a humid climate. Agric. Water Manag. 2019, 225, 105731. [Google Scholar] [CrossRef]
- Tahmasebi, P.; Mahmudy-Gharaie, M.H.; Ghassemzadeh, F.; Karimi Karouyeh, A. Assessment of groundwater suitability for irrigation in a gold mine surrounding area, NE Iran. Environ. Earth Sci. 2018, 77, 766. [Google Scholar] [CrossRef]
- Li, K.; Liu, H.; He, X.; Li, X. Simulation of water and salt transport in soil under pipe drainage and drip irrigation conditions in Xinjiang. Water 2019, 11, 2456. [Google Scholar] [CrossRef]
- Alegría, M.E.O.; Schütze, N.; Niyogi, D. Evaluation of hydroclimatic variability and prospective irrigation strategies in the U.S. corn belt. Water 2019, 11, 1–18. [Google Scholar]
- Jia, H.; Qian, H.; Zheng, L.; Feng, W.; Wang, H.; Gao, Y. Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci. Total Environ. 2020, 717, 137170. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Tian, R.; He, S.; He, X.; Xue, C.; Zhang, K. Geochemistry, Hydraulic Connectivity and Quality Appraisal of Multilayered Groundwater in the Hongdunzi Coal Mine, Northwest China. Mine Water Environ. 2018, 37, 222–237. [Google Scholar] [CrossRef]
- Khanoranga; Khalid, S. An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Balochistan province, Pakistan, through water quality index and multivariate statistical approaches. J. Geochemical Explor. 2019, 197, 14–26. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, M.; Sun, J.; Zhang, Z. Response of water-salt migration to brackish water irrigation with different irrigation intervals and sequences. Wate 2019, 11, 2089. [Google Scholar] [CrossRef]
- Wang, L.; Mei, Y.; Yu, K.; Li, Y.; Meng, X.; Hu, F. Anthropogenic effects on hydrogeochemical characterization of the Shallow Groundwater in an arid irrigated plain in Northwestern China. Water 2019, 11, 2247. [Google Scholar] [CrossRef]
- Xu, P.; Feng, W.; Qian, H.; Zhang, Q. Hydrogeochemical characterization and irrigation quality assessment of shallow groundwater in the central-Western Guanzhong basin, China. Int. J. Environ. Res. Public Health 2019, 16, 1492. [Google Scholar] [CrossRef]
- Molle, F.; Rap, E. Irrigation improvement projects in the Nile Delta: Promises, challenges, surprises. Agric. Water Manag. 2019, 216, 425–435. [Google Scholar] [CrossRef]
- Chi, Y.; Sun, J.; Liu, W.; Wang, J.; Zhao, M. Mapping coastal wetland soil salinity in different seasons using an improved comprehensive land surface factor system. Ecol. Indic. 2019, 107, 105517. [Google Scholar] [CrossRef]
- Katuri, J.R.; Trifonov, P.; Arye, G. Spatial distribution of salinity and sodicity in arid climate following long term brackish water drip irrigated olive orchard. Water 2019, 11, 2556. [Google Scholar] [CrossRef]
- Minhas, P.S.; Qadir, M.; Yadav, R.K. Groundwater irrigation induced soil sodification and response options. Agric. Water Manag. 2019, 215, 74–85. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, S.; Zhu, Z.; Jiang, H.; Zhang, X. Assessing the effects of plant density and plastic film mulch on maize evaporation and transpiration using dual crop coefficient approach. Agric. Water Manag. 2019, 225, 105765. [Google Scholar] [CrossRef]
- van Straten, G.; de Vos, A.C.; Rozema, J.; Bruning, B.; van Bodegom, P.M. An improved methodology to evaluate crop salt tolerance from field trials. Agric. Water Manag. 2019, 213, 375–387. [Google Scholar] [CrossRef]
- Lwimbo, Z.D.; Komakech, H.C.; Muzuka, A.N.N. Impacts of emerging agricultural practices on groundwater quality in Kahe catchment, Tanzania. Water 2019, 11, 2263. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance–current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar]
- Srivastava, S.K. Assessment of groundwater quality for the suitability of irrigation and its impacts on crop yields in the Guna district, India. Agric. Water Manag. 2019, 216, 224–241. [Google Scholar] [CrossRef]
- Zhang, W.; Du, X.; Huang, A.; Yin, H. Analysis and comprehensive evaluation of water use efficiency in China. Water 2019, 11, 2620. [Google Scholar] [CrossRef]
- Martin, D.A. Linking fire and the United Nations Sustainable Development Goals. Sci. Total Environ. 2019, 662, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture. Irrigation and Drainage Paper No. 29; Food and Agriculture Organization: Rome, Italy, 1985. [Google Scholar]
- da Silva Leite, R.; do Nascimento, M.N.; Tanan, T.T.; Neto, L.P.G.; da Silva Ramos, C.A.; da Silva, A.L. Alleviation of water deficit in Physalis angulata plants by nitric oxide exogenous donor. Agric. Water Manag. 2019, 216, 98–104. [Google Scholar] [CrossRef]
- Duan, L.; Wang, W.; Sun, Y.; Zhang, C. Iodine in groundwater of the Guanzhong Basin, China: Sources and hydrogeochemical controls on its distribution. Environ. Earth Sci. 2016, 75, 970. (In Chinese) [Google Scholar] [CrossRef]
- Qin, P. Characteristics of Main Agricultural Meteorological Disasters and Changes of Main Grain Yields in Shaanxi under Climate Change. Master’s Thesis, Lanzhou University, Lanzhou, China, 2016. (In Chinese). [Google Scholar]
- Wang, C. Change Characteristics of Crop Climate Production Potential Under the Background of Climate Change in Western Guanzhong of Shaanxi. Chinese Agric. Sci. Bull. 2016, 32, 170–176. (In Chinese) [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Vikas, C.; Kushwaha, R.; Ahmad, W.; Prasannakumar, V.; Dhanya, P.V.; Reghunath, R. Hydrochemical Appraisal and Geochemical Evolution of Groundwater with Special Reference to Nitrate Contamination in Aquifers of a Semi-Arid Terrain of NW India. Water Qual. Exp. Health 2015, 7, 347–361. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Sun, G.; Wu, Y.; Chen, Y. Assessment of Lake water quality and eutrophication risk in an agricultural irrigation area: A case study of the Chagan Lake in Northeast China. Water 2019, 11, 2380. [Google Scholar] [CrossRef]
- Zhang, J. Study on Dynamic Characteristics of Groundwater in Baoji Gorge and Wool Bay Irrigation District. Master’s Thesis, Chang’an University, Xi’an, China, 2017. (In Chinese). [Google Scholar]
- Ministry of Environmental Protection of the P.R. China (MEPC). Water Quality Sampling—Technical Regulation of the Preservation and Handling of Samples (HJ 493–2009); China Environmental Science Press: Beijing, China, 2009. (In Chinese) [Google Scholar]
- Wang, Y.; Zheng, C.; Ma, R. Review: Safe and sustainable groundwater supply in China. Hydrogeol. J. 2018, 26, 1301–1324. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Xu, B. Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ. Earth Sci. 2018, 77, 273. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Xue, C.; Wu, J. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef]
- Li, D.; Du, T.; Sun, Q.; Cao, Y. The key driving factors of irrigation water productivity based on soil spatio-temporal characteristics. Agric. Water Manag. 2019, 216, 351–360. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; LWW: Philadelphia, PA, USA, 1954; Volume 78, ISBN 0038-075X. [Google Scholar]
- Wilcox, L.V. The Quality of Water for Irrigation Use; US Department of Agriculture: Washington DC, USA, 1948.
- WHO. Guidelines for Drinking Water Quality, 4th ed.; incorporating 1st addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Li, P.; Qian, H.; Wu, J. Conjunctive use of groundwater and surface water to reduce soil salinization in the Yinchuan Plain, North-West China. Int. J. Water Resour. Dev. 2018, 34, 337–353. [Google Scholar] [CrossRef]
- Rao, P.N.; Prasad, K.M.; Madhusudhan, B.J.; Krishna, V.S.R.; Anand, A.V.S.S.; Madhnure, P. Impact of urbanization on groundwater quality in Vijayawada urban agglomeration, the new capital region of Andhra Pradesh, India–A baseline study. J. Geol. Soc. India 2016, 87, 539–552. [Google Scholar] [CrossRef]
- Onwuka, O.S.; Omonona, O. V Hydrogeochemical characteristics of coastal aquifers from Port Harcourt, southern Nigeria. Environ. Earth Sci. 2017, 76, 609. [Google Scholar] [CrossRef]
- Zaidi, F.K.; Al-Bassam, A.M.; Kassem, O.M.K.; Alfaifi, H.J.; Alhumidan, S.M. Factors influencing the major ion chemistry in the Tihama coastal plain of southern Saudi Arabia: Evidences from hydrochemical facies analyses and ionic relationships. Environ. Earth Sci. 2017, 76, 472. [Google Scholar] [CrossRef]
- Nemčić-Jurec, J.; Singh, S.K.; Jazbec, A.; Gautam, S.K.; Kovač, I. Hydrochemical investigations of groundwater quality for drinking and irrigational purposes: Two case studies of Koprivnica-Križevci County (Croatia) and district Allahabad (India). Sustain. Water Resour. Manag. 2017, 17, 1–24. [Google Scholar] [CrossRef]
- Mora, A.; Mahlknecht, J.; Rosales-Lagarde, L.; Hernández-Antonio, A. Assessment of major ions and trace elements in groundwater supplied to the Monterrey metropolitan area, Nuevo León, Mexico. Environ. Monit. Assess. 2017, 189, 394. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Zheng, Y.; Song, C.; Long, M.; Chen, T.; Ren, Z.; Yang, M.; Li, X.; Guo, J. Hydrochemical characteristics and mixing behavior of thermal springs along the Bijiang River in the Lanping basin of China. Environ. Earth Sci. 2017, 76, 487. [Google Scholar] [CrossRef]
- Avci, H.; Dokuz, U.E.; Avci, A.S. Hydrochemistry and groundwater quality in a semiarid calcareous area: An evaluation of major ion chemistry using a stoichiometric approach. Environ. Monit. Assess. 2018, 190, 641. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the P.R. China (MEPC). Standard for Groundwater Quality; China Stand Press: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Kawo, N.S.; Karuppannan, S. Groundwater quality assessment using water quality index and GIS technique in Modjo River Basin, central Ethiopia. J. African Earth Sci. 2018, 147, 300–311. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: A case study in and around Hua County, China. Arab. J. Geosci. 2016, 9, 1–17. [Google Scholar] [CrossRef]
- Doneen, L.D. Notes on water quality in agriculture. In Water Science and Engineering; Department of Water Sciences and Engineering, University of California: Oakland, CA, USA, 1964; p. 4001. [Google Scholar]
- Doneen, L.D. Water quality for irrigated agriculture. In Plants in Saline Environments; Springer: Berlin, Germany, 1975; pp. 56–76. [Google Scholar]
- Elliott, E.M.; Yu, Z.; Cole, A.S.; Coughlin, J.G. Isotopic advances in understanding reactive nitrogen deposition and atmospheric processing. Sci. Total Environ. 2019, 662, 393–403. [Google Scholar] [CrossRef]
- Rabeiy, R.E. Assessment and modeling of groundwater quality using WQI and GIS in Upper Egypt area. Environ. Sci. Pollut. Res. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Qian, H. Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ. Earth Sci. 2015, 73, 8575–8588. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, X.; Wang, L.; Zhang, Y.; Shen, X.; Zhou, H.; Ye, S.; Fang, B. Hydrogeochemical characteristics and sources of salinity of the springs near Wenquanzhen in the eastern Sichuan Basin, China. Hydrogeol. J. 2018, 26, 1137–1151. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, M.; Chen, X.; Liu, J. Health Risk Assessment and Risk Control: Drinking Groundwater in Yinchuan Plain, China. Exp. Health 2019, 11, 59–72. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigations and Development; UNESCO: Paris, France, 1965; pp. 54–83. [Google Scholar]
- Ma, L.; Wang, X.; Gao, Z.; Youke, W.; Nie, Z.; Liu, X. Canopy pruning as a strategy for saving water in a dry land jujube plantation in a loess hilly region of China. Agric. Water Manag. 2019, 216, 436–443. [Google Scholar] [CrossRef]

| Ca2+ | Mg2+ | Na+ | HCO3− | SO42− | Cl− | TDS | pH | TH | EC | |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (µs/cm) | ||
| West of Qishui River (WQ) n = 51 | ||||||||||
| Minimum | 22.04 | 2.65 | 11.03 | 207.46 | 0 | 4.96 | 203 | 7.3 | 4.77 | 316.72 |
| Maximum | 195.39 | 97.86 | 133.72 | 594.9 | 127.28 | 292.49 | 888 | 8.1 | 522 | 1387.5 |
| Mean | 63.43 | 27.93 | 46.41 | 364.77 | 16.4 | 26.12 | 543 | 7.61 | 207.01 | 847.26 |
| Standard deviation | 26.81 | 14.35 | 25.68 | 62.24 | 23.31 | 44.06 | 151.26 | 0.21 | 93.48 | 236.35 |
| East of Qishui River (EQ) n = 172 | ||||||||||
| Minimum | 6.01 | 2.67 | 2.07 | 225.76 | 7.20 | 8.80 | 252 | 7.10 | 5.19 | 393.75 |
| Maximum | 3701.00 | 307.55 | 700.72 | 979.32 | 1261.29 | 1680.00 | 3208 | 9.60 | 487.90 | 5012.95 |
| Mean | 52.54 | 56.10 | 220.02 | 526.33 | 148.99 | 123.60 | 763 | 7.65 | 23.53 | 1191.88 |
| Standard deviation | 280.71 | 49.86 | 98.97 | 139.71 | 171.34 | 201.17 | 466.46 | 0.33 | 43.74 | 728.84 |
| National standard | - | - | 200 | - | 250 | 250 | 1000 | 6.5–8.5 | 450 | - |
| WHO standard | - | - | 200 | - | 250 | 250 | 1000 | 6.5–8.5 | 500 | - |
| Parameters (meq/L) | Sample Range | Range | Classification | Number of Samples | |||
|---|---|---|---|---|---|---|---|
| Min | Max | Average | STD | ||||
| Alkalinity hazard (SAR) [37] | 0.04 | 26.79 | 4.82 | 3.35 | <10 | Excellent | 223 |
| 10~18 | Good | 12 | |||||
| 18~26 | Doubtful | 0 | |||||
| >26 | Unsuitable | 1 | |||||
| EC | 361.72 | 2636.5 | 1017.314 | 482.26 | <250 | Excellent | 24 |
| 250~750 | Good | 38 | |||||
| 750~2250 | Acceptable | 66 | |||||
| >2250 | Unacceptable | 19 | |||||
| Hydrochemical Parameter | pH | EC | Cl− | TDS | Ca2+ | Mg2+ | Na+ | HCO3− | SO42− |
|---|---|---|---|---|---|---|---|---|---|
| pH | 1.0000 | 0.2517 | 0.1401 | 0.2517 | 0.0736 | 0.0481 | −0.0754 | −0.3106 | −0.0394 |
| EC | 0.2517 | 1.0000 | 0.1988 | 0.8634 | −0.0223 | 0.1317 | 0.0530 | −0.1708 | 0.1365 |
| Cl− | 0.1401 | 0.1988 | 1.0000 | 0.1988 | 0.1632 | 0.7706 | 0.6109 | −0.0911 | 0.6670 |
| TDS | 0.2517 | 0.8634 | 0.1988 | 1.0000 | −0.0223 | 0.1317 | 0.0530 | −0.1708 | 0.1365 |
| Ca2+ | 0.0736 | −0.0223 | 0.1632 | −0.0223 | 1.0000 | 0.2719 | −0.4003 | −0.4584 | 0.0405 |
| Mg2+ | 0.0481 | 0.1317 | 0.7706 | 0.1317 | 0.2719 | 1.0000 | 0.4312 | 0.1123 | 0.6311 |
| Na+ | −0.0754 | 0.0530 | 0.6109 | 0.0530 | −0.4003 | 0.4312 | 1.0000 | 0.4273 | 0.6618 |
| HCO3− | −0.3106 | −0.1708 | −0.0911 | −0.1708 | −0.4584 | 0.1123 | 0.4273 | 1.0000 | 0.0113 |
| SO42− | −0.0394 | 0.1365 | 0.6670 | 0.1365 | 0.0405 | 0.6311 | 0.6618 | 0.0113 | 1.0000 |
| Factors | Initial Eigenvalue | Extract Load Sum of Squares | Sum of Rotation Load Squares | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Percentage Variance | Accumulate (%) | Total | Percentage Variance | Accumulate (%) | Total | Percentage Variance | Accumulate (%) | |
| 1 | 2.95 | 36.85 | 36.85 | 2.95 | 36.85 | 36.85 | 2.89 | 36.11 | 36.11 |
| 2 | 1.92 | 23.98 | 60.83 | 1.92 | 23.98 | 60.83 | 1.79 | 22.33 | 58.44 |
| 3 | 1.18 | 14.80 | 75.63 | 1.18 | 14.80 | 75.63 | 1.38 | 17.19 | 75.63 |
| 4 | 0.73 | 9.17 | 84.80 | ||||||
| 5 | 0.60 | 7.49 | 92.29 | ||||||
| 6 | 0.31 | 3.82 | 96.12 | ||||||
| 7 | 0.23 | 2.93 | 99.04 | ||||||
| 8 | 0.08 | 0.96 | 100.00 | ||||||
| Factors | 1 | 2 | 3 |
|---|---|---|---|
| pH | 0.040 | 0.513 | 0.590 |
| TDS | 0.221 | 0.332 | 0.671 |
| Ca2+ | 0.008 | 0.747 | −0.546 |
| Mg2+ | 0.835 | 0.196 | −0.230 |
| Na+ | 0.796 | −0.463 | 0.160 |
| Cl− | 0.894 | 0.239 | −0.033 |
| HCO3− | 0.158 | −0.823 | −0.014 |
| SO42− | 0.862 | 0.025 | −0.083 |
| Crops | Common Names | Yield Potential (Salinity Tolerance Limit) | |||
|---|---|---|---|---|---|
| Botanical Name | 100% | 90% | 75% | 50% | |
| Zea mays | Corn | 75.17 (1.1) | 90.60 (1.7) | 95.30 (2.5) | 97.99 (3.9) |
| Triticum | Wheat | 99.30 (4.0) | 100 (4.9) | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Qian, H.; Xu, P.; Hou, K. Hydrochemical Characteristic of Groundwater and Its Impact on Crop Yields in the Baojixia Irrigation Area, China. Water 2020, 12, 1443. https://doi.org/10.3390/w12051443
Feng W, Qian H, Xu P, Hou K. Hydrochemical Characteristic of Groundwater and Its Impact on Crop Yields in the Baojixia Irrigation Area, China. Water. 2020; 12(5):1443. https://doi.org/10.3390/w12051443
Chicago/Turabian StyleFeng, Wenwen, Hui Qian, Panpan Xu, and Kai Hou. 2020. "Hydrochemical Characteristic of Groundwater and Its Impact on Crop Yields in the Baojixia Irrigation Area, China" Water 12, no. 5: 1443. https://doi.org/10.3390/w12051443
APA StyleFeng, W., Qian, H., Xu, P., & Hou, K. (2020). Hydrochemical Characteristic of Groundwater and Its Impact on Crop Yields in the Baojixia Irrigation Area, China. Water, 12(5), 1443. https://doi.org/10.3390/w12051443





