Assessing the Benefits of Forested Riparian Zones: A Qualitative Index of Riparian Integrity Is Positively Associated with Ecological Status in European Streams
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sampling Overview
2.3. Riparian Habitat Assessment
2.4. Water Quality
2.5. Macroinvertebrates
2.6. Data Analysis
3. Results
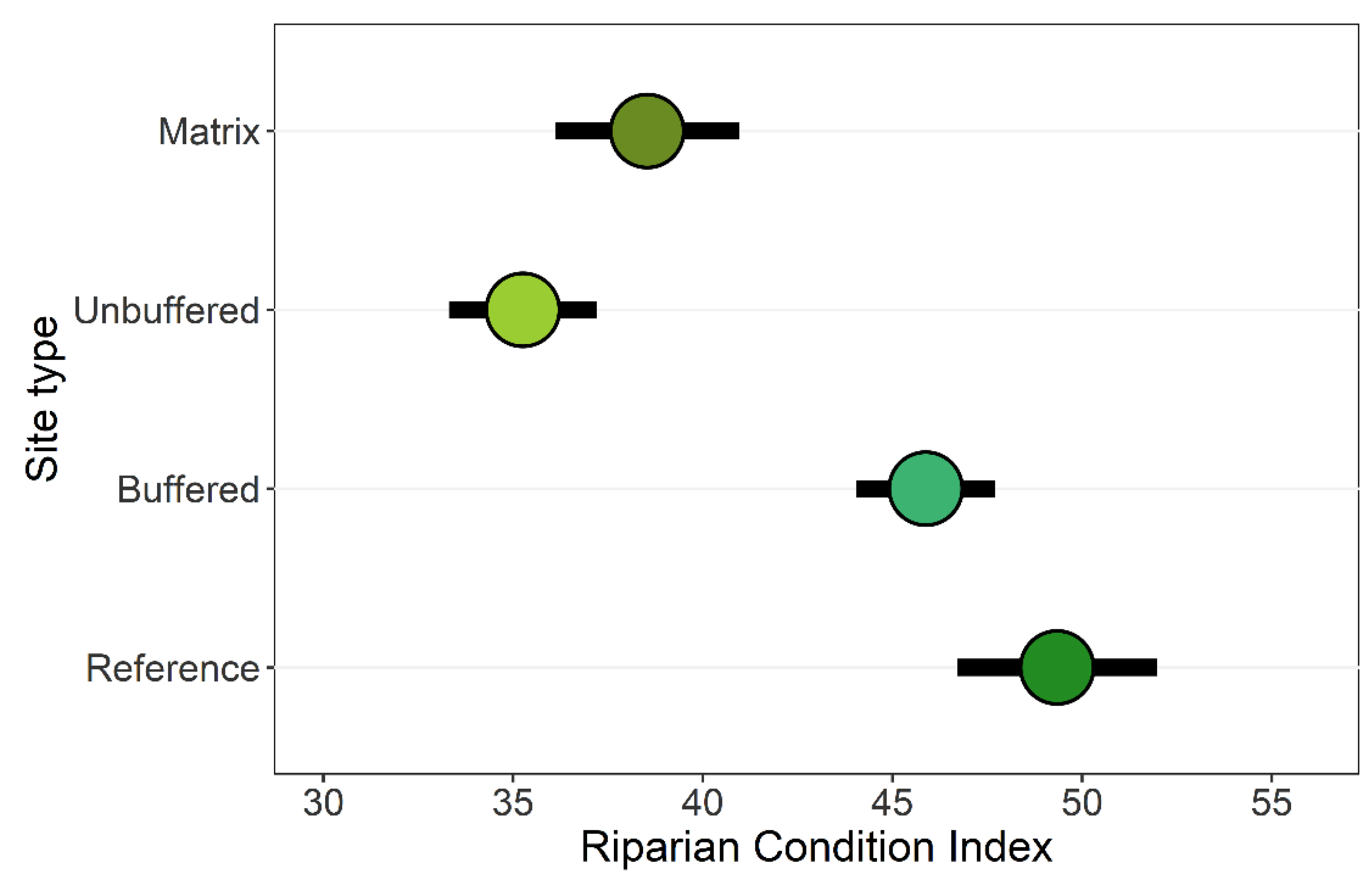
3.1. Riparian Integrity across Case-Study Basins
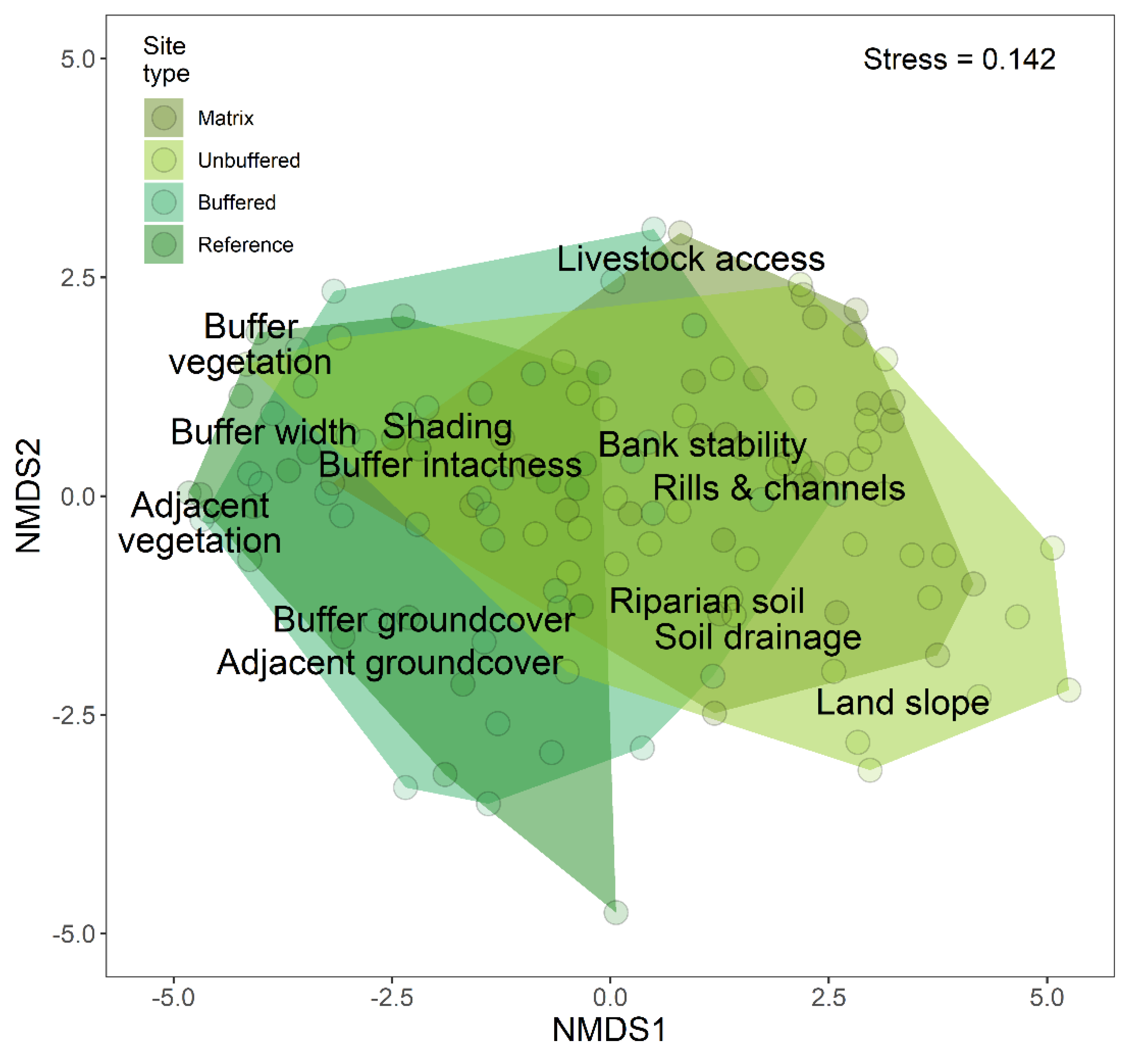
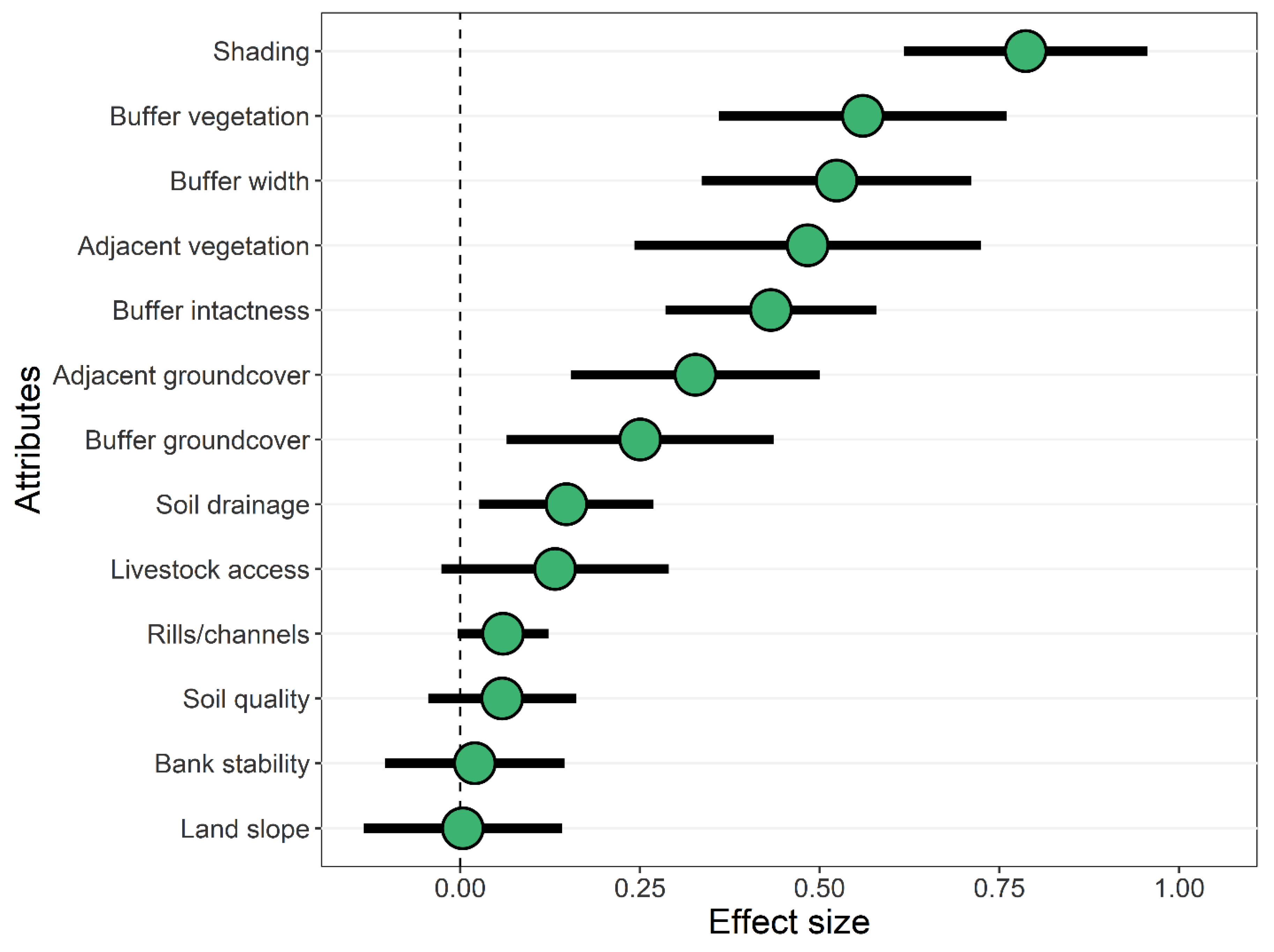
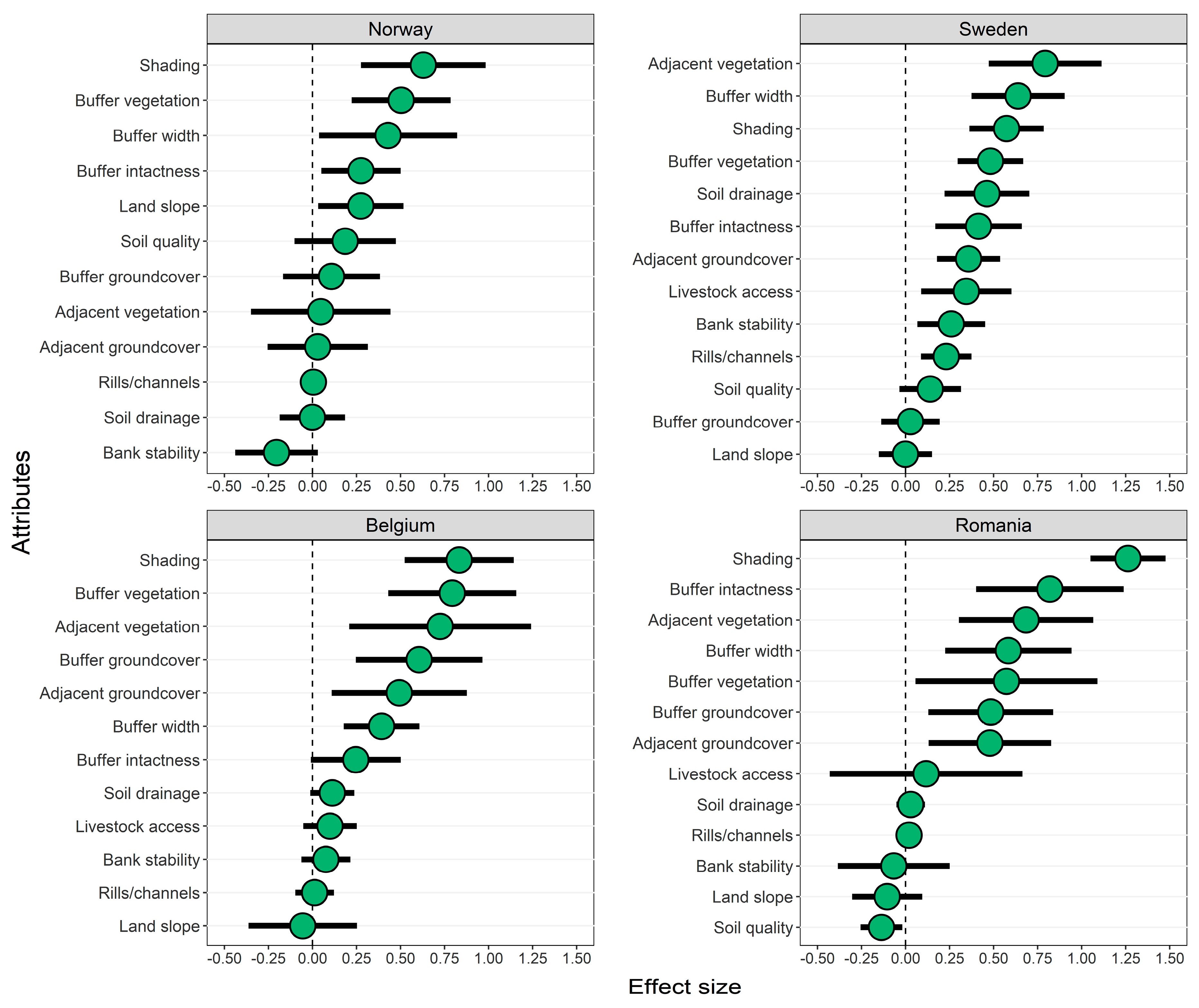
3.2. Effects of Forested Riparian Buffers
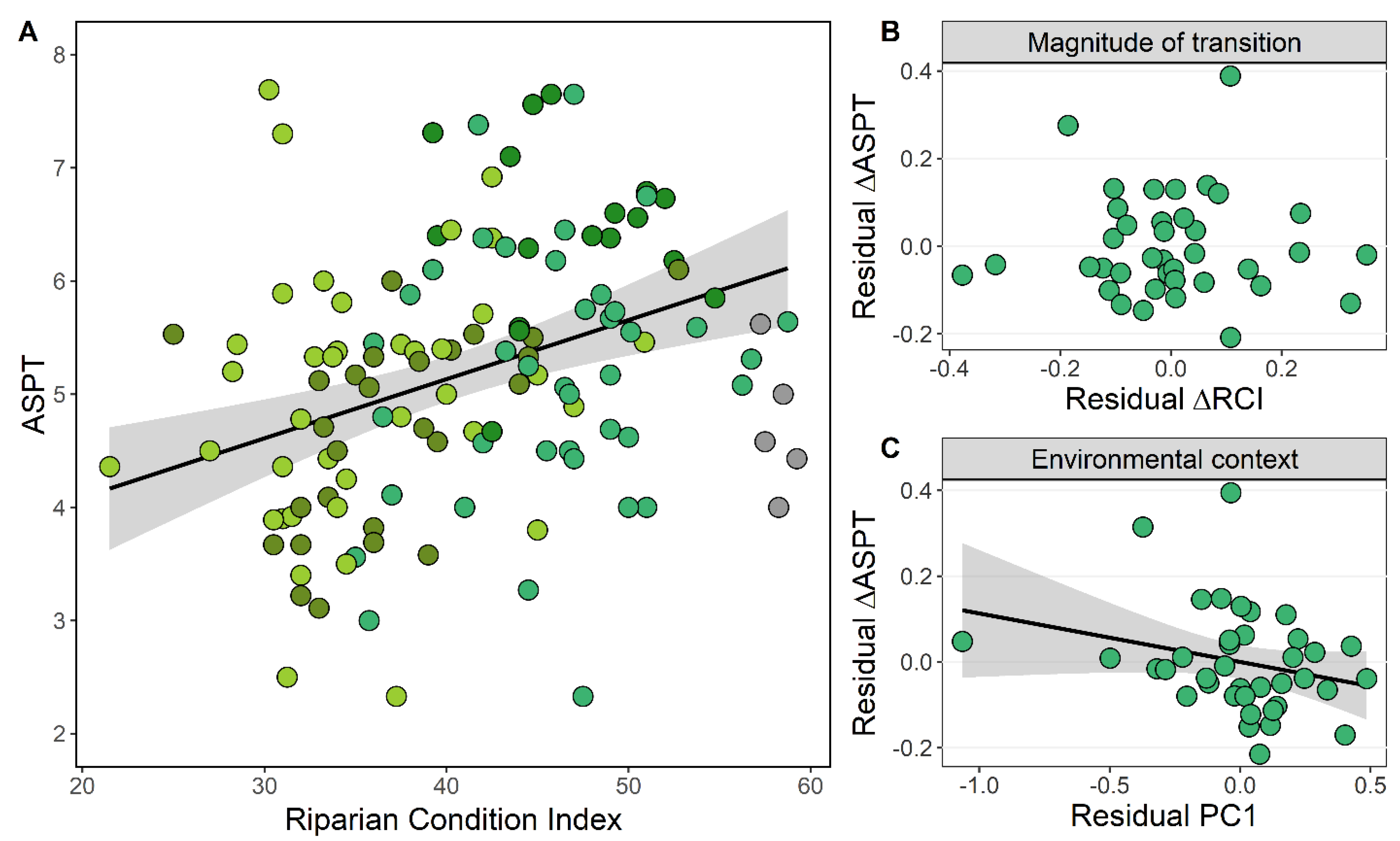
3.3. Riparian Condition and Stream Ecological Status
4. Discussion
4.1. The Riparian Condition Index (RCI) in the European Context
4.2. Effects of Shading by Forested Riparian Buffers
4.3. Magnitude of Transition and Environmental Context
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gregory, S.V.; Swanson, F.J.; McKee, W.A.; Cummins, K.W. An ecosystem perspective of riparian zones: Focus on links between land and water. Bioscience 1991, 41, 540–551. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H. The ecology of interfaces: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Power, M.E.; Rainey, W.E. Food webs and resource sheds: Towards spatially delimiting trophic interactions. In The Ecological Consequences of Environmental Heterogeneity; Hutchings, M.J., John, E.A., Stewart, A.J., Eds.; Blackwell Science: London, UK, 2000; pp. 291–314. [Google Scholar]
- Marcarelli, A.M.; Baxter, C.V.; Benjamin, J.R.; Miyake, Y.; Murakami, M.; Fausch, K.D.; Nakano, S. Magnitude and direction of stream–forest community interactions change with time scale. Ecology 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Leroux, S.J.; Loreau, M. Subsidy hypothesis and strength of trophic cascades across ecosystems. Ecol. Lett. 2008, 11, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Naiman, R.J.; Decamps, H.; Pollock, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.E.L.; Raudsepp-Hearne, C.; Bennett, E.M. Effects of land use, cover, and protection on stream and riparian ecosystem services and biodiversity. Conserv. Biol. 2020, 34, 244–255. [Google Scholar] [CrossRef]
- Nakano, S.; Murakami, M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl. Acad. Sci. USA 2001, 98, 166–170. [Google Scholar] [CrossRef]
- Burdon, F.J.; McIntosh, A.R.; Harding, J.S. Mechanisms of trophic niche compression: Evidence from landscape disturbance. J. Anim. Ecol. 2020, 89, 730–744. [Google Scholar] [CrossRef]
- Polis, G.A.; Anderson, W.B.; Holt, R.D. Toward an intergration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 1997, 28, 289–316. [Google Scholar] [CrossRef]
- Baxter, C.V.; Fausch, K.D.; Carl Saunders, W. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshw. Biol. 2005, 50, 201–220. [Google Scholar] [CrossRef]
- Burdon, F.J.; Harding, J.S. The linkage between riparian predators and aquatic insects across a stream-resource spectrum. Freshw. Biol. 2008, 53, 330–346. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Carlson, P.E.; McKie, B.G.; Sandin, L.; Johnson, R.K. Strong land-use effects on the dispersal patterns of adult stream insects: Implications for transfers of aquatic subsidies to terrestrial consumers. Freshw. Biol. 2016, 61, 848–861. [Google Scholar] [CrossRef]
- Steffen, W.; Grinevald, J.; Crutzen, P.; McNeill, J. The Anthropocene: Conceptual and historical perspectives. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 842–867. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.; Abell, R.; Darwall, W.; Thieme, M.L.; Tickner, D.; Timboe, I. The freshwater biodiversity crisis. Science 2018, 362, 1369. [Google Scholar] [CrossRef] [PubMed]
- Vorosmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Correll, D.L. Principles of planning and establishment of buffer zones. Ecol. Eng. 2005, 24, 433–439. [Google Scholar] [CrossRef]
- Greenwood, M.J.; Harding, J.S.; Niyogi, D.K.; McIntosh, A.R. Improving the effectiveness of riparian management for aquatic invertebrates in a degraded agricultural landscape: Stream size and land-use legacies. J. Appl. Ecol. 2012, 49, 213–222. [Google Scholar] [CrossRef]
- European Commission. Towards an EU Research and Innovation Policy Agenda for Nature-Based Solutions & Re-Naturing Cities; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Friberg, N.; Angelopoulos, N.V.; Buijse, A.D.; Cowx, I.G.; Kail, J.; Moe, T.F.; Moir, H.; O’Hare, M.T.; Verdonschot, P.F.M.; Wolter, C. Effective river restoration in the 21st Century: From trial and error to novel evidence-based approaches. In Advances in Ecological Research; Dumbrell, A.J., Kordas, R.L., Woodward, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 55, pp. 535–611. [Google Scholar]
- Honnay, O.; Jacquemyn, H.; Nackaerts, K.; Breyne, P.; Van Looy, K. Patterns of population genetic diversity in riparian and aquatic plant species along rivers. J. Biogeogr. 2010, 37, 1730–1739. [Google Scholar] [CrossRef]
- Tagwireyi, P.; Sullivan, S.M.P. Riverine landscape patches influence trophic dynamics of riparian ants. River Res. Appl. 2016, 32, 1721–1729. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Padoa-Schioppa, E.; De Bernardi, F. Influence of landscape elements in riparian buffers on the conservation of semiaquatic amphibians. Conserv. Biol. 2009, 23, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Hasselquist, E.M.; Laudon, H. Towards ecologically functional riparian zones: A meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. J. Environ. Manag. 2019, 249, 109391. [Google Scholar] [CrossRef] [PubMed]
- Lowrance, R.; Todd, R.; Fail, J.; Hendrickson, O.; Leonard, R.; Asmussen, L. Riparian forests as nutrient filters in agricultural watersheds. BioScience 1984, 34, 374–377. [Google Scholar] [CrossRef]
- Parkyn, S.M.; Davies-Colley, R.J.; Halliday, N.J.; Costley, K.J.; Croker, G.F. Planted Riparian Buffer Zones in New Zealand: Do They Live Up to Expectations? Restor. Ecol. 2003, 11, 436–447. [Google Scholar] [CrossRef]
- Wahl, C.M.; Neils, A.; Hooper, D. Impacts of land use at the catchment scale constrain the habitat benefits of stream riparian buffers. Freshw. Biol. 2013, 58, 2310–2324. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Palmer, M.A. River restoration: The fuzzy logic of repairing reaches to reverse catchment scale degradation. Ecol. Appl. 2011, 21, 1926–1931. [Google Scholar] [CrossRef]
- Mander, Ü.; Hayakawa, Y.; Kuusemets, V. Purification processes, ecological functions, planning and design of riparian buffer zones in agricultural watersheds. Ecol. Eng. 2005, 24, 421–432. [Google Scholar] [CrossRef]
- Cole, L.J.; Stockan, J.; Helliwell, R. Managing riparian buffer strips to optimise ecosystem services: A review. Agric. Ecosyst. Environ. 2020, in press. [Google Scholar] [CrossRef]
- Friberg, N. Pressure-response relationships in stream ecology: Introduction and synthesis. Freshw. Biol. 2010, 55, 1367–1381. [Google Scholar] [CrossRef]
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Seppelt, R.; Müller, F.; Schröder, B.; Volk, M. Challenges of simulating complex environmental systems at the landscape scale: A controversial dialogue between two cups of espresso. Ecol. Model. 2009, 220, 3481–3489. [Google Scholar] [CrossRef]
- Harding, J.S.; Clapcott, J.; Quinn, J.; Hayes, J.; Joy, M.; Storey, R.; Greig, H.; Hay, J.; James, T.; Beech, M.; et al. Stream Habitat Assessment Protocols for Wadeable Rivers and Streams of New Zealand; School of Biological Sciences, University of Canterbury: Christchurch, New Zealand, 2009. [Google Scholar]
- Burdon, F.J.; McIntosh, A.R.; Harding, J.S. Habitat loss drives threshold response of benthic invertebrate communities to deposited sediment in agricultural streams. Ecol. Appl. 2013, 23, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Friberg, N.; Bonada, N.; Bradley, D.C.; Dunbar, M.J.; Edwards, F.K.; Grey, J.; Hayes, R.B.; Hildrew, A.G.; Lamouroux, N.; Trimmer, M.; et al. Biomonitoring of Human Impacts in Freshwater Ecosystems: The Good, the Bad and the Ugly. In Advances in Ecological Research; Woodward, G., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 44, pp. 1–68. [Google Scholar]
- Davy-Bowker, J.; Clarke, R.T.; Johnson, R.K.; Kokes, J.; Murphy, J.F.; Zahrádková, S. A comparison of the European Water Framework Directive physical typology and RIVPACS-type models as alternative methods of establishing reference conditions for benthic macroinvertebrates. Hydrobiologia 2006, 566, 91–105. [Google Scholar] [CrossRef]
- Birk, S.; Hering, D. Direct comparison of assessment methods using benthic macroinvertebrates: A contribution to the EU Water Framework Directive intercalibration exercise. Hydrobiologia 2006, 566, 401. [Google Scholar] [CrossRef]
- Burdon, F.J.; Reyes, M.; Alder, A.C.; Joss, A.; Ort, C.; Räsänen, K.; Jokela, J.; Eggen, R.I.L.; Stamm, C. Environmental context and magnitude of disturbance influence trait-mediated community responses to wastewater in streams. Ecol. Evol. 2016, 6, 3923–3939. [Google Scholar] [CrossRef]
- Lake, P.S. Resistance, Resilience and Restoration. Ecol. Manag. Restor. 2013, 14, 20–24. [Google Scholar] [CrossRef]
- Sörensen, J. Urban, Pluvial Flooding: Blue-Green Infrastructure as a Strategy for Resilience; Lund University: Lund, Sweden, 2018. [Google Scholar]
- Lennon, M. Green infrastructure and planning policy: A critical assessment. Local Environ. 2015, 20, 957–980. [Google Scholar] [CrossRef]
- Turner, T. Greenways, blueways, skyways and other ways to a better London. Landsc. Urban Plan. 1995, 33, 269–282. [Google Scholar] [CrossRef]
- Walsh, C.J.; Booth, D.B.; Burns, M.J.; Fletcher, T.D.; Hale, R.L.; Hoang, L.N.; Livingston, G.; Rippy, M.A.; Roy, A.H.; Scoggins, M.; et al. Principles for urban stormwater management to protect stream ecosystems. Freshw. Sci. 2016, 35, 398–411. [Google Scholar] [CrossRef]
- Sörensen, J.; Persson, A.; Sternudd, C.; Aspegren, H.; Nilsson, J.; Nordström, J.; Jönsson, K.; Mottaghi, M.; Becker, P.; Pilesjö, P.; et al. Re-Thinking Urban Flood Management—Time for a Regime Shift. Water 2016, 8, 332. [Google Scholar] [CrossRef]
- Ossa-Moreno, J.; Smith, K.M.; Mijic, A. Economic analysis of wider benefits to facilitate SuDS uptake in London, UK. Sustain. Cities Soc. 2017, 28, 411–419. [Google Scholar] [CrossRef]
- Fölster, J.; Johnson, R.K.; Futter, M.N.; Wilander, A. The Swedish monitoring of surface waters: 50 years of adaptive monitoring. AMBIO 2014, 43, 3–18. [Google Scholar] [CrossRef]
- Hauer, F.R.; Resh, V.H. Chapter 20—Macroinvertebrates. In Methods in Stream Ecology, 2nd ed.; Academic Press: San Diego, CA, USA, 2007; pp. 435–454. [Google Scholar]
- Briers, R. Biotic: Calculation of Freshwater Biotic Indices. R Package Version 0.1.2. 2016. Available online: https://github.com/robbriers/biotic (accessed on 1 April 2020).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Swan, D.M.; Pustejovsky, J.E. A gradual effects model for single-case designs. Multivar. Behav. Res. 2018, 53, 574–593. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, M.J. On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology 2011, 92, 2049–2055. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- CLC. Corine Land Cover (CLC) Inventory; European Environment Agency, EEA: Copenhagen, Denmark, 2018; Available online: https://land.copernicus.eu/ (accessed on 1 April 2020).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 1 April 2020).
- Pfankuch, D.J. Stream Reach Inventory and Channel Stability Evaluation; USDA Forest Service, Northern Region, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1975; p. 26.
- Death, R.G.; Winterbourn, M.J. Diversity patterns in stream benthic invertebrate communities: The influence of habitat stability. Ecology 1995, 76, 1446–1460. [Google Scholar] [CrossRef]
- Rosgen, D. A Watershed Assessment for River Stability and Sediment Supply (WARSSS); Wildland Hydrology: Fort Collins, CO, USA, 2006. [Google Scholar]
- Schwendel, A.C.; Death, R.G.; Fuller, I.C.; Joy, M.K. Linking disturbance and stream invertebrate communities: How best to measure bed stability. J. North Am. Benthol. Soc. 2011, 30, 11–24. [Google Scholar] [CrossRef]
- Rutherford, J.C.; Davies-Colley, R.J.; Quinn, J.; Stroud, M.J.; Cooper, A.B. Stream Shade: Towards a Restoration Strategy; National Institute of Water & Atmospheric Research Ltd.: Hamilton, New Zealand, 1997. [Google Scholar]
- Clews, E.; Vaughan, I.P.; Ormerod, S.J. Evaluating the effects of riparian restoration on a temperate river-system using standardized habitat survey. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, S96–S104. [Google Scholar] [CrossRef]
- Quinn, J.M.; Cooper, A.B.; Stroud, M.J.; Burrell, G.P. Shade effects on stream periphyton and invertebrates: An experiment in streamside channels. N. Zeal. J. Mar. Freshw. Res. 1997, 31, 665–683. [Google Scholar] [CrossRef]
- Collins, K.E.; Febria, C.M.; Warburton, H.J.; Devlin, H.S.; Hogsden, K.L.; Goeller, B.C.; McIntosh, A.R.; Harding, J.S. Evaluating practical macrophyte control tools on small agricultural waterways in Canterbury, New Zealand. N. Zeal. J. Mar. Freshw. Res. 2019, 53, 182–200. [Google Scholar] [CrossRef]
- Burrell, T.K.; O’Brien, J.M.; Graham, S.E.; Simon, K.S.; Harding, J.S.; McIntosh, A.R. Riparian shading mitigates stream eutrophication in agricultural catchments. Freshw. Sci. 2013, 33, 73–84. [Google Scholar] [CrossRef]
- Rixen, T.; Baum, A.; Sepryani, H.; Pohlmann, T.; Jose, C.; Samiaji, J. Dissolved oxygen and its response to eutrophication in a tropical black water river. J. Environ. Manag. 2010, 91, 1730–1737. [Google Scholar] [CrossRef]
- Jacobsen, D.; Rostgaard, S.; Vásconez, J.J. Are macroinvertebrates in high altitude streams affected by oxygen deficiency? Freshw. Biol. 2003, 48, 2025–2032. [Google Scholar] [CrossRef]
- Bray, J.P.; Kilroy, C.; Gerbeaux, P.; Burdon, F.J.; Harding, J.S. Ecological processes mediate the effects of the invasive bloom-forming diatom Didymosphenia geminata on stream algal and invertebrate assemblages. Hydrobiologia 2020, 847, 177–190. [Google Scholar] [CrossRef]
- Palmer, M.A.; Lettenmaier, D.P.; Poff, N.L.; Postel, S.L.; Richter, B.; Warner, R. Climate change and river ecosystems: Protection and adaptation options. Environ. Manag. 2009, 44, 1053–1068. [Google Scholar] [CrossRef]
- Thomas, S.M.; Griffiths, S.W.; Ormerod, S.J. Beyond cool: Adapting upland streams for climate change using riparian woodlands. Glob. Chang. Biol. 2016, 22, 310–324. [Google Scholar] [CrossRef]
- Salo, T.; Stamm, C.; Burdon, F.J.; Räsänen, K.; Seppälä, O. Resilience to heat waves in the aquatic snail Lymnaea stagnalis: Additive and interactive effects with micropollutants. Freshw. Biol. 2017, 62, 1831–1846. [Google Scholar] [CrossRef]
- Broadmeadow, S.B.; Jones, J.G.; Langford, T.E.L.; Shaw, P.J.; Nisbet, T.R. The influence of riparian shade on lowland stream water temperatures in southern England and their viability for brown trout. River Res. Appl. 2011, 27, 226–237. [Google Scholar] [CrossRef]
- Battin, J.; Wiley, M.W.; Ruckelshaus, M.H.; Palmer, R.N.; Korb, E.; Bartz, K.K.; Imaki, H. Projected impacts of climate change on salmon habitat restoration. Proc. Natl. Acad. Sci. USA 2007, 104, 6720. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K.; Almlöf, K. Adapting boreal streams to climate change: Effects of riparian vegetation on water temperature and biological assemblages. Freshw. Sci. 2016, 35, 984–997. [Google Scholar] [CrossRef]
- Kristensen, P.B.; Kristensen, E.A.; Riis, T.; Baisner, A.J.; Larsen, S.E.; Verdonschot, P.F.M.; Baattrup-Pedersen, A. Riparian forest as a management tool for moderating future thermal conditions of lowland temperate streams. Hydrol. Earth Syst. Sci. Discuss. 2013, 10, 6081–6106. [Google Scholar] [CrossRef]
- Göthe, E.; Degerman, E.; Sandin, L.; Segersten, J.; Tamario, C.; Mckie, B.G. Flow restoration and the impacts of multiple stressors on fish communities in regulated rivers. J. Appl. Ecol. 2019, 56, 1687–1702. [Google Scholar] [CrossRef]
- Niyogi, D.K.; Koren, M.; Arbuckle, C.J.; Townsend, C.R. Stream communities along a catchment land-use gradient: Subsidy-stress responses to pastoral development. Environ. Manag. 2007, 39, 213–225. [Google Scholar] [CrossRef]
- Odum, E.P.; Finn, J.T.; Eldon, H.F. Perturbation theory and the subsidy-stress gradient. BioScience 1979, 29, 349–352. [Google Scholar] [CrossRef]
- Jacobsen, D.; Friberg, N. Macroinvertebrate Communities in Danish Streams—The Effect of Riparian Forest Cover. In Freshwater Biology: Priorities and Development in Danish Research; Sand-Jensen, K., Pedersen, O., Eds.; Gad: Copenhagen, Denmark, 1997; pp. 208–222. [Google Scholar]
- Stutter, M.I.; Chardon, W.J.; Kronvang, B. Riparian buffer strips as a multifunctional management tool in agricultural landscapes: Introduction. J. Environ. Qual. 2012, 41, 297–303. [Google Scholar] [CrossRef]
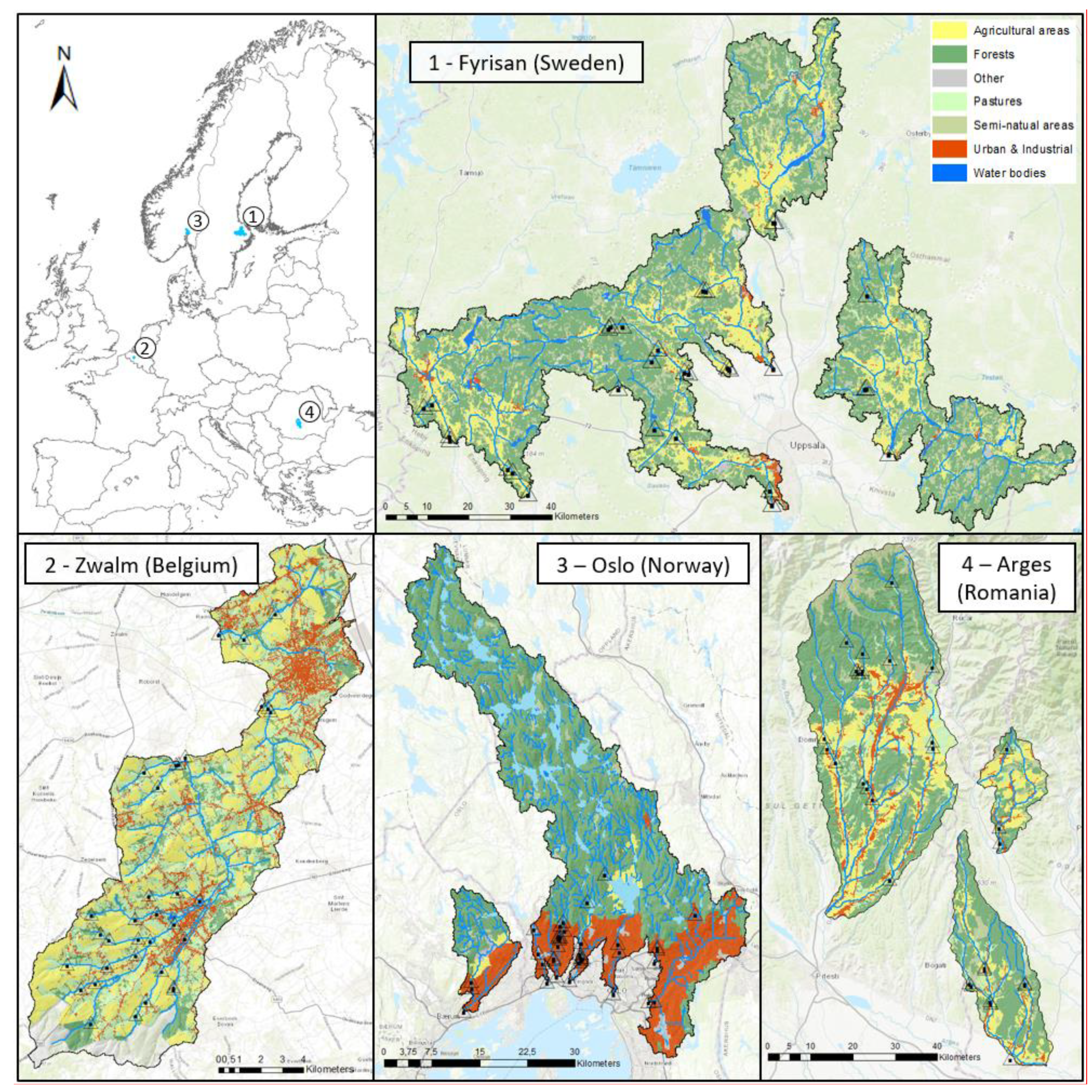

| Term | Definition |
|---|---|
| BiodivERsA | BiodivERsA is a network of national and regional funding organizations promoting pan-European research on biodiversity and ecosystem services, funded under the Horizon 2020 European Research Area (ERA-NET) COFUND scheme. |
| CROSSLINK | The full title of the CROSSLINK project is “Understanding cross-habitat linkages between blue and green infrastructure to optimize management of biodiversity, ecosystem services and multiple human uses.” The CROSSLINK project is funded under the 2015 pan-European BiodivErSA call for international research projects on “Promoting synergies and reducing trade-offs between food supply, biodiversity and ecosystem services.” Specifically, CROSSLINK addresses the theme “Understanding and managing biodiversity dynamics in land-, river- and seascapes (habitat connectivity, green and blue infrastructures, and naturing cities) to improve ecosystem functioning and delivery of ecosystem services.” |
| Blue-green infrastructure (BGI) | The concept of blue-green infrastructure emphasizes the importance of both “blue” (water) and “green” (vegetation) and the interaction between them [44]. The word infrastructure underscores the need for these different elements to be interlinked to work as a connected web of measures [45]. Elements of BGI are nature-based solutions that deliver multiple co-benefits to impacted environments such as urban (“grey”) cityscapes; benefits include water supply, flood mitigation, terrestrial biodiversity, cooling and climate change resilience, and human well-being [46,47,48]. |
| Nature-based solution (NBS) | Nature-based solutions are “living solutions inspired and supported by nature that simultaneously provide environmental, social and economic benefits and help build resilience” [21]. BGI can be NBS by providing natural ways to manage water resources and the environment [21]. The economic benefits of NBS have been promoted by the European Commission, as well as advocated by researchers [49]. |
| Riparian buffer | A riparian buffer is a vegetated area (a “buffer strip”) that helps to protect the stream from the impact of adjacent land uses [26]. A forested riparian buffer is a buffer strip dominated by woody vegetation, which in addition to helping protect the stream from human land-use impacts can also provide stream shading and crucial habitat diversity in fragmented landscapes. |
| Water Framework Directive (WFD) | The European Water Framework Directive (2000/60/EC; WFD) is a policy statement that establishes a framework for water protection so that all waterbodies in Europe reach “good ecological status” by 2021 or 2027. |
| Group | Response | Description |
|---|---|---|
| Environmental (Protocol S2) | Water quality | Grab water samples and spot measurements for a wide range of water chemistry parameters |
| Thermal dynamics | Spot measurements and continuous logging of stream and riparian temperatures | |
| Instream habitat | Transect measurements of channel profiles and benthic habitat assessment | |
| Hydromorphological impacts | Assessment of human activities affecting hydrogeomorphic integrity | |
| Riparian habitat | Assessment of riparian condition and measurement of key habitat properties in six 50 m2 plots (Figure S3, Supplementary Materials) | |
| Land use | Use of CORINE land cover inventory to describe catchment land uses | |
| Biodiversity (Protocol S3) | Microbial | Environmental samples for microbial (e.g., bacterial) diversity from stream and riparian zone in effective sampling reach (ESR) |
| Diatoms | Semi-quantitative sampling of benthic diatoms in ESR | |
| Macroinvertebrates | Quantitative sampling of aquatic macroinvertebrates in ESR | |
| Riparian invertebrates | Semi-quantitative sampling of terrestrial arachnids and predatory ground beetles in riparian plots | |
| Trees | Recording trees species and size (DBH) in riparian plots | |
| Ecosystem functions (Protocol S4) | Algal accrual | Measurement of periphyton biomass on standardized substrates in ESR |
| Sediment dynamics | Measurement of near-bed organic and inorganic particulate accrual on standardized substrates in ESR | |
| Organic-matter processing | Measuring stream and riparian organic-matter decomposition rates using litter bags and the cotton-strip assay | |
| Carbon sequestration | Using allometric scaling relationships to estimate tree biomass and carbon sequestration potential in riparian plots | |
| Food webs (Protocol S5) | Trophic diversity | Use of stable isotopes (C and N) to describe community trophic niche breadths |
| Energy flow | Using Bayesian mixing models to estimate consumer diets based on stable isotope measurements of basal resources and prey | |
| Trophic connectivity | Use of fatty acid biomarkers (e.g., poly-unsaturated FAs) to describe trophic connectivity between stream and riparian food-web compartments | |
| Societal needs (Protocol S6) | Optimization framework | Applying collected data as objective functions in an optimization framework to balance land-user needs with biodiversity and ecosystem benefits of forested riparian buffers |
| Attributes | Score 1 | Score 2 | Score 3 | Score 4 | Score 5 | ||
|---|---|---|---|---|---|---|---|
| Shading of water | Little or no shading | 10%–25% shading | 25%–50% | 50%–80% | >80% | ||
| Buffer width | <1 m | 1–5 m | 5–15 m | 15–30 m | >30 m | ||
| Buffer intactness | Buffer absent | 50%–99% gaps | 20%–50% gaps | 1%–20% gaps | Completely intact | ||
| Vegetation comp. of buffer and/or adjacent land to 30 m from streambank | Buffer | Short grazed pasture grasses to stream edge, or impervious surfaces | Weedy shrubs or mainly long grasses 0.3–2 m or herbs/forbs | Deciduous tree dominated; small tree dom. (2–5 m); or forest plantation with < 25% cover of > 5 m trees; or natural grassy veg. | Regenerating forest or woodlot evergreens with > 25% cover sub-canopy (>5 m) trees but < 10% canopy trees (>12 m); or natural grassy veg. | Maturing forest including >10% cover canopy trees (>12 m); or natural wetland or natural grassy vegetation | |
| Adj. land | |||||||
| Bank stability | Very low: uncohesive sediments and few roots and > 40% recently eroded | Low: uncohesive sediments and few roots/low veg. cover and > 15%–40% recently eroded | Moderate: stabilized by geology (e.g., cobbles), veg. cover and/or roots and > 5%–15% recently eroded | High: stabilized by geology (e.g., bedrock), veg. cover and/or roots; and 1%–5% recently eroded | Very high: stabilized by geology (e.g., bedrock), veg. cover and/or roots; < 1% recently eroded | ||
| Livestock access | High: unfenced and unmanaged with active livestock use | Moderate: some livestock access | Limited: unfenced but low stocking, bridges, troughs, natural deterrents | Very limited: temporary fencing of all livestock or naturally very limited access | None: permanent fencing or no livestock | ||
| Riparian soil denitrification potential | Soils dry/firm underfoot or moist–wet but frequent tile drains bypass riparian soils (≥3 per 100 m) | 1%–30% streambank soils moist but firm or moist-wet with infrequent bypass drains (1–2 per 100 m) | ≥30% streambank soils moist but firm underfoot. No drains. | 1%–30% streambank soils water-logged, soft underfoot with black soil. No drains. | ≥30% of streambanks water-logged, surface moist/fluid underfoot. No drains. | ||
| Land slope 0–30 m from stream bank | >35° | >20–35° | >10–20° | >5–10° | 0–5° | ||
| Groundcover of buffer and/or adjacent land to 30 m from streambank | Buffer | Bare | Short/regularly grazed pasture (<3 cm) | Pasture grasses or crops with bare flow paths or 2–3 cm tree litter layer | Moderate density grass or crops dense (>3 cm) tree litter layer | High density long grasses or crops | |
| Adj. land | |||||||
| Soil drainage | Impervious (e.g., sealed) or extensively pugged and/or compacted soil | Low permeability (e.g., high clay content) or moderately pugged/compacted soil | Low-moderate permeability (e.g., silt/loam) and not pugged/compacted | Mod-high permeability (e.g., sandy loam) and not pugged/compacted | Very high permeability (e.g., pumice/sand) and not pugged/compacted | ||
| Rills/channels | Frequent rills (>9 per 100 m) or larger channels carry most runoff | Common rills (4–9 per 100 m) or 1–2 larger channels carry some runoff | Infrequent rills (2–3 per 100 m) and no larger channels | Rare rills (1 per 100 m) and no larger channels | None | ||
| Response | Predictors | Estimates | CI | P | Marginal R2 | Conditional R2 |
|---|---|---|---|---|---|---|
| ASPT | (Intercept) | 0.997 | 0.497–1.498 | <0.001 | 0.278 | 0.887 |
| log (RCI) | 0.174 | 0.048–0.300 | 0.007 | |||
| PC1 | −0.267 | −0.365–−0.169 | <0.001 | |||
| ΔASPT | (Intercept) | 0.265 | −0.043–0.430 | 0.050 | 0.140 | 0.467 |
| ΔRCI | −0.675 | −1.387–0.206 | 0.093 | |||
| PC1 | −0.177 | −0.268–0.025 | 0.046 | |||
| ΔRCI × PC1 | 0.531 | −0.035–0.922 | 0.031 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burdon, F.J.; Ramberg, E.; Sargac, J.; Forio, M.A.E.; de Saeyer, N.; Mutinova, P.T.; Moe, T.F.; Pavelescu, M.O.; Dinu, V.; Cazacu, C.; et al. Assessing the Benefits of Forested Riparian Zones: A Qualitative Index of Riparian Integrity Is Positively Associated with Ecological Status in European Streams. Water 2020, 12, 1178. https://doi.org/10.3390/w12041178
Burdon FJ, Ramberg E, Sargac J, Forio MAE, de Saeyer N, Mutinova PT, Moe TF, Pavelescu MO, Dinu V, Cazacu C, et al. Assessing the Benefits of Forested Riparian Zones: A Qualitative Index of Riparian Integrity Is Positively Associated with Ecological Status in European Streams. Water. 2020; 12(4):1178. https://doi.org/10.3390/w12041178
Chicago/Turabian StyleBurdon, Francis J., Ellinor Ramberg, Jasmina Sargac, Marie Anne Eurie Forio, Nancy de Saeyer, Petra Thea Mutinova, Therese Fosholt Moe, Mihaela Oprina Pavelescu, Valentin Dinu, Constantin Cazacu, and et al. 2020. "Assessing the Benefits of Forested Riparian Zones: A Qualitative Index of Riparian Integrity Is Positively Associated with Ecological Status in European Streams" Water 12, no. 4: 1178. https://doi.org/10.3390/w12041178
APA StyleBurdon, F. J., Ramberg, E., Sargac, J., Forio, M. A. E., de Saeyer, N., Mutinova, P. T., Moe, T. F., Pavelescu, M. O., Dinu, V., Cazacu, C., Witing, F., Kupilas, B., Grandin, U., Volk, M., Rîşnoveanu, G., Goethals, P., Friberg, N., Johnson, R. K., & McKie, B. G. (2020). Assessing the Benefits of Forested Riparian Zones: A Qualitative Index of Riparian Integrity Is Positively Associated with Ecological Status in European Streams. Water, 12(4), 1178. https://doi.org/10.3390/w12041178












