A Pilot Study Combining Ultrafiltration with Ozonation for the Treatment of Secondary Urban Wastewater: Organic Micropollutants, Microbial Load and Biological Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Secondary Effluent and Treated Samples
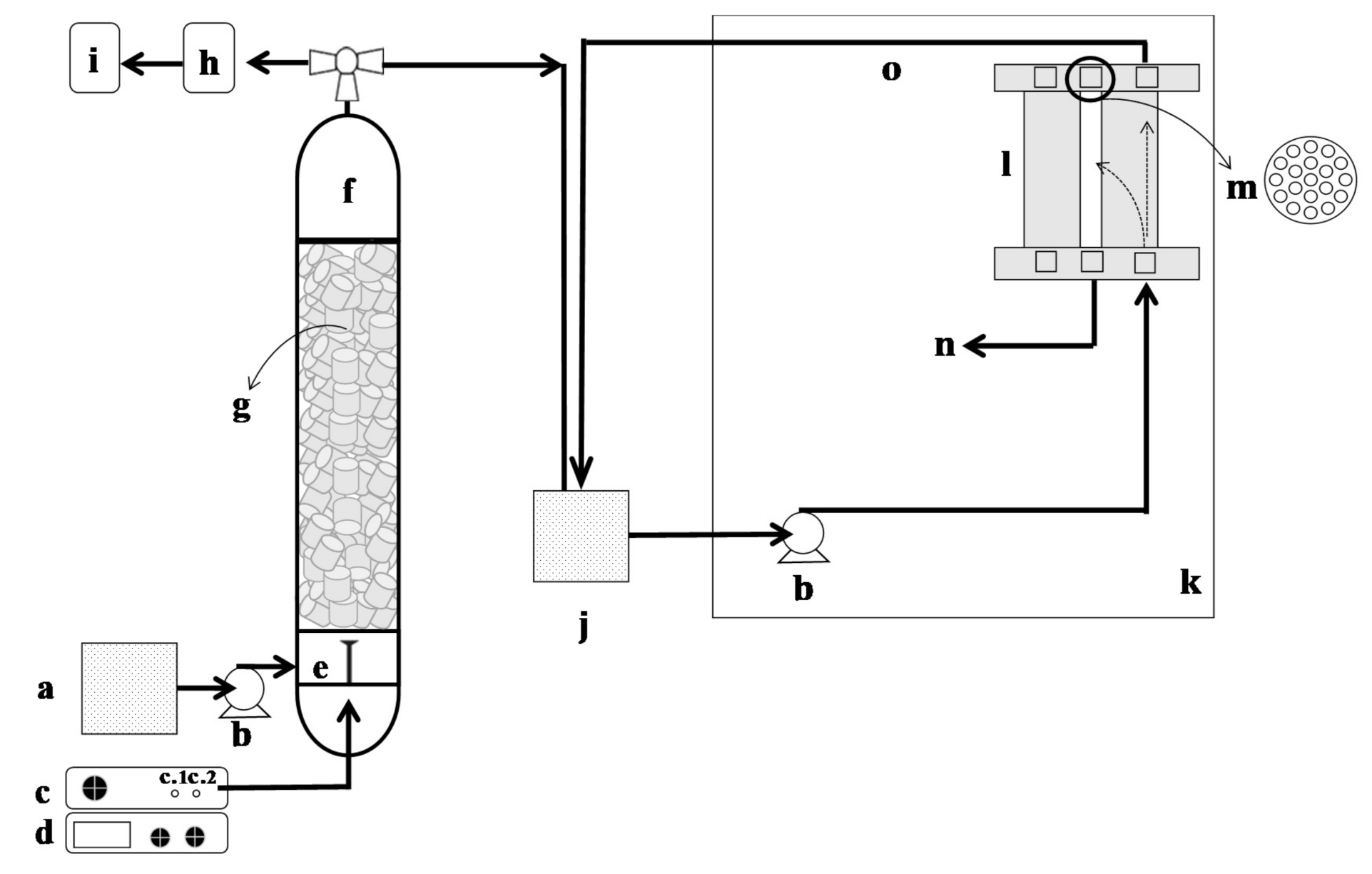
2.3. Experimental Setup and Procedure
2.4. Chemical Analyses
2.5. Microbial Culture Analyses
2.6. DNA Extraction, 16S rRNA and Inti1 Genes Quantification
2.7. Biological Effect Assays
2.7.1. Cell Culture and Incubation with Water Samples
2.7.2. Thiazolyl Blue Tetrazolium Reduction (MTT) and Lactate Dehydrogenase (LDH) Assays
2.7.3. Yeast Estrogen Screen (YES) Assay for Estrogenic Activity Assessment
3. Results and Discussion
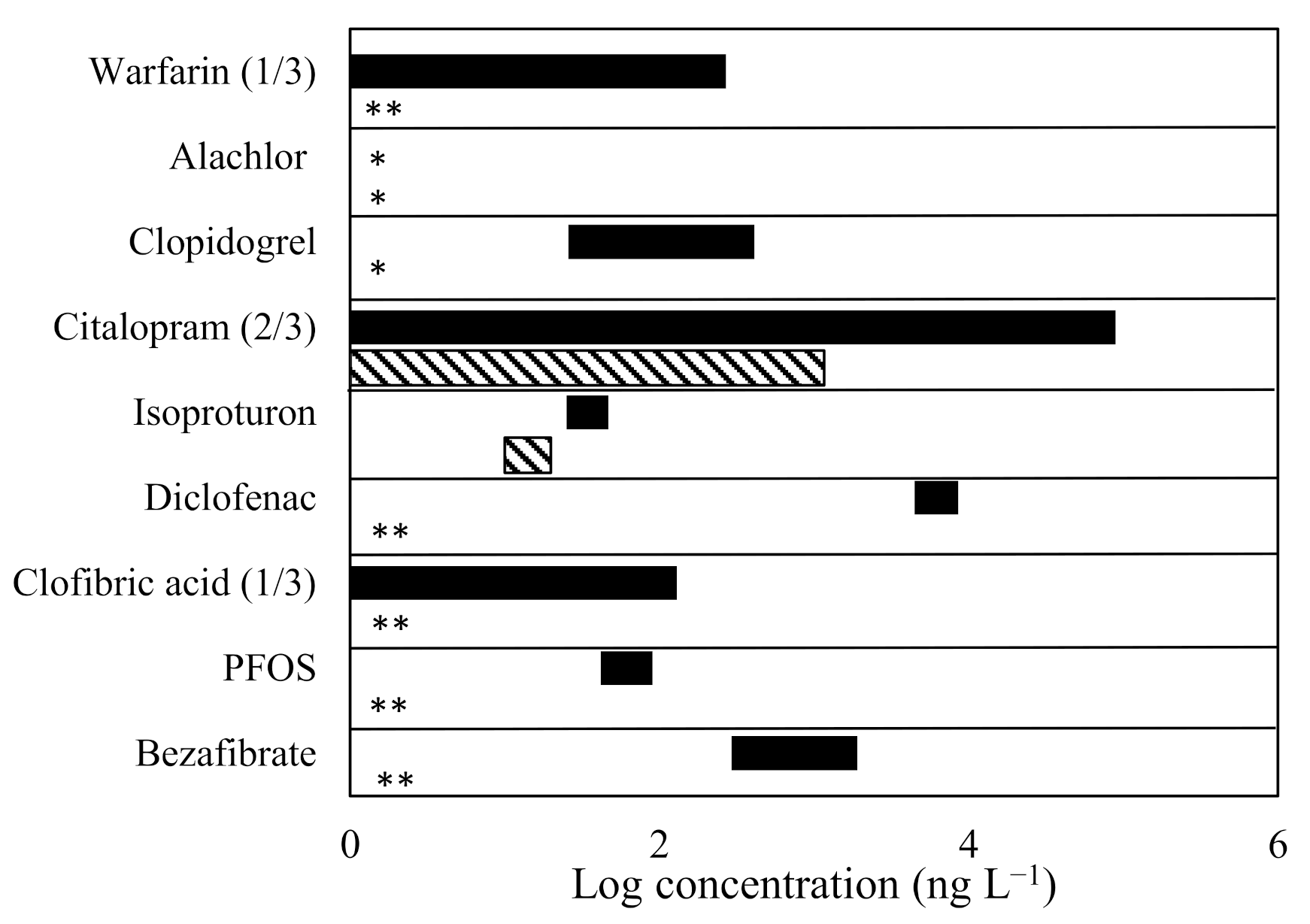
3.1. Micropollutant Removal, Mineralisation, and Other Physico-Chemical Parameters
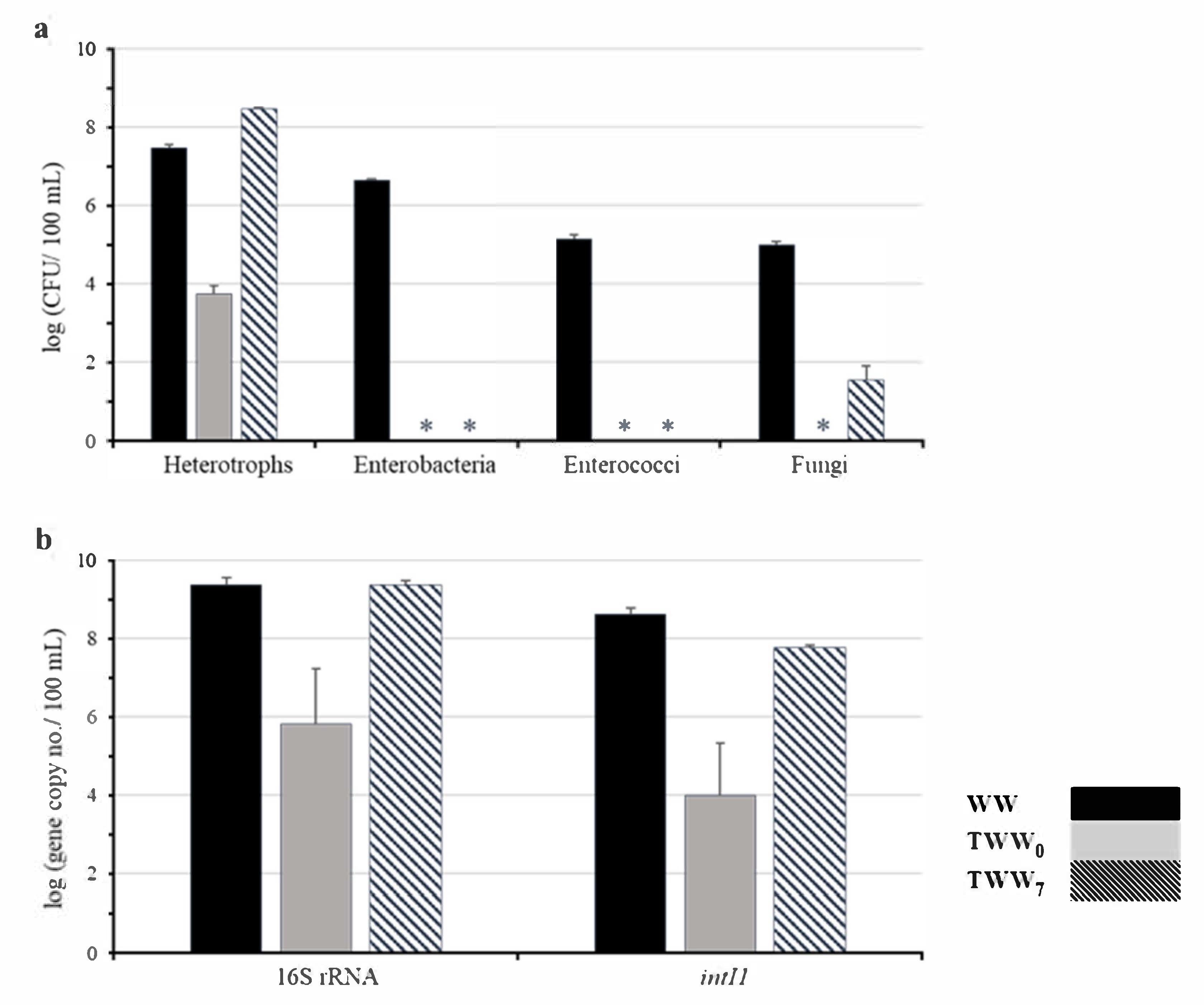
3.2. Microbial Inactivation and Regrowth
3.3. Evaluation of Biological Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development, 21 October 2015, A/RES/70/1. Available online: https://www.refworld.org/docid/57b6e3e44.html (accessed on 22 April 2020).
- Jimenez, B.; Asano, T. Water Reuse: An International Survey of Current Practice, Issues and Needs (Scientific and Technical Report); IWA Publishing: London, UK, 2018. [Google Scholar]
- Bixio, D.; De Heyder, B.; Cicurel, H.; Muston, M.; Miska, V.; Joksimovic, D.; Schäfer, A.I.; Ravazzini, A.; Aharoni, A.; Savic, D.; et al. Municipal wastewater reclamation: Where do we stand? An overview of treatment technology and management practice. Water Sci. Tech. W. Sup. 2005, 5, 77–85. [Google Scholar] [CrossRef]
- Pedrero, F.; Kalavrouziotis, I.; Alarcón, J.J.; Koukoulakis, P.; Asano, T. Use of treated municipal wastewater in irrigated agriculture-Review of some practices in Spain and Greece. Agric. Water Manag. 2010, 97, 1233–1241. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev. 2014, 38, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on minimum requirements for water reuse. Off. J. Eur. Union L 2020, 177, 32–55. [Google Scholar]
- Michael-Kordatou, I.; Karaolia, P.; Fatta-Kassinos, D. The role of operating parameters and oxidative damage mechanisms of advanced chemical oxidation processes in the combat against antibiotic-resistant bacteria and resistance genes present in urban. Water Res. 2018, 129, 208–230. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2016, 33, 49–90. [Google Scholar] [CrossRef]
- Von Gunten, U. Oxidation processes in water treatment: Are we on track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part, I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sousa, J.M.; Macedo, G.; Ribeiro, A.R.; Barreiros, L.; Pedrosa, M.; Faria, J.L.; Pereira, M.F.R.; Castro-Silva, S.; Segundo, M.A.; et al. Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: Micropollutants, antibiotic resistance genes and estrogenic activity. Water Res. 2016, 94, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254 nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T. Continuous ozonation of urban wastewater: Removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hembach, N.; Alexander, J.; Hiller, C.; Wieland, A.; Schwartz, T. Dissemination prevention of antibiotic resistant and facultative pathogenic bacteria by ultrafiltration and ozone treatment at an urban wastewater treatment plant. Sci. Rep. 2019, 9, 12843. [Google Scholar] [CrossRef] [Green Version]
- Czekalski, N.; Imminger, S.; Salhi, E.; Veljkovic, M.; Kleffel, K.; Drissner, D.; Von Gunten, U. Inactivation of antibiotic resistant bacteria and resistance genes by ozone: From laboratory experiments to full-scale wastewater treatment. Environ. Sci. Technol. 2016, 50, 11862–11871. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Sanchez Perez, J.A.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Tot. Env. 2020, 710, 136312. [Google Scholar] [CrossRef]
- EUR-Lex. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union L 2013, 226, 1–17. [Google Scholar]
- EU Decision 495/2015. Commission implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union L 2015, 78, 40–42. [Google Scholar]
- Spencer, P.; Domingos, S.; Edwards, B.; Howes, D.; Shorney-Darby, H.; Scheerman, H.; Milton, G.; . Clement, J. Ozone enhanced ceramic membrane filtration for wastewater recycling. Water Pract. Technol 2019, 14, 331–340. [Google Scholar] [CrossRef]
- Si, X.; Hu, Z.; Huang, S. Combined process of ozone oxidation and ultrafiltration as an effective treatment technology for the removal of endocrine-disrupting chemicals. Appl. Sci. 2018, 8, 1240. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Park, M.; Liang, H.; Wu, S.; Lopez, I.J.; Ji, W.; Li, G.; Snyder, S.A. Reducing ultrafiltration membrane fouling during potable water reuse using pre-ozonation. Water Res. 2017, 125, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; Rodriguez, E. Elimination of selected emerging contaminants by the combination of membrane filtration and chemical oxidation processes. Water Air Soil Pollut. 2015, 226, 139. [Google Scholar] [CrossRef]
- FAOLEX. Decree-Law 236/98 Establishing Water Quality Standards; Republic Diary No. 176/1998, Series I-A of 1998-08-01; Portuguese Presidency of the Council of Ministers: Lisboa, Portugal, 1998; pp. 3676–3716.
- FAOLEX. Decree-Law No. 119/2019 Establishing the Legal Scheme of the Production of Water for Reuse; Republic Diary No. 159/2019, Series I of 2019-08-21; Portuguese Presidency of the Council of Ministers: Lisboa, Portugal, 2019; pp. 21–44.
- WHO. A Compendium for Standards for Wastewater Reuse in the Eastern Mediterranean Region; World Health Organisation (WHO): Cairo, Egypt, 2006. [Google Scholar]
- Becerra-Castro, C.; Rita, A.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater reuse in irrigation: A microbiological perspective on implications in soil fertility and human and environmental health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef] [Green Version]
- Graça, C.A.L.; Lima, R.B.; Pereira, M.F.R.; Silva, A.M.T.; Ferreira, A. Intensification of the ozone-water mass transfer in an oscillatory flow reactor with innovative design of periodic constrictions: Optimization and application in ozonation water treatment. Chem. Eng. J. 2020, 389, 124412. [Google Scholar]
- Marchese, J.; Ochoa, N.A.; Pagliero, C.; Almandoz, C. Pilot-scale ultrafiltration of an emulsified oil wastewater. Environ. Sci. Technol. 2000, 34, 2990–2996. [Google Scholar] [CrossRef]
- Mansas, C.; Mendret, J.; Brosillon, S.; Ayral, A. Coupling catalytic ozonation and membrane separation: A review. Sep. Purif. Technol. 2020, 236, 1161221. [Google Scholar] [CrossRef]
- You, S.-H.; Tseng, D.-H.; Hsu, W.-C. Effect and mechanism of ultrafiltration membrane fouling removal by ozonation. Desalination 2007, 202, 224–230. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Barbosa, M.O.; Ribeiro, A.R.; Ratola, N.; Hain, E.; Homem, V.; Pereira, M.F.R.; Blaney, L.; Silva, A.M.T. Spatial and seasonal occurrence of micropollutants in four Portuguese rivers and a case study for fluorescence excitation-emission matrices. Sci. Total Environ. 2018, 644, 1128–1140. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Pedrosa, M.; Moreira, N.F.F.; Pereira, M.F.R.; Silva, A.M.T. Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU. J. Chromatogr. A 2015, 1418, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Manaia, C.M. Cell-based internal standard for qPCR determinations of antibiotic resistance indicators in environmental water samples. Ecol. Indic. 2020, 113, 106194. [Google Scholar] [CrossRef]
- Narciso-da-Rocha, C.; Rocha, J.; Vaz-Moreira, I.; Lira, F.; Tamames, J.; Henriques, J.L.; Manaia, C.M. Bacterial lineages putatively associated with the dissemination of antibiotic resistance genes in a full-scale urban wastewater treatment plant. Environ. Int. 2018, 118, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, K.M.M.; Narciso-da-Rocha, C.; Kneis, D.; Berendonk, T.U.; Cacace, D.; Do, T.T.; Elpers, C.; Fatta-Kassinos, D.; Henriques, I.; Jaeger, T.; et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci. Adv. 2019, 5, eaau9124. [Google Scholar]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Barraud, O.; Baclet, M.C.; Denis, F.; Ploy, M.C. Quantitative multiplex real-time PCR for detecting class 1, 2 and 3 integrons. J. Antimicrob. Chemother. 2010, 65, 1642–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brankatschk, R.; Bodenhausen, N.; Zeyer, J.; Bürgmann, H. Simple absolute quantification method correcting for quantitative PCR efficiency variations for microbial community samples. Appl. Environ. Microbiol. 2012, 78, 4481–4489. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.; Cacace, D.; Kampouris, I.; Guilloteau, H.; Jäger, T.; Marano, R.B.M.; Karaolia, P.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; et al. Inter-laboratory calibration of quantitative analyses of antibiotic resistance genes. J. Environ. Chem. Eng. 2018, 8, 102214. [Google Scholar] [CrossRef]
- Ferreira, M.; Chaves, L.L.; Lima, S.A.C.; Reis, S. Optimization of nanostructured lipid carriers loaded with methotrexate: A tool for inflammatory and cancer therapy. Int. J. Pharm. 2015, 492, 65–72. [Google Scholar] [CrossRef]
- Dorais, M.; Alsanius, B.W.; Voogt, W.; Pepin, S.; Tüzel, H.; Tüzel, Y.; Möller, K. Impact of Water Quality and Irrigation Management on Organic Greenhouse Horticulture; BioGreenhouse COST Action FA1105: Bleiswijk, Netherlands, 2020; ISBN 978-94-6257-538-7. [Google Scholar]
- Water Salinity and Plant Irrigation. Available online: https://www.agric.wa.gov.au/water-management/water-salinity-and-plant-irrigation (accessed on 8 October 2020).
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Removal of Ammonia from Water by Ozone Microbubbles. Ind. Eng. Chem. Res. 2013, 52, 318–326. [Google Scholar] [CrossRef]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Lucheta, A.R.; Lambais, M.R. Sulfur in agriculture. Rev. Bras. Ciênc. Solo 2012, 36, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Mousel, D.; Palmowski, L.; Pinnekamp, J. Energy demand for elimination of organic micropollutants in municipal wastewater treatment plants. Sci. Total Environ. 2017, 575, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Zhang, W.; Xiong, W.; Ye, Q.; Hou, X.; Wang, C.; Wang, P. Life cycle assessment of advanced wastewater treatment processes: Involving 126 pharmaceuticals and personal care products in life cycle inventory. J. Environ. Manag. 2019, 238, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Arzate, S.; Pfister, S.; Oberschelp, C.; Sánchez-Pérez, J.A. Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci. Total Environ. 2019, 694, 133572. [Google Scholar] [CrossRef] [PubMed]
- Pesqueira, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T.S. Environmental impact assessment of advanced urban wastewater treatment technologies for the removal of priority substances and contaminants of emerging concern: A review. J. Clean. Prod. 2020, 261, 121078. [Google Scholar] [CrossRef]
- Spuhler, D.; Andrés Rengifo-Herrera, J.; Pulgarin, C. The effect of Fe2+, Fe3+, H2O2 and the photo-Fenton reagent at near neutral pH on the solar disinfection (SODIS) at low temperatures of water containing Escherichia coli K12. Appl. Catal. B Environ. 2010, 96, 126–141. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.-Y.; Yu, T.; Su, C.; Jiang, H.; Liu, S. Effect of different molecular weight organic components on the increase of microbial growth potential of secondary effluent by ozonation. J. Environ. Sci. 2014, 26, 2190–2197. [Google Scholar] [CrossRef]
- Giannakis, S.; Merino Gamo, A.I.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Monitoring the post-irradiation E. coli survival patterns in environmental water matrices: Implications in handling solar disinfected wastewater. Chem. Eng. J. 2014, 253, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Ubomba-Jaswa, E.; Navntoft, C.; Polo-López, M.I.; Fernandez-Ibáñez, P.; McGuigan, K.G. Solar disinfection of drinking water (SODIS): An investigation of the effect of UV-A dose on inactivation efficiency. Photochem. Photobiol. Sci. 2009, 8, 587–595. [Google Scholar] [CrossRef]
- Clancy, S. DNA damage & repair: Mechanisms for maintaining DNA integrity. Nat. Educ. 2008, 1, 103. [Google Scholar]
- EPA. Guidelines for Water Reuse; Environmental Protection Agency (EPA): Wasghinton, DC, USA, 2012; (EPA/600/R-12/618).
- Alcalde-Sanz, L.; Gawlik, B.M. Minimum Quality Requirements for Water Reuse in Agricultural Irrigation and Aquifer Recharge—Towards A Water Reuse Regulatory Instrument at EU Level, EUR 28962 EN; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Trintinaglia, L.; Bianchi, E.; Silva, L.; Nascimento, C.; Spilki, F.; Ziulkoski, A. Cytotoxicity assays as tools to assess water quality in the Sinos River basin. Braz. J. Biol. 2015, 75, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yuan, Y.X.; Wang, Y.F.; Li, C.; Zhu, J.; Li, R.F.; Wu, Y.H. Comprehensive evaluation on the bio-toxicity of three advanced wastewater treatment processes. Water Air Soil Pollut. 2020, 231, 110. [Google Scholar] [CrossRef]
- Nahim-Granados, S.; Rivas-Ibanez, G.; Perez, J.A.S.; Oller, I.; Malato, S.; Polo-Lopez, M.I. Synthetic fresh-cut wastewater disinfection and decontamination by ozonation at pilot scale. Water Res. 2020, 170, 115304. [Google Scholar] [CrossRef] [PubMed]
- Affek, K.; Muszynski, A.; Zaleska-Radziwill, M.; Doskocz, N.; Zietkowska, A.; Widomski, M. Evaluation of ecotoxicity and inactivation of bacteria during ozonation of treated wastewater. Desalin. Water Treat. 2020, 192, 176–184. [Google Scholar] [CrossRef]
- Pohl, J.; Ahrens, L.; Carlsson, G.; Golovko, O.; Norrgren, L.; Weiss, J.; Orn, S. Embryotoxicity of ozonated diclofenac, carbamazepine, and oxazepam in zebrafish (Danio rerio). Chemosphere 2019, 225, 191–199. [Google Scholar] [CrossRef]
| Decree-Law | WHO | ||||
|---|---|---|---|---|---|
| Parameters | UWWTP Secondary Effluent (WW) | After O3 + UF Treatment (TWW0) | 236/98 [27] MVR | 119/2019 [28] PV | 2016 [29] MVR |
| Al (mg/L) | 9.55 × 10−5 | 6.10 × 10−5 | 5.0 | 5 | 5.0 |
| As (mg/L) | 1.12 × 10−5 | <5 × 10−6 | 0.1 | n.a | 0.1 |
| Ba (mg/L) | 4.25 × 10−5 | 1.52 × 10−5 | 1.0 | n.a | n.a |
| Be (mg/L) | <5 × 10−6 | <5 × 10−6 | 0.5 | 0.1 | 0.1 |
| B (mg/L) | 1.29 × 10−4 | 1.06 × 10−4 | 0.3 | variable | n.a |
| Cd (mg/L) | <5 × 10−6 | <5 × 10−6 | 0.01 | n.a | 0.1 |
| Pb (mg/L) | 6.73 × 10−6 | 6.55 × 10−6 | 5.0 | n.a | 5.0 |
| Cl- (mg/L) | 80.8 | 79.5 | 70 | n.a | 142 b |
| Co (mg/L) | <5 × 10−6 | <5 × 10−6 | 0.05 | 0.05 | 0.05 |
| Cu (mg/L) | 1.26 × 10−5 | 6.28 × 10−5 | 0.2 | n.a | 0.2 |
| Total Cr (mg/L) | <5 × 10−6 | <5 × 10−6 | 0.1 | n.a | 0.1 |
| Sn (mg/L) | <5 × 10−6 | <5 × 10−6 | 2.0 | n.a | n.a |
| Fe (mg/L) | 1.06 × 10−4 | 2.36 × 10−5 | 5.0 | 2.0 | 5.0 |
| F− (mg/L) | <DL | <DL | 1.0 | 2.0 | 1.0 |
| Li (mg/L) | 1.98 × 10−5 | 1.96 × 10−5 | 2.5 | 2.5 | 2.5 |
| Mn (mg/L) | 4.56 × 10−5 | 3.77 × 10−5 | 0.2 | 0.2 | 0.2 |
| Mo (mg/L) | 2.45 × 10−5 | 8.60 × 10−5 | 0.005 | 0.01 | 0.01 |
| Ni (mg/L) | <5 × 10−6 | <5 × 10−6 | 0.5 | n.a | 0.2 |
| NO3− (mg/L) | 0.9 ± 0.4 | 7.70 | 50 | n.a | 9.5 b |
| Salinity (μS/cm) | 848 | 782 | 1000 | variable | 700 b |
| TDS (mg/L) | 335 | 191 | 640 | n.a | 450 b |
| SAR (meq/L) | 2.49 | 1.50 | 8 | variable | 3.0 |
| Se (mg/L) | <5 × 10−6 | <5 × 10−6 | 0.02 | 0.02 | 0.02 |
| TSS (mg/L) | 24.50 | 0.00 | 60 | ≤10 | 20 c |
| SO42− (mg/L) | 45.2 | 50.0 | 575 | n.a | n.a |
| V (mg/L) | <5 × 10−6 | <5 × 10−6 | 0.1 | n.a | 0.1 |
| Zn (mg/L) | 4.70 × 10−5 | 2.61 × 10−5 | 2 | n.a | 2.0 |
| pH | 7.0 ± 1.0 | 8.0 ± 0.2 | 6.5–8.4 | n.a | 6.5–8.4 |
| E. coli (log CFU/100 mL) | 6.67 | <DL | 2.0 | ≤10 | 2.3 c |
| Intestinal parasite eggs a | 0.00 | 0.00 | n.a | ≤1 | n.a |
| Becerra et al., 2015 [30] | Decree-Law 119/2019 [28] | ||||
|---|---|---|---|---|---|
| Additional Analyses | UWWTP Secondary Effluent (WW) | After O3 + UF Treatment (TWW0) | MVA | MVR | PV |
| Dissolved organic carbon (DOC, mg/L) | 11.0 ± 0.8 | 9.6 ± 0.8 | n.a. | n.a. | n.a. |
| Biological oxygen demand (BOD5, mg/L) | 15.1 ± 1.1 | 0 | 10 b | n.a | ≤10 c |
| Chemical oxygen demand (COD, mg/L) | 22.7 ± 0.7 | 5.4 ± 0.8 | 60–200 | n.a | n.a. |
| Turbidity (NTU) | 3.25 ± 0.15 | 0.28 ± 0.02 | 2 | n.a | ≤5 |
| NH4+ | <DL | 0.59 | n.a | n.a | 10 |
| PO43− | <DL | <DL | n.a | n.a | n.a |
| Cell Line | MTT Assay | LDH Assay c | ||
|---|---|---|---|---|
| WW | TWW0 | WW | TWW0 | |
| L929 | 102 ± 13 | 112 ± 15 | 20.7 ± 2.0 (28.3 ± 3.2) | 19.7 ± 1.6 (32.6 ± 4.5) |
| Caco-2 | 116 ± 8 | 96 ± 9 | 58.6 ± 4.4 (59.1 ± 6.8) | 53.2 ± 7.7 (59.5 ± 5.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graça, C.A.L.; Ribeirinho-Soares, S.; Abreu-Silva, J.; Ramos, I.I.; Ribeiro, A.R.; Castro-Silva, S.M.; Segundo, M.A.; Manaia, C.M.; Nunes, O.C.; Silva, A.M.T. A Pilot Study Combining Ultrafiltration with Ozonation for the Treatment of Secondary Urban Wastewater: Organic Micropollutants, Microbial Load and Biological Effects. Water 2020, 12, 3458. https://doi.org/10.3390/w12123458
Graça CAL, Ribeirinho-Soares S, Abreu-Silva J, Ramos II, Ribeiro AR, Castro-Silva SM, Segundo MA, Manaia CM, Nunes OC, Silva AMT. A Pilot Study Combining Ultrafiltration with Ozonation for the Treatment of Secondary Urban Wastewater: Organic Micropollutants, Microbial Load and Biological Effects. Water. 2020; 12(12):3458. https://doi.org/10.3390/w12123458
Chicago/Turabian StyleGraça, Cátia A. L., Sara Ribeirinho-Soares, Joana Abreu-Silva, Inês I. Ramos, Ana R. Ribeiro, Sérgio M. Castro-Silva, Marcela A. Segundo, Célia M. Manaia, Olga C. Nunes, and Adrián M. T. Silva. 2020. "A Pilot Study Combining Ultrafiltration with Ozonation for the Treatment of Secondary Urban Wastewater: Organic Micropollutants, Microbial Load and Biological Effects" Water 12, no. 12: 3458. https://doi.org/10.3390/w12123458
APA StyleGraça, C. A. L., Ribeirinho-Soares, S., Abreu-Silva, J., Ramos, I. I., Ribeiro, A. R., Castro-Silva, S. M., Segundo, M. A., Manaia, C. M., Nunes, O. C., & Silva, A. M. T. (2020). A Pilot Study Combining Ultrafiltration with Ozonation for the Treatment of Secondary Urban Wastewater: Organic Micropollutants, Microbial Load and Biological Effects. Water, 12(12), 3458. https://doi.org/10.3390/w12123458











