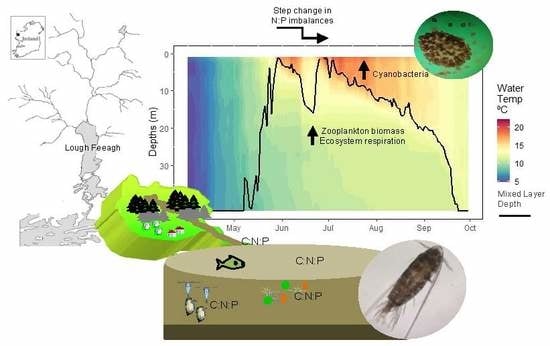
Effects of Consecutive Extreme Weather Events on a Temperate Dystrophic Lake: A Detailed Insight into Physical, Chemical and Biological Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and High-Frequency Monitoring
2.2. Seasonal Chemical and Biological Sampling
2.3. Chlorophyll a and Nutrient Analyses
2.4. Phytoplankton Community Identification and Enumeration
2.5. Zooplankton Community Identification and Enumeration
2.6. Data Analyses
3. Results
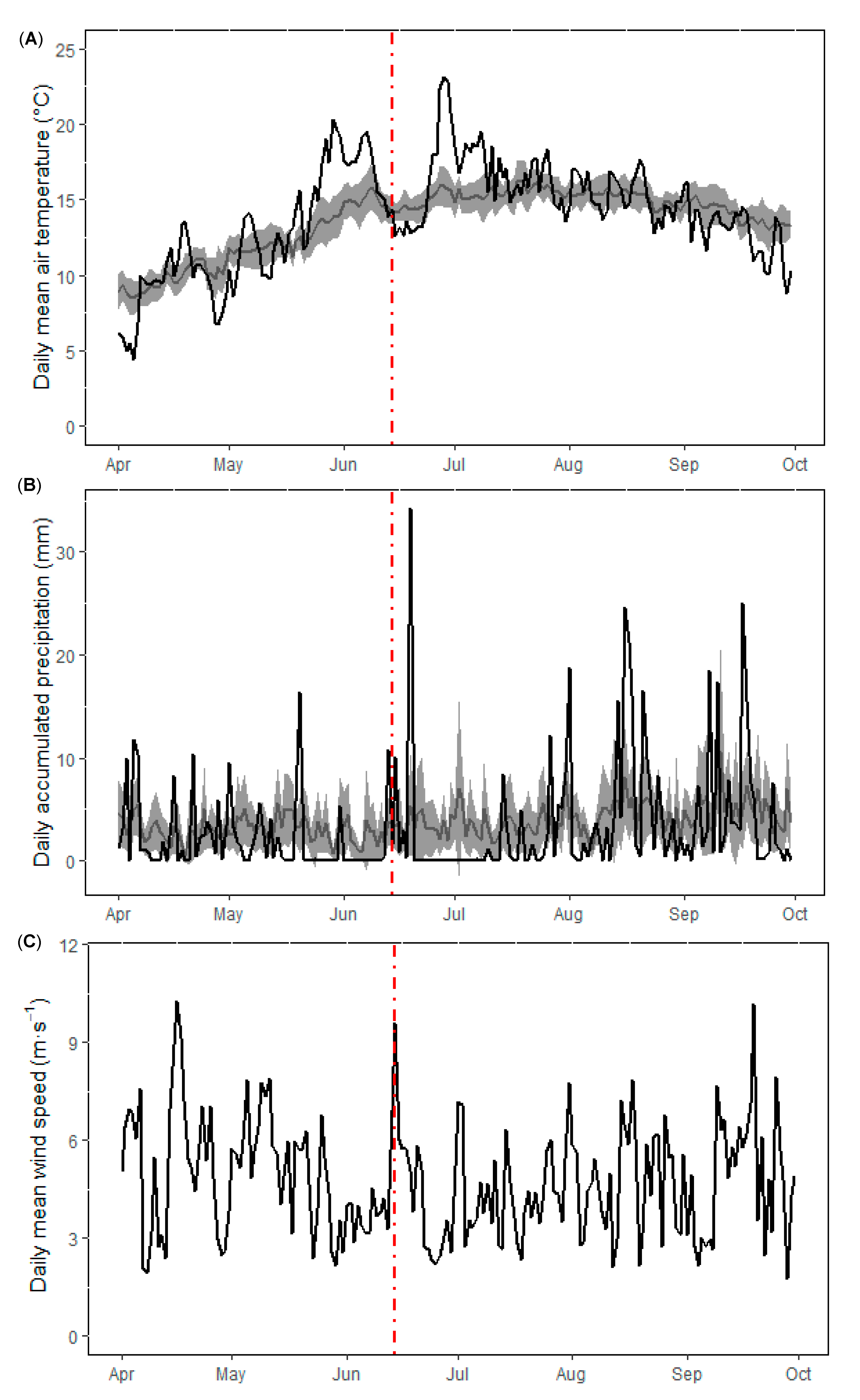
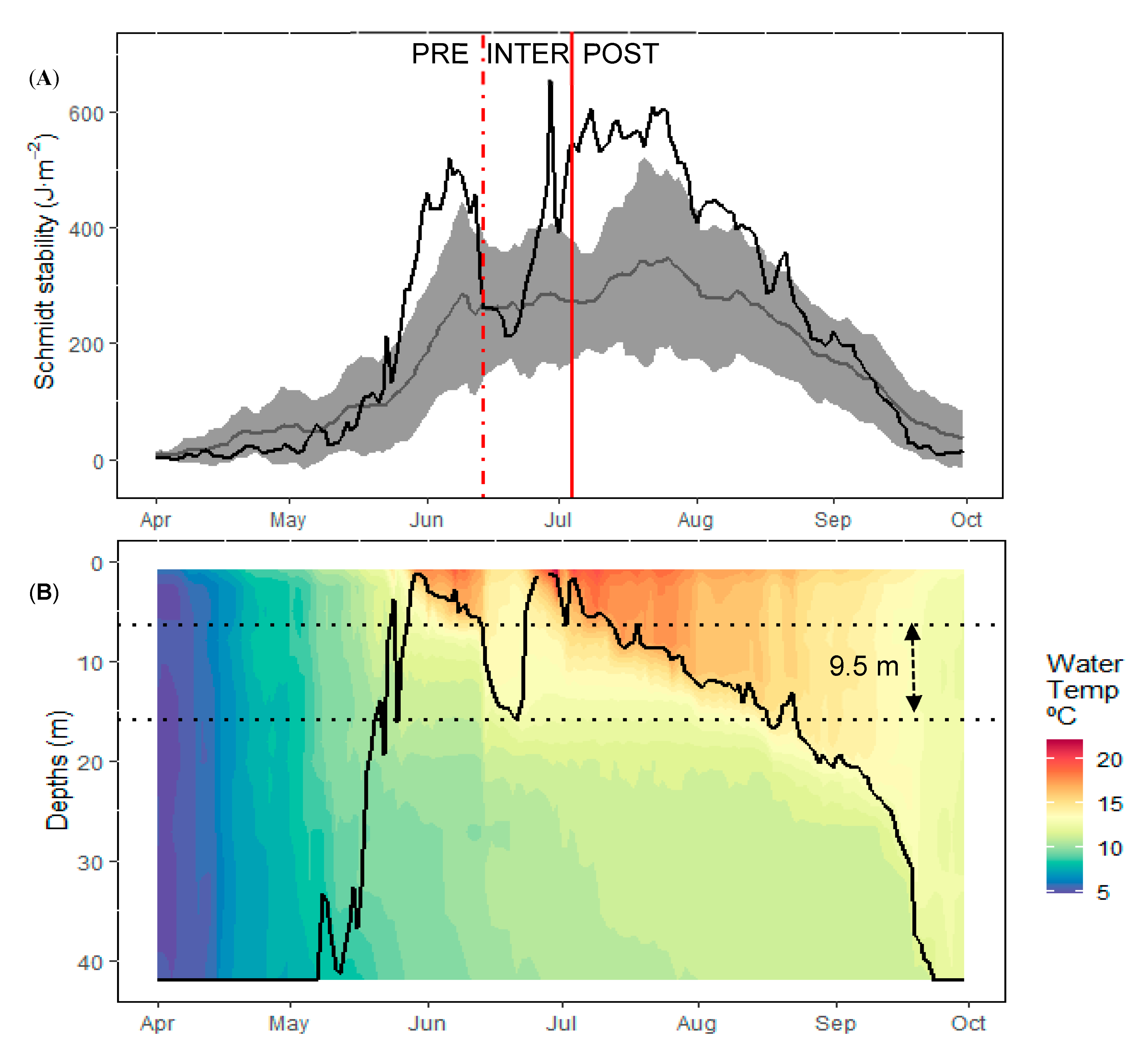
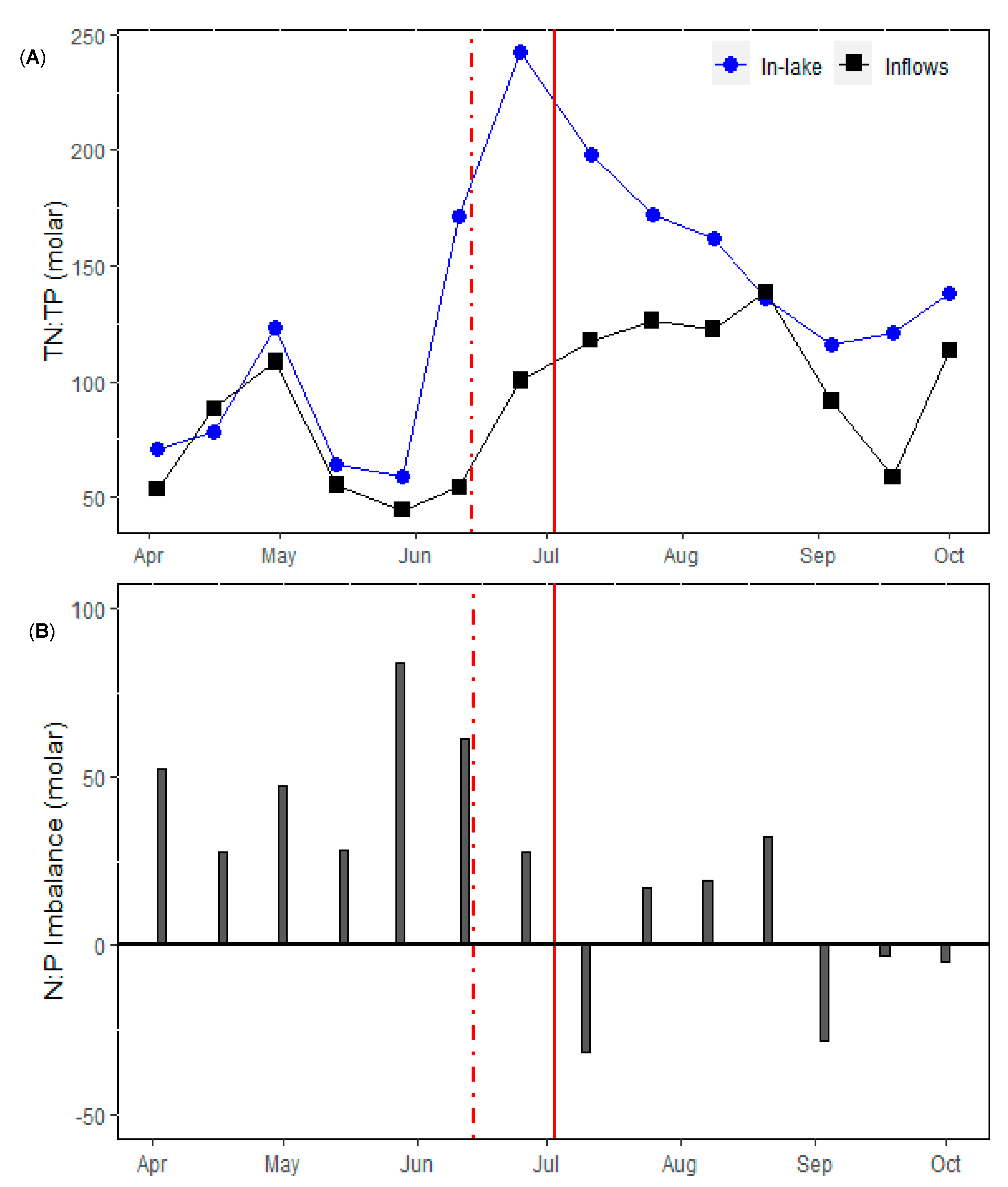
3.1. Physical and Nutrient Dynamics
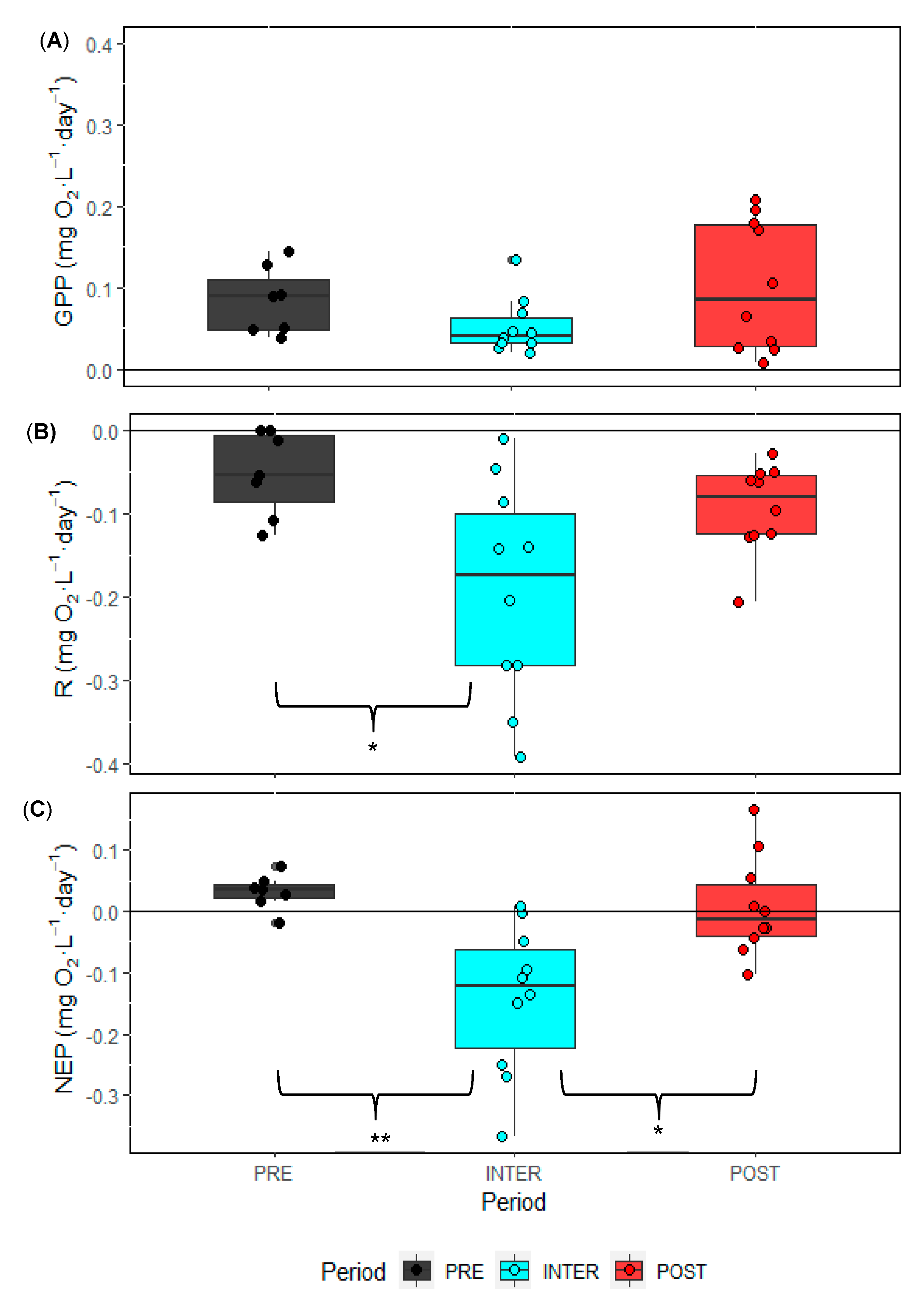
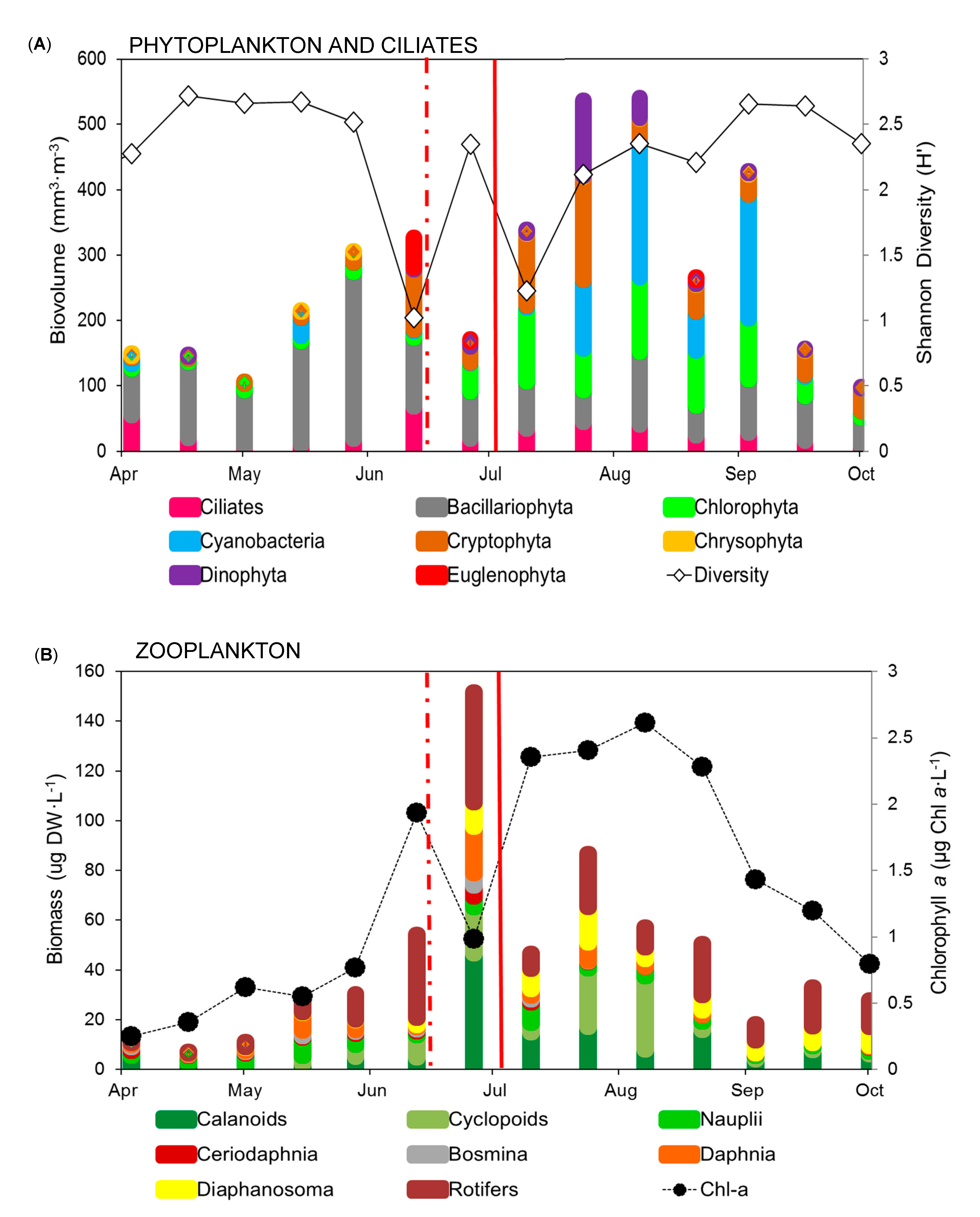
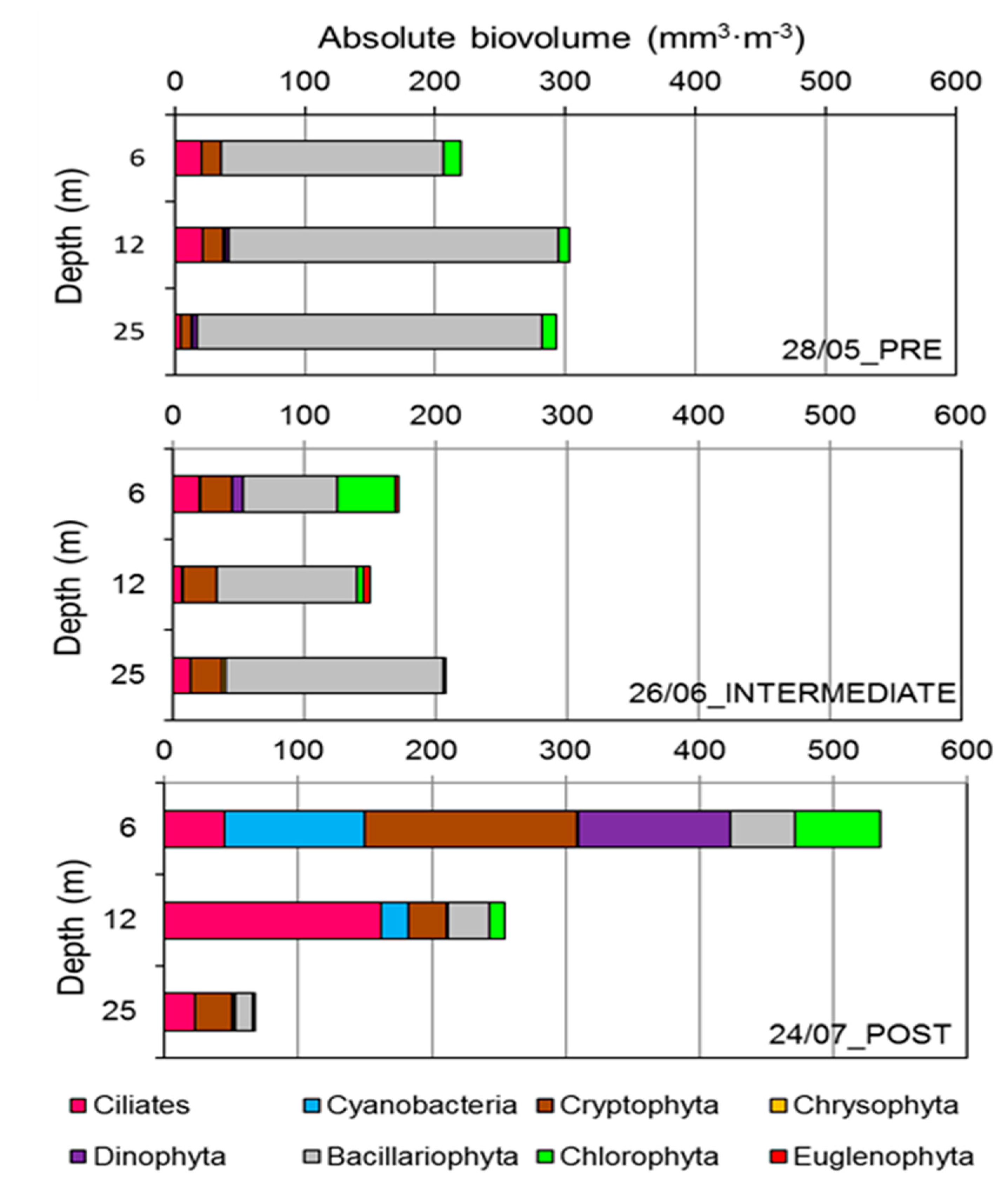
3.2. Phytoplankton and Ciliate Dynamics
3.3. Zooplankton Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- IPCC. The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Stephenson, D.B.; Diaz, H.F.; Murnane, R.J. Definition, diagnosis, and origin of extreme weather and climate events. Clim. Extrem. Soc. 2008, 340, 11–23. [Google Scholar]
- Woolway, R.I.; Merchant, C.J.; Hoek, J.V.D.; Azorin-Molina, C.; Nõges, P.; Laas, A.; Mackay, E.B.; Jones, I.D. Northern Hemisphere Atmospheric Stilling Accelerates Lake Thermal Responses to a Warming World. Geophys. Res. Lett. 2019, 46, 11983–11992. [Google Scholar] [CrossRef]
- Jennings, E.; Jones, S.; Arvola, L.; Staehr, P.A.; Gaiser, E.; Jones, I.D.; Weathers, K.C.; Weyhenmeyer, G.A.; Chiu, C.-Y.; Eyto, E.D. Effects of weather-related episodic events in lakes: An analysis based on high-frequency data. Freshw. Biol. 2012, 57, 589–601. [Google Scholar] [CrossRef]
- Quante, M.; Colijn, F. North Sea Region Climate Change Assessment; Springer Open: Cham, Swhitzerland, 2016. [Google Scholar]
- Ryo, M.; Aguilar-Trigueros, C.A.; Pinek, L.; Muller, L.A.H.; Rillig, M.C. Basic Principles of Temporal Dynamics. Trends Ecol. Evol. 2019, 34, 723–733. [Google Scholar] [CrossRef]
- Adrian, R.; Gerten, D.; Huber, V.; Wagner, C.; Schmidt, S.R. Windows of change: Temporal scale of analysis is decisive to detect ecosystem responses to climate change. Mar. Biol. 2012, 159, 2533–2542. [Google Scholar] [CrossRef]
- Weathers, K.C.; Hanson, P.C.; Arzberger, P.; Brentrup, J.; Brookes, J.; Carey, C.C.; Gaiser, E.; Gaiser, E.; Hamilton, D.P.; Hong, G.S.; et al. The Global Lake Ecological Observatory Network (gleon): The Evolution of Grassroots Network Science. Limnol. Oceanogr. Bull. 2013, 22, 71–73. [Google Scholar] [CrossRef]
- De Eyto, E.; Jennings, E.; Ryder, E.; Sparber, K.; Dillane, M.; Dalton, C.; Poole, R. Response of a humic lake ecosystem to an extreme precipitation event: Physical, chemical, and biological implications. Inland Waters 2016, 6, 483–498. [Google Scholar] [CrossRef]
- Nürnberg, G.K.; Shaw, M. Productivity of clear and humic lakes: Nutrients, phytoplankton, bacteria. Hydrobiologia 1998, 382, 97–112. [Google Scholar] [CrossRef]
- Chapman, P.J.; McDonald, A.T.; Tyson, R.; Palmer, S.M.; Mitchell, G.; Irvine, B. Changes in water colour between 1986 and 2006 in the headwaters of the River Nidd, Yorkshire, UK. Biogeochemistry 2010, 101, 281–294. [Google Scholar] [CrossRef]
- Williamson, C.E.; Morris, D.P. Dissolved organic carbon and nutrients as regulators of lake ecosystems: Resurrection of a more integrated paradigm. Limnol. Oceanogr. 1999, 44, 795–803. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Cole, J.J.; Kitchell, J.F.; Pace, M.L. Impact of dissolved organic carbon, phosphorus, and grazing on phytoplankton biomass and production in experimental lakes. Limnol. Oceanogr. 1998, 43, 73–80. [Google Scholar] [CrossRef]
- Jansson, M.; Bergström, A.-K.; Blomqvist, P.; Drakare, S. Allochthonous Organic Carbon and Phytoplankton/Bacterioplankton Production Relationships in Lakes. Ecology 2000, 81, 3250–3255. [Google Scholar] [CrossRef]
- Faithfull, C.L.; Mathisen, P.; Wenzel, A.; Bergström, A.-K.; Vrede, T. Food web efficiency differs between humic and clear water lake communities in response to nutrients and light. Oecologia 2015, 177, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. PEATMAP: Refining estimates of global peatland distribution based on a meta-analysis. Catena 2018, 160, 134–140. [Google Scholar] [CrossRef]
- Montanarella, L.; Jones, R.J.A.; Hiederer, R. The distribution of peatland in Europe. Mires Peat 2006, 1, 1. [Google Scholar]
- Kelly, P.T.; Renwick, W.H.; Knoll, L.; Vanni, M.J. Stream Nitrogen and Phosphorus Loads Are Differentially Affected by Storm Events and the Difference May Be Exacerbated by Conservation Tillage. Environ. Sci. Technol. 2019, 53, 5613–5621. [Google Scholar] [CrossRef]
- Williamson, T.J.; Vanni, M.J.; Renwick, W.H. Spatial and Temporal Variability of Nutrient Dynamics and Ecosystem Metabolism in a Hyper-eutrophic Reservoir Differ between a Wet and Dry Year. Ecosystems 2020. [Google Scholar] [CrossRef]
- Asam, Z.-U.-Z. Cycling and Transport of Phosphorus and Nitrogen from Harvested Peatland Forests and Possible Mitigation and Methods; Civil Engineering Department, National University of Ireland: Galway, Ireland, 2012. [Google Scholar]
- Schade, J.D.; MacNeill, K.; Thomas, S.A.; McNelly, F.C.; Welter, J.R.; Hood, J.; Goodrich, M.; Power, M.E.; Finlay, J.C. The stoichiometry of nitrogen and phosphorus spiralling in heterotrophic and autotrophic streams. Freshw. Biol. 2011, 56, 424–436. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 978-0-691-07491-7. [Google Scholar]
- Berger, S.A.; Diehl, S.; Kunz, T.J.; Albrecht, D.; Oucible, A.M.; Ritzer, S. Light supply, plankton biomass, and seston stoichiometry in a gradient of lake mixing depths. Limnol. Oceanogr. 2006, 51, 1898–1905. [Google Scholar] [CrossRef]
- Peeters, F.; Straile, D.; Lorke, A.; Ollinger, D. Turbulent mixing and phytoplankton spring bloom development in a deep lake. Limnol. Oceanogr. 2007, 52, 286–298. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Jones, J.I.; Trimmer, M.; Woodward, G.; Montoya, J.M. Warming alters the metabolic balance of ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.; Beisner, B.E.; Gunn, J.M.; Prairie, Y.T.; Winter, J.G. Effects of thermocline deepening on lake plankton communities. Can. J. Fish. Aquat. Sci. 2011, 68, 260–276. [Google Scholar] [CrossRef]
- Kraemer, B.M.; Mehner, T.; Adrian, R. Reconciling the opposing effects of warming on phytoplankton biomass in 188 large lakes. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Foreman, C.M.; Wolf, C.F.; Priscu, J.C. Impact of episodic warming events on the physical, chemical and biological relationships of lakes in the McMurdo Dry Valleys. Antarct. Aquat. Geochem. 2004, 10, 239–268. [Google Scholar] [CrossRef]
- Klug, J.L.; Richardson, D.C.; Ewing, H.A.; Hargreaves, B.R.; Samal, N.R.; Vachon, D.; Pierson, D.C.; Lindsey, A.M.; O’Donnell, D.M.; Effler, S.W. Ecosystem effects of a tropical cyclone on a network of lakes in northeastern North America. Environ. Sci. Technol. 2012, 46, 11693–11701. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, P.; Shatwell, T.; Gessner, M.O.; Gonsiorczyk, T.; Kirillin, G.; Selmeczy, G.; Padisák, J.; Engelhardt, C. Extreme weather event triggers cascade towards extreme turbidity in a clear-water lake. Ecosystems 2017, 20, 1407–1420. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- Sverdrup, H.U. On conditions for the vernal blooming of phytoplankton. J. Cons. Int. Explor. Mer. 1953, 18, 287–295. [Google Scholar] [CrossRef]
- Huisman, J.E.F.; van Oostveen, P.; Weissing, F.J. Critical depth and critical turbulence: Two different mechanisms for the development of phytoplankton blooms. Limnol. Oceanogr. 1999, 44, 1781–1787. [Google Scholar] [CrossRef]
- Huisman, J.; Sharples, J.; Stroom, J.M.; Visser, P.M.; Kardinaal, W.E.A.; Verspagen, J.M.; Sommeijer, B. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 2004, 85, 2960–2970. [Google Scholar] [CrossRef]
- Litchman, E. Growth rates of phytoplankton under fluctuating light. Freshw. Biol. 2000, 44, 223–235. [Google Scholar] [CrossRef]
- Diehl, S.; Berger, S.; Ptacnik, R.; Wild, A. Phytoplankton, light, and nutrients in a gradient of mixing depths: Field experiments. Ecology 2002, 83, 399–411. [Google Scholar] [CrossRef]
- Winder, M.; Reuter, J.E.; Schladow, S.G. Lake warming favours small-sized planktonic diatom species. Proc. R. Soc. B Biol. Sci. 2009, 276, 427–435. [Google Scholar] [CrossRef]
- Sanders, R.W.; Porter, K.G. Phagotrophic phytoflagellates. In Advances in Microbial Ecology; Springer: Berlin, Germany, 1988; pp. 167–192. [Google Scholar]
- Olrik, K. Ecology of mixotrophic flagellates with special reference to Chrysophyceae in Danish lakes. In Phytoplankton Trophic Gradients; Springer: Dordrecht, The Netherlands, 1998; pp. 329–338. [Google Scholar]
- Mitra, A.; Flynn, K.J.; Tillmann, U.; Raven, J.A.; Caron, D.; Stoecker, D.K.; Not, F.; Hansen, P.J.; Hallegraeff, G.; Sanders, R. Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: Incorporation of diverse mixotrophic strategies. Protist 2016, 167, 106–120. [Google Scholar] [CrossRef]
- Ptacnik, R.; Diehl, S.; Berger, S. Performance of sinking and nonsinking phytoplankton taxa in a gradient of mixing depths. Limnol. Oceanogr. 2003, 48, 1903–1912. [Google Scholar] [CrossRef]
- Giling, D.P.; Nejstgaard, J.C.; Berger, S.A.; Grossart, H.-P.; Kirillin, G.; Penske, A.; Lentz, M.; Casper, P.; Sareyka, J.; Gessner, M.O. Thermocline deepening boosts ecosystem metabolism: Evidence from a large-scale lake enclosure experiment simulating a summer storm. Glob. Chang. Biol. 2017, 23, 1448–1462. [Google Scholar] [CrossRef]
- Perga, M.-E.; Bruel, R.; Rodriguez, L.; Guénand, Y.; Bouffard, D. Storm impacts on alpine lakes: Antecedent weather conditions matter more than the event intensity. Glob. Chang. Biol. 2018, 24, 5004–5016. [Google Scholar] [CrossRef]
- Segatto, P.L.; Battin, T.J.; Bertuzzo, E. Modeling the coupled dynamics of stream metabolism and microbial biomass. Limnol. Oceanogr. 2020. [Google Scholar] [CrossRef]
- Sadro, S.; Melack, J.M. The effect of an extreme rain event on the biogeochemistry and ecosystem metabolism of an oligotrophic high-elevation lake. Arct. Antarct. Alp. Res. 2012, 44, 222–231. [Google Scholar] [CrossRef]
- Solomon, C.T.; Bruesewitz, D.A.; Richardson, D.C.; Rose, K.C.; Van de Bogert, M.C.; Hanson, P.C.; Kratz, T.K.; Larget, B.; Adrian, R.; Babin, B.L. Ecosystem respiration: Drivers of daily variability and background respiration in lakes around the globe. Limnol. Oceanogr. 2013, 58, 849–866. [Google Scholar] [CrossRef]
- Guillemette, F.; McCallister, S.L.; del Giorgio, P.A. Selective consumption and metabolic allocation of terrestrial and algal carbon determine allochthony in lake bacteria. ISME J. 2016, 10, 1373–1382. [Google Scholar] [CrossRef]
- Vachon, D.; Del Giorgio, P.A. Whole-lake CO2 dynamics in response to storm events in two morphologically different lakes. Ecosystems 2014, 17, 1338–1353. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Allen, A.P.; Gillooly, J.F.; Brown, J.H. Linking the global carbon cycle to individual metabolism. Funct. Ecol. 2005, 19, 202–213. [Google Scholar] [CrossRef]
- Gómez-Gener, L.; Lupon, A.; Laudon, H.; Sponseller, R.A. Drought alters the biogeochemistry of boreal stream networks. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Havens, K.E.; Beaver, J.R.; Casamatta, D.A.; East, T.L.; James, R.T.; Mccormick, P.; Phlips, E.J.; Rodusky, A.J. Hurricane effects on the planktonic food web of a large subtropical lake. J. Plankton Res. 2011, 33, 1081–1094. [Google Scholar] [CrossRef]
- Tóth, G.L.; Parpala, L.; Balogh, C.; Tàtrai, I.; Baranyai, E. Zooplankton community response to enhanced turbulence generated by water-level decrease in Lake Balaton, the largest shallow lake in Central Europe. Limnol. Oceanogr. 2011, 56, 2211–2222. [Google Scholar] [CrossRef]
- Beaver, J.R.; Casamatta, D.A.; East, T.L.; Havens, K.E.; Rodusky, A.J.; James, R.T.; Tausz, C.E.; Buccier, K.M. Extreme weather events influence the phytoplankton community structure in a large lowland subtropical lake (Lake Okeechobee, Florida, USA). Hydrobiologia 2013, 709, 213–226. [Google Scholar] [CrossRef]
- Ji, G.; Havens, K.E.; Beaver, J.R.; East, T.L. Recovery of plankton from hurricane impacts in a large shallow lake. Freshw. Biol. 2018, 63, 366–379. [Google Scholar] [CrossRef]
- Gauthier, J.; Prairie, Y.T.; Beisner, B.E. Thermocline deepening and mixing alter zooplankton phenology, biomass and body size in a whole-lake experiment. Freshw. Biol. 2014, 59, 998–1011. [Google Scholar] [CrossRef]
- Sastri, A.R.; Gauthier, J.; Juneau, P.; Beisner, B.E. Biomass and productivity responses of zooplankton communities to experimental thermocline deepening. Limnol. Oceanogr. 2014, 59, 1–16. [Google Scholar] [CrossRef]
- Zhou, J.; Han, X.; Qin, B.; Casenave, C.; Yang, G. Response of zooplankton community to turbulence in large, shallow Lake Taihu: A mesocosm experiment. Fundam. Appl. Limnol. Hydrobiol. 2016, 187, 315–324. [Google Scholar] [CrossRef]
- DeMott, W.R. Optimal foraging theory as a predictor of chemically mediated food selection by suspension-feeding copepods. Limnol. Oceanogr. 1989, 34, 140–154. [Google Scholar] [CrossRef]
- Dobberfuhl, D.R.; Elser, J.J. Elemental stoichiometry of lower food web components in arctic and temperate lakes. J. Plankton Res. 2000, 22, 1341–1354. [Google Scholar] [CrossRef]
- Met Éireann. 2018, A Summer of Heat Waves and Droughts; Irish Meteorological Sevice: Dublin, Ireland, 2018. [Google Scholar]
- Andersen, M.R.; de Eyto, E.; Dillane, M.; Poole, R.; Jennings, E. 13 Years of Storms: An Analysis of the Effects of Storms on Lake Physics on the Atlantic Fringe of Europe. Water 2020, 12, 318. [Google Scholar] [CrossRef]
- Woolway, R.I.; Jennings, E.; Carrea, L. Impact of the 2018 European heatwave on lake surface water temperature. Inland Waters 2020, 1–11. [Google Scholar] [CrossRef]
- Sparber, K.; Dalton, C.; de Eyto, E.; Jennings, E.; Lenihan, D.; Cassina, F. Contrasting pelagic plankton in temperate Irish lakes: The relative contribution of heterotrophic, mixotrophic, and autotrophic components, and the effects of extreme rainfall events. Inland Waters 2015, 5, 295–310. [Google Scholar] [CrossRef]
- Ryder, E. Estimating Carbon Pools and Processing in a Humic Irish Lake; School of Health and Science, Dundalk Institute of Technology: Dundalk, Ireland, 2015. [Google Scholar]
- Irvine, K.; Allott, N.; de Eyto, E.; Free, G.; White, J.; Caroni, R.; Kennelly, C.; Keaney, J.; Lennon, C.; Kemp, A.; et al. The Ecological Assessment of Irish Lakes: The development of a new methodology suited to the needs of the EU directive for Surface Waters; Environmental Protection Agency: Wexport, Ireland, 2001. [Google Scholar]
- Byrne, K.; Farrell, E.P.; Papen, H.; Burrerbach-Bahl, K. The influence of temperature on carbon dioxide production in laboratory columns of virgin and forested blanket bog. Int. Peat J. 2001, 11, 35–42. [Google Scholar]
- Allott, N.; McGinnity, P.; O’Hea, B. Factors influencing the downstream transport of sediment in the Lough Feeagh catchment, Burrishoole, Co. Mayo, Ireland. Freshw. Forum 2005, 23, 126–138. [Google Scholar]
- Fealy, R.M.; Buckley, C.; Mechan, S.; Melland, A.; Mellander, P.E.; Shortle, G.; Wall, D.; Jordan, P. The Irish Agricultural Catchments Programme: Catchment selection using spatial multi-criteria decision analysis. Soil Use Manag. 2010, 26, 225–236. [Google Scholar] [CrossRef]
- Sottocornola, M.; Laine, A.; Kiely, G.; Byrne, K.A.; Tuittila, E.-S. Vegetation and environmental variation in an Atlantic blanket bog in South-western Ireland. Plant Ecol. 2008, 203, 69. [Google Scholar] [CrossRef]
- De Eyto, E.; Dillane, M.; Cooney, J.; Hughes, P.; Murphy, M.; Nixon, P.; Sweeney, D.; Poole, R.; Rouen, M. Water quality and meteorological data from the Lough Feeagh Automatic Water Quality Monitoring Station (AWQMS), 2004–2017 Marine Institute, Ireland. Available online: http://data.marine.ie/geonetwork/srv/eng/catalog.search#/metadata/ie.marine.data:dataset.3757 (accessed on 6 April 2020).
- Doyle, B.C.; de Eyto, E.; Dillane, M.; Poole, R.; McCarthy, V.; Ryder, E.; Jennings, E. Synchrony in catchment stream colour levels is driven by both local and regional climate. Biogeosciences 2019, 16, 1053–1071. [Google Scholar] [CrossRef]
- Irish Marine Institute. BurrishooleLTER-Public. Available online: https://github.com/IrishMarineInstitute/BurishooleLTER-Public (accessed on 14 May 2020).
- Met Éireann. Display and Download Historical Data from Current Stations. Available online: https://www.met.ie/climate/available-data/historical-data (accessed on 6 April 2020).
- FWE; APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-3-527-61399-1. [Google Scholar]
- Eisenreich, S.J.; Bannerman, R.T.; Armstrong, D.E. A Simplified Phosphorus Analysis Technique. Environ. Lett. 1975, 9, 43–53. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, V.; Donohue, I.A.N.; Irvine, K. Field evidence for stoichiometric relationships between zooplankton and N and P availability in a shallow calcareous lake. Freshw. Biol. 2006, 51, 1589–1604. [Google Scholar] [CrossRef]
- Utermohl, H. Zur Vervollkommung der quantitativen phytoplankton-methodik. Mitt Int. Ver Limnol. 1958, 9, 38. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002; Volume 25, pp. 2002–2702. [Google Scholar]
- Cox, E.J. Identification of Freshwater Diatoms from Live Material; Chapman & Hall: London, UK, 1996; Volume 158. [Google Scholar]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Bpttrell, H.H. A review of some problems in zooplankton production studies. Nor. J. Zool. 1976, 24, 419–456. [Google Scholar]
- Scourfield, D.J.; Harding, J.P. A Key to the British Species of Freshwater Cladocera, with Notes on their Ecology; Freshwater Biological Association: Ambleside, UK, 1958. [Google Scholar]
- Harding, J.P.; Smith, W.A. A key to the British Freshwater Cyclopoid and Calanoid Copepods; Freshwater Biological Association: Ambleside, UK, 1974; pp. 1–55. [Google Scholar]
- McCarthy, V. The Relationship between Resource Elemental Deficiencies and Zooplankton Community Structure and Dynamics; School of Natural Science, The University of Dublin: Dublin, Ireland, 2007. [Google Scholar]
- De Eyto, E.; Irvine, K. Variation in the Biomass of Functional Groups Comprising the Open-Water Plankton of Shallow Lakes in Ireland. Biol. Environ. Proc. R. Ir. Acad. 2005, 105B, 53–58. [Google Scholar] [CrossRef]
- Dumont, H.J.; Van de Velde, I.; Dumont, S. The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 1975, 19, 75–97. [Google Scholar] [CrossRef]
- Zambrano–Bigiarini, M. Time Series Management, Analysis and Interpolation for Hydrological Modeling; R Package Version 04-2-1; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Winslow, L.; Read, J.; Woolway, R.; Brentrup, J.; Leach, T.; Zwart, Z.; Albers, S.; Collinge, D. rLakeAnalyzer: Lake Physics Tools; R Package Version 1110; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Read, J.S.; Hamilton, D.P.; Jones, I.D.; Muraoka, K.; Winslow, L.A.; Kroiss, R.; Wu, C.H.; Gaiser, E. Derivation of lake mixing and stratification indices from high-resolution lake buoy data. Environ. Model. Softw. 2011, 26, 1325–1336. [Google Scholar] [CrossRef]
- Wilson, H.L.; Ayala, A.I.; Jones, I.D.; Rolston, A.; Pierson, D.; de Eyto, E.; Grossart, H.P.; Perga, M.E.; Woolway, R.I.; Jennings, J. Variability in epilimnion depth estimations in lakes. Hydrol. Earth Syst. Sci. 2020. in prep. [Google Scholar]
- Gray, E.; Mackay, E.B.; Elliott, J.A.; Folkard, A.M.; Jones, I.D. Wide-spread inconsistency in estimation of lake mixed depth impacts interpretation of limnological processes. Water Res. 2020, 168, 115136. [Google Scholar] [CrossRef]
- Winslow, L.; Zwart, J.; Batt, R.; Corman, J.; Dugan, H.; Hanson, P.; Holtgrieve, G.; Jaimes, A.; Read, J.; Woolway, R. LakeMetabolizer: Tools for the Analysis of Ecosystem Metabolism. R Package Version 15. 2016. [Google Scholar]
- Cole, J.J.; Caraco, N.F. Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6. Limnol. Oceanogr. 1998, 43, 647–656. [Google Scholar] [CrossRef]
- Helsel, D.R.; Hirsch, R.M. Statistical methods in water resources, chapter A3. In Hydrologic Analysis and Interpretation; U.S. Geological Survey: Reston, VA, USA, 2002; Volume 4. [Google Scholar]
- Dinno, A. Nonparametric Pairwise Multiple Comparisons in Independent Groups using Dunn’s Test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis; R Package Version 08309000; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Byrne, M.J.; Wood, M.S. Concentrations and Loads of Nutrients in the Tributaries of the Lake Okeechobee Watershed, South–Central Florida, Water Years 2004–2008; U.S. Geological Survey: Recon, VA, USA, 2012.
- Peterson, B.G.; Carl, P.; Boudt, K.; Bennett, R.; Ulrich, J.; Zivot, E.; Cornilly, D.; Hung, E.; Lestel, M.; Balkissoon, K.; et al. Econometric Tools for Performance and Risk Analyses; R Package ‘PerformanceAnalytics’ Version 153; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Oksanen, J. Vegan: Ecological Diversity; R Package Version 25-5; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Magurran, A.E.; McGill, B.J. Biological Diversity: Frontiers in Measurement and Assessment; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Berggren, M.; Sponseller, R.A.; Alves Soares, A.R.; Bergström, A.-K. Toward an ecologically meaningful view of resource stoichiometry in DOM-dominated aquatic systems. J. Plankton Res. 2015, 37, 489–499. [Google Scholar] [CrossRef]
- Maranger, R.; Jones, S.E.; Cotner, J.B. Stoichiometry of carbon, nitrogen, and phosphorus through the freshwater pipe. Limnol. Oceanogr. Lett. 2018, 3, 89–101. [Google Scholar] [CrossRef]
- Wagner, C.; Adrian, R. Consequences of changes in thermal regime for plankton diversity and trait composition in a polymictic lake: A matter of temporal scale. Freshw. Biol. 2011, 56, 1949–1961. [Google Scholar] [CrossRef]
- Huber, V.; Wagner, C.; Gerten, D.; Adrian, R. To bloom or not to bloom: Contrasting responses of cyanobacteria to recent heat waves explained by critical thresholds of abiotic drivers. Oecologia 2012, 169, 245–256. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; De Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef]
- Burford, M.A.; Carey, C.C.; Hamilton, D.P.; Huisman, J.; Paerl, H.W.; Wood, S.A.; Wulff, A. Perspective: Advancing the research agenda for improving understanding of cyanobacteria in a future of global change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Shi, X.; Kong, F.; Ma, R.; Yu, Y. Nutrient reduction magnifies the impact of extreme weather on cyanobacterial bloom formation in large shallow Lake Taihu (China). Water Res. 2016, 103, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A. The seasonal sensitivity of Cyanobacteria and other phytoplankton to changes in flushing rate and water temperature. Glob. Chang. Biol. 2010, 16, 864–876. [Google Scholar] [CrossRef]
- Isles, P.D.F.; Giles, C.D.; Gearhart, T.A.; Xu, Y.; Druschel, G.K.; Schroth, A.W. Dynamic internal drivers of a historically severe cyanobacteria bloom in Lake Champlain revealed through comprehensive monitoring. J. Gt. Lakes Res. 2015, 41, 818–829. [Google Scholar] [CrossRef]
- Bucak, T.; Trolle, D.; Tavşanoğlu, Ü.N.; Çakıroğlu, A.İ.; Özen, A.; Jeppesen, E.; Beklioğlu, M. Modeling the effects of climatic and land use changes on phytoplankton and water quality of the largest Turkish freshwater lake: Lake Beyşehir. Sci. Total Environ. 2018, 621, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Bober, B.; Bialczyk, J. Determination of the toxicity of the freshwater cyanobacterium Woronichinia naegeliana (Unger) Elenkin. J. Appl. Phycol. 2017, 29, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L. Algae, Cyanobacteria Blooms, and Climate Change; The Climate Institute: Washington, DC, USA, 2017. [Google Scholar]
- Hallstan, S.; Trigal, C.; Johansson, K.S.L.; Johnson, R.K. The impact of climate on the geographical distribution of phytoplankton species in boreal lakes. Oecologia 2013, 173, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, J.D.; Doubek, J.P.; Adrian, R.; Anneville, O.; Carey, C.C.; Carvalho, L.; de Senerpont Domis, L.N.; Dur, G.; Frassl, M.A.; Grossart, H.P.; et al. Storm impacts on phytoplankton community dynamics in lakes. Glob. Chang. Biol. 2020. [Google Scholar] [CrossRef]
- Meersche, K.V.; den Rijswijk, P.V.; Soetaert, K.; Middelburg, J.J. Autochthonous and allochthonous contributions to mesozooplankton diet in a tidal river and estuary: Integrating carbon isotope and fatty acid constraints. Limnol. Oceanogr. 2009, 54, 62–74. [Google Scholar] [CrossRef]
- De Kluijver, A.; Schoon, P.L.; Downing, J.A.; Schouten, S.; Middelburg, J.J. Stable carbon isotope biogeochemistry of lakes along a trophic gradient. Biogeosciences 2014, 11, 6265. [Google Scholar] [CrossRef]
- Leitão, E.; Ger, K.A.; Panosso, R. Selective Grazing by a Tropical Copepod (Notodiaptomus iheringi) Facilitates Microcystis Dominance. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Hartmann, H.J.; Taleb, H.; Aleya, L.; Lair, N. Predation on Ciliates by the Suspension-Feeding Calanoid Copepod Acanthodiaptomus denticornis. Can. J. Fish. Aquat. Sci. 1993, 50, 1382–1393. [Google Scholar] [CrossRef]
- Fenchel, T. The microbial loop—25 years later. J. Exp. Mar. Biol. Ecol. 2008, 366, 99–103. [Google Scholar] [CrossRef]
- Lydersen, E.; Aanes, K.J.; Andersen, S.; Andersen, T.; Brettum, P.; Baekken, T.; Lien, L.; Lindstrøm, E.A.; Løvik, J.E.; Mjelde, M.; et al. Ecosystem effects of thermal manipulation of a whole lake, Lake Breisjøen, southern Norway (THERMOS project). Hydrol. Earth Syst. Sci. Discuss. 2008, 12, 509–522. [Google Scholar] [CrossRef]
- Visser, A.W.; Mariani, P.; Pigolotti, S. Swimming in turbulence: Zooplankton fitness in terms of foraging efficiency and predation risk. J. Plankton Res. 2009, 31, 121–133. [Google Scholar] [CrossRef]
- Mehner, T.; Thiel, R. A Review of Predation Impact by 0+ Fish on Zooplankton in Fresh and Brackish Waters of the Temperate Northern Hemisphere. Environ. Biol. Fishes 1999, 56, 169–181. [Google Scholar] [CrossRef]
- Härkönen, L.; Pekcan-Hekim, Z.; Hellén, N.; Ojala, A.; Horppila, J. Combined Effects of Turbulence and Different Predation Regimes on Zooplankton in Highly Colored Water—Implications for Environmental Change in Lakes. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Main, T.M.; Dobberfuhl, D.R.; Elser, J.J. N: P stoichiometry and ontogeny of crustacean zooplankton: A test of the growth rate hypothesis. Limnol. Oceanogr. 1997, 42, 1474–1478. [Google Scholar] [CrossRef]
- Burns, C.W.; Brett, M.T.; Schallenberg, M. A comparison of the trophic transfer of fatty acids in freshwater plankton by cladocerans and calanoid copepods. Freshw. Biol. 2011, 56, 889–903. [Google Scholar] [CrossRef]
- Galloway, A.W.E.; Taipale, S.J.; Hiltunen, M.; Peltomaa, E.; Strandberg, U.; Brett, M.T.; Kankaala, P. Diet-specific biomarkers show that high-quality phytoplankton fuels herbivorous zooplankton in large boreal lakes. Freshw. Biol. 2014, 59, 1902–1915. [Google Scholar] [CrossRef]
- Porter, K.G. The Plant-Animal Interface in Freshwater Ecosystems: Microscopic grazers feed differentially on planktonic algae and can influence their community structure and succession in ways that are analogous to the effects of herbivores on terrestrial plant communities. Am. Sci. 1977, 65, 159–170. [Google Scholar]
- De Eyto, E.; Doyle, B.; King, N.; Kilbane, T.; Finlay, R.; Sibigtroth, L.; Poole, R.; Dillane, M.; Jennings, E. Characterisation of salmonid food webs in the rivers and lakes of an Irish peatland ecosystem. Biol. Environ. Proc. R. Ir. Acad. 2020. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderó-Pascual, M.; de Eyto, E.; Jennings, E.; Dillane, M.; Andersen, M.R.; Kelly, S.; Wilson, H.L.; McCarthy, V. Effects of Consecutive Extreme Weather Events on a Temperate Dystrophic Lake: A Detailed Insight into Physical, Chemical and Biological Responses. Water 2020, 12, 1411. https://doi.org/10.3390/w12051411
Calderó-Pascual M, de Eyto E, Jennings E, Dillane M, Andersen MR, Kelly S, Wilson HL, McCarthy V. Effects of Consecutive Extreme Weather Events on a Temperate Dystrophic Lake: A Detailed Insight into Physical, Chemical and Biological Responses. Water. 2020; 12(5):1411. https://doi.org/10.3390/w12051411
Chicago/Turabian StyleCalderó-Pascual, Maria, Elvira de Eyto, Eleanor Jennings, Mary Dillane, Mikkel René Andersen, Sean Kelly, Harriet Louise Wilson, and Valerie McCarthy. 2020. "Effects of Consecutive Extreme Weather Events on a Temperate Dystrophic Lake: A Detailed Insight into Physical, Chemical and Biological Responses" Water 12, no. 5: 1411. https://doi.org/10.3390/w12051411
APA StyleCalderó-Pascual, M., de Eyto, E., Jennings, E., Dillane, M., Andersen, M. R., Kelly, S., Wilson, H. L., & McCarthy, V. (2020). Effects of Consecutive Extreme Weather Events on a Temperate Dystrophic Lake: A Detailed Insight into Physical, Chemical and Biological Responses. Water, 12(5), 1411. https://doi.org/10.3390/w12051411