Depression of Groundwater Table and Reduced Nitrogen Application Jointly Regulate the Bacterial Composition of nirS-Type and nirK-Type Genes in Agricultural Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site and Experimental Description
2.2. Soil Sampling and Physicochemical Properties Analysis
2.3. DNA Extraction, PCR Amplification and Illumina-Based Sequencing
2.4. Amplicon Sequencing and Sequencing Data Processing
2.5. Statistical Analyses
3. Results
3.1. Soil Environmental Parameters
3.2. Soil Bacterial Composition of nirS-Type and nirK-Type Genes
3.3. Influences of Depression of Groundwater Table, Reduced Nitrogen Application and Soil Environmental Parameters on the Bacterial Composition of nirS-Type and nirK-Type Genes
4. Discussion
4.1. Effects of Depression of Groundwater Table and Reduced Nitrogen Application on Soil Attributes
4.2. Effects of Altered Groundwater Table and Reduced Nitrogen Application on Bacterial Composition of Denitrifier Communities
4.3. Influences of Soil Attributes on Bacterial Composition of Denitrifier Communities
4.4. Research Limitation and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stockert, F.T.; Qin, D.; Plattner, G.K. Ipcc: The Physical Science Basis. Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Handa, I.T.; Rien, A.; Frank, B.; Matty, P.B.; Andreas, B.; Olaf, B.; Eric, C.; Mark, O.G.; Jérémy, J.; Marika, M.; et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koranda, M.; Kaiser, C.; Fuchslueger, L.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S.; Richter, A. Seasonal variation in functional properties of microbial communities in beech forest soil. Seas. Var. Funct. Prop. Microb. Communities Beech Soil 2013, 60, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, N.; Bouwman, A. Denitrification in agricultural soils: Summarizing published data and estimating global annual rates. Nutr. Cycl. Agroecosyst. 2005, 72, 267–278. [Google Scholar] [CrossRef]
- Wertz, S.; Goyer, C.; Zebarth, B.J.; Burton, D.L.; Tatti, E.; Chantigny, M.H.; Filion, M. Effects of temperatures near the freezing point on N2O emissions, denitrification and on the abundance and structure of nitrifying and denitrifying soil communities. FEMS Microbiol. Ecol. 2013, 83, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Cocco, E.; Bertora, C.; Squartini, A.; Delle Vedove, G.; Berti, A.; Grignani, C.; Lazzaro, B.; Morari, F. How shallow water table conditions affect N2O emissions and associated microbial abundances under different nitrogen fertilisations. Agric. Ecosyst. Environ. 2018, 261, 1–11. [Google Scholar] [CrossRef]
- Wiśniewski, P. Assessment of nitrous oxide emissions from agricultural soils at local level in Poland. Int. Agrophys. 2019, 33, 303–311. [Google Scholar] [CrossRef]
- Sun, R.; Guo, X.; Wang, D.; Chu, H. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. Sect. Agric. Ecosyst. Environ. 2015, 95, 171–178. [Google Scholar] [CrossRef]
- Hou, S.; Ai, C.; Zhou, W.; Liang, G.; He, P. Structure and assembly cues for rhizospheric nirk- and nirs-type denitrifier communities in long-term fertilized soils. Soil Biol. Biochem. 2018, 119, 32–40. [Google Scholar] [CrossRef]
- Zhang, W.-F.; Dou, Z.-X.; He, P.; Ju, X.-T.; Powlson, D.; Chadwick, D.; Norse, D.; Lu, Y.-L.; Zhang, Y.; Wu, L.; et al. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc. Natl. Acad. Sci. USA 2013, 110, 8375–8380. [Google Scholar] [CrossRef] [Green Version]
- Lasagna, M.; Luca, D.A.; Franchino, E. Nitrate contamination of groundwater in the western po plain (Italy): The effects of groundwater and surface water interactions. Environ. Earth Sci. 2016, 75, 1–16. [Google Scholar] [CrossRef]
- Han, H.; Chen, C.; Bai, M.; Xu, T.; Yang, H.; Shi, A.; Ding, G.-C.; Li, J. Abundance and diversity of denitrifying bacterial communities associated with N2O emission under long-term organic farming. Eur. J. Soil Biol. 2020, 97, 103153. [Google Scholar] [CrossRef]
- Sieczka, A.; Bujakowski, F.; Falkowski, T.; Koda, E. Morphogenesis of a floodplain as a criterion for assessing the susceptibility to water pollution in an agriculturally rich valley of a lowland river. Water 2018, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- Paladino, O.; Massabò, M.; Gandoglia, E. Assessment of nitrate hazards in Umbria region (Italy) using field datasets: Good agriculture practices and farms sustainability. Sustainability 2020, 12, 9497. [Google Scholar] [CrossRef]
- She, W.; Bai, Y.; Zhang, Y.; Qin, S.; Feng, W.; Sun, Y.; Zheng, J.; Wu, B. Resource availability drives responses of soil microbial communities to short-term precipitation and nitrogen addition in a desert shrubland. Front. Microbiol. 2018, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Tiehang, W.; Ashley, G.; Gan, L.; Hilary, K.; Bolanle Osi, E.; Doug, P.A. Groundwater depth overrides tree-species effects on the structure of soil microbial communities involved in nitrogen cycling in plantation forests. Forests 2020, 11, 275. [Google Scholar]
- Luo, S.; Yu, L.; Liu, Y.; Zhang, Y.; Yang, W.; Li, Z.; Wang, J. Effects of reduced nitrogen input on productivity and N2O emissions in a sugarcane/soybean intercropping system. Eur. J. Agron. 2016, 81, 78–85. [Google Scholar] [CrossRef]
- Chen, J.; Arafat, Y.; Wu, L.; Xiao, Z.; Li, Q.; Khan, M.A.; Khan, M.U.; Lin, S.; Lin, W. Shifts in soil microbial community, soil enzymes and crop yield under peanut/maize intercropping with reduced nitrogen levels. Appl. Soil Ecol. Sect. Agric. Ecosyst. Environ. 2018, 124, 327–334. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.A.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; Wang, G. Chronic effects of different fertilization regimes on nirs-type denitrifier communities across the black soil region of Northeast China. Pedosphere 2020, 30, 73–86. [Google Scholar] [CrossRef]
- Maeda, K.; Morioka, R.; Hanajima, D.; Osada, T. The impact of using mature compost on nitrous oxide emission and the denitrifier community in the cattle manure composting process. Microb. Ecol. 2010, 59, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, X.; Li, D.; Wang, H.; Chen, F.; Fu, X.; Fang, X.; Sun, X.; Yu, G. Impacts of nitrogen and phosphorus additions on the abundance and community structure of ammonia oxidizers and denitrifying bacteria in Chinese fir plantations. Soil Biol. Biochem. 2016, 103, 284–293. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Jiang, Y.; Hu, Y.; Zhang, M.; Zeng, Z. Response of bacteria harboring nirS and nirK genes to different N fertilization rates in an alkaline northern Chinese soil. Eur. J. Soil Biol. 2017, 82, 1–9. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Eloise, K. Groundwater exploitation and its impact on the environment in the North China Plain. Water Int. 2001, 26, 265–272. [Google Scholar]
- Zhang, D.; Cui, R.; Fu, B.; Yang, Y.; Wang, P.; Mao, Y.; Chen, A.; Lei, B. Shallow groundwater table fluctuations affect bacterial communities and nitrogen functional genes along the soil profile in a vegetable field. Appl. Soil Ecol. 2020, 146, 103368. [Google Scholar] [CrossRef]
- Xu, X.; Huang, G.; Pereira, L.S.; Ramos, T.B.; Huang, Q.; Hao, Y. Assessing the effects of water table depth on water use, soil salinity and wheat yield: Searching for a target depth for irrigated areas in the upper yellow river basin. Agric. Water Manag. 2013, 125, 46–60. [Google Scholar] [CrossRef]
- Abliz, A.; Tiyip, T.; Ghulam, A.; Halik, Ü.; Ding, J.-I.; Sawut, M.; Zhang, F.; Nurmemet, I.; Abliz, A. Effects of shallow groundwater table and salinity on soil salt dynamics in the Keriya Oasis, Northwestern China. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, M.; Liu, H.; Wang, R.; Zhao, J.; Yang, Y.; Cui, R.; Chen, A. Effects of shallow groundwater table fluctuations on nitrogen in the groundwater and soil profile in the nearshore vegetable fields of Erhai Lake, southwest China. J. Soils Sediments 2020, 20, 42–51. [Google Scholar] [CrossRef]
- Matzeu, A.; Secci, R.; Uras, G. Methodological approach to assessment of groundwater contamination risk in an agricultural area. Agric. Water Manag. 2017, 184, 46–58. [Google Scholar] [CrossRef]
- Soylu, M.E.; Kucharik, C.J.; Loheide, S.P. Influence of groundwater on plant water use and productivity: Development of an integrated ecosystem—Variably saturated soil water flow model. Agric. For. Meteorol. 2014, 189–190, 198–210. [Google Scholar] [CrossRef]
- Askri, B.; Ahmed, A.T.; Abichou, T.; Bouhlila, R. Effects of shallow water table, salinity and frequency of irrigation water on the date palm water use. J. Hydrol. 2014, 513, 81–90. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; He, N.; Ye, Z.; Chen, C.; Zang, R.; Feng, Y.; Lu, Q.; Li, J. Plant functional traits regulate soil bacterial diversity across temperate deserts. Sci. Total Environ. 2020, 715. [Google Scholar] [CrossRef] [PubMed]
- Weike, L.; Shukui, N.; Xiaodong, L.; Jianming, W. Short-term response of the soil bacterial community to differing wildfire severity in pinus tabulaeformis stands. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Coughlan, J.; Hooper, D.; Mullen, M. Structural equation modelling: Guidelines for determining model fit. Electron. J. Bus. Res. Methods 2008, 6, 53–60. [Google Scholar]
- Yves, R. Iavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.; Xi, B.; Yuan, Z.; Zhu, X.; Zhang, X. Effects of groundwater level variations on the nitrate content of groundwater: A case study in Luoyang area, China. Environ. Earth Sci. 2015, 74, 3969–3983. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, J.; Li, F. Effects of shallow groundwater table and fertilization level on soil physico-chemical properties, enzyme activities, and winter wheat yield. Agric. Water Manag. 2018, 208, 307–317. [Google Scholar] [CrossRef]
- Creze, C.M.; Madramootoo, C.A. Water table management and fertilizer application impacts on CO2, N2O and CH4 fluxes in a corn agro-ecosystem. Sci. Rep. 2019, 9, 2692. [Google Scholar] [CrossRef]
- Anuradha, M.; Sivaraju, K.; Krishnamurthy, V. Effect of waterlogging on physiological characteristics, yield and quality of flue-cured tobacco. Indian J. Plant Physiol. 2013, 18, 67–70. [Google Scholar] [CrossRef]
- Kumar, P.; Pal, M.; Joshi, R.; Sairam, R. Yield, growth and physiological responses of mung bean [Vigna radiata (l.) Wilczek] genotypes to waterlogging at vegetative stage. Physiol. Mol. Biol. Plants 2013, 19, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Trillo, N.; Fernández, R. Wheat plant hydraulic properties under prolonged experimental drought: Stronger decline in root-system conductance than in leaf area. Plant Soil 2005, 277, 277–284. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Li, J.; Schneider, R.; Lamberts, W. Growth dynamics of Chinese wingnut (Pterocarya stenoptera) seedlings and its effects on soil chemical properties under simulated water change in the Three Gorges Reservoir region of Yangtze River. Environ. Sci. Pollut. Res. 2013, 20, 7112–7123. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, I.; Fleige, H.; Horn, R. Longtime effects of deep groundwater extraction management on water table levels in surface aquifers. J. Soils Sediments 2017, 17, 133–143. [Google Scholar] [CrossRef]
- Ramos, T.B.; Simionesei, L.; Jauch, E.; Almeida, C.; Neves, R. Modelling soil water and maize growth dynamics influenced by shallow groundwater conditions in the Sorraia Valley region, Portugal. Agric. Water Manag. 2017, 185, 27–42. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Bailey, M.; Whiteley, A.S. The bacterial biogeography of British soils. Environ. Microbiol. 2011, 13, 1642–1654. [Google Scholar] [CrossRef]
- Hu, Y.; Xiang, D.; Veresoglou, S.D.; Chen, F.; Chen, Y.; Hao, Z.; Zhang, X.; Chen, B. Soil organic carbon and soil structure are driving microbial abundance and community composition across the arid and semi-arid grasslands in northern China. Soil Biol. Biochem. 2014, 77, 51–57. [Google Scholar] [CrossRef]
- Liu, Y.; Priscu, J.C.; Xiong, J.; Conrad, R.; Vick-Majors, T.; Chu, H.; Hou, J.; Wagner, D. Salinity drives archaeal distribution patterns in high altitude lake sediments on the Tibetan Plateau. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Li, L.; Li, J.; Feng, Y.; Lu, Q. The patterns and drivers of bacterial and fungal β-diversity in a typical dryland ecosystem of northwest China. Front. Microbiol. 2017, 8, 2126. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Dai, Z.; Wang, H.; Dsouza, M.; Liu, X.; He, Y.; Wu, J.; Rodrigues, J.L.M.; Gilbert, J.A.; Brookes, P.C.; et al. Distinct biogeographic patterns for archaea, bacteria, and fungi along the vegetation gradient at the continental scale in eastern China. mSystems 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Van Nostrand, J.D.; Deng, Y.; Lu, X.; Wang, C.; Zhou, J.; Han, X. Scale-dependent effects of climate and geographic distance on bacterial diversity patterns across northern China’s grasslands. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Wu, L.; Liu, W.; Ge, Y.; Mu, T.; Zhou, T.; Li, Z.; Zhou, J.; Sun, X.; Luo, Y.; et al. Biogeography and diversity patterns of abundant and rare bacterial communities in rice paddy soils across China. Sci. Total Environ. 2020, 730, 139116. [Google Scholar] [CrossRef]


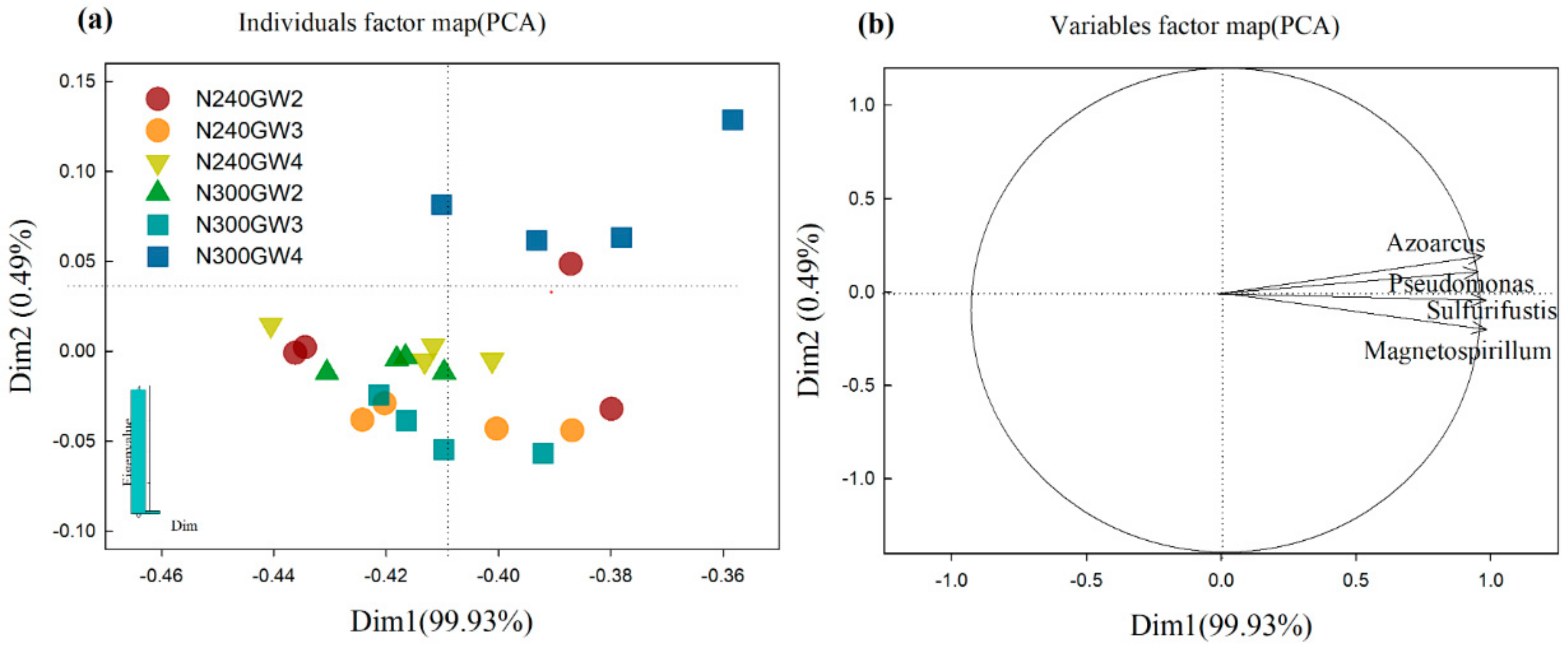
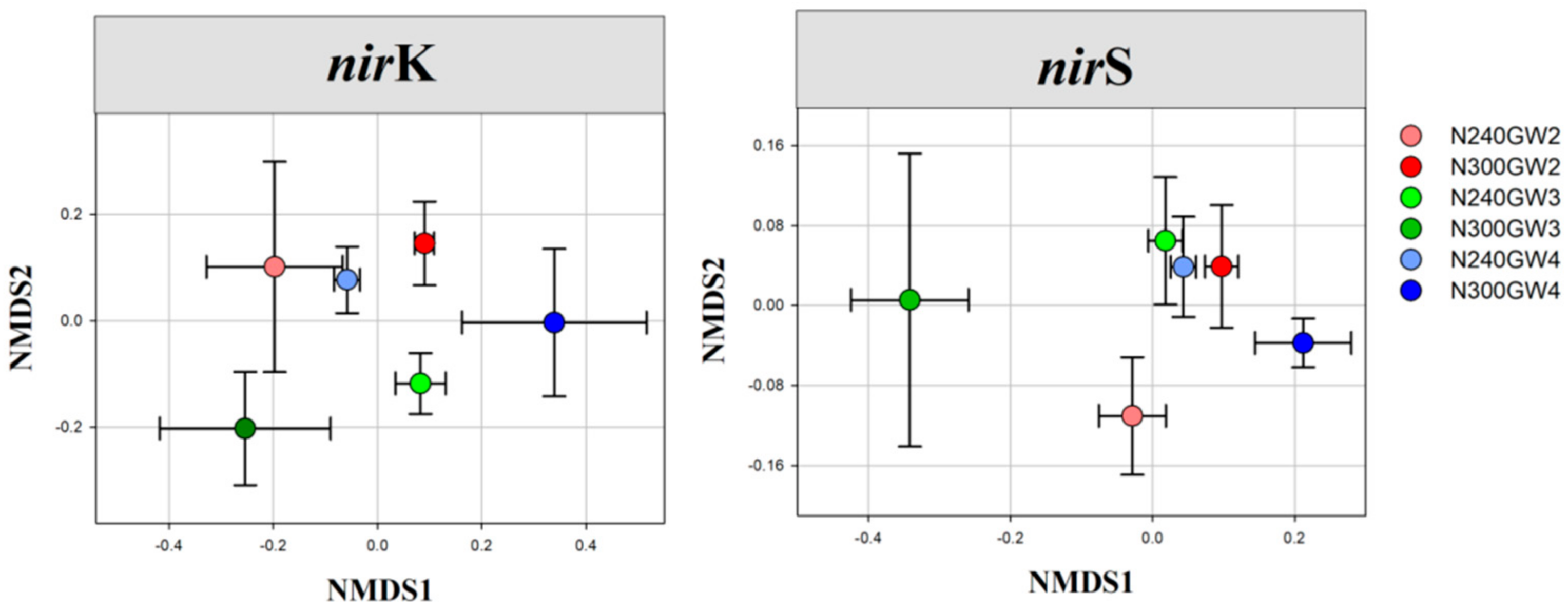

| Source | NO3−–N | NH4+–N | pH | EC | SOC | TSN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Ninput | 519.10 | <0.001 | 11.27 | <0.01 | 376.15 | <0.001 | 365.49 | <0.001 | 52.65 | <0.001 | 16.12 | <0.001 |
| GWD | 121.74 | <0.001 | 4.03 | <0.05 | 172.96 | <0.001 | 18.33 | <0.001 | 44.26 | <0.001 | 39.91 | <0.001 |
| Ninput*GWD | 0.22 | 0.80 | 1.53 | 0.24 | 26.73 | <0.001 | 27.90 | <0.001 | 12.85 | <0.001 | 2.75 | 0.09 |
| Source | Sinorhizobium | Mesorhizobium | Xanthomonas | Bosea | ||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| Ninput | 0.12 | 0.74 | 4.51 | <0.05 | 5.02 | <0.05 | 0.08 | 0.78 |
| GWD | 32.35 | <0.001 | 25.77 | <0.001 | 1.56 | 0.24 | 11.00 | <0.001 |
| Ninput*GWD | 79.60 | <0.001 | 11.83 | <0.001 | 17.58 | <0.001 | 3.88 | <0.05 |
| Source | Pseudomonas | Azoarcus | Sulfurifustis | Magnetospirillum | ||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| Ninput | 4.63 | <0.05 | 5.36 | <0.05 | 0.04 | 0.84 | 6.11 | <0.05 |
| GWD | 4.33 | <0.05 | 29.42 | <0.001 | 5.46 | <0.05 | 4.30 | <0.05 |
| Ninput*GWD | 0.22 | 0.80 | 15.50 | <0.001 | 2.27 | 0.13 | 0.22 | 0.81 |
| Genotype | Ninput | GWD | Ninput*GWD | |
|---|---|---|---|---|
| nirK-type | F | 1.52 | 1.75 | 1.96 |
| P | <0.05 | <0.01 | <0.01 | |
| nirS-type | F | 1.61 | 2.10 | 2.11 |
| P | <0.05 | <0.01 | <0.01 |
| Variables | nirK-Type | nirS-Type | ||
|---|---|---|---|---|
| r | P | r | P | |
| NO3−–N | 0.36 | <0.001 | 0.34 | <0.01 |
| NH4+–N | −0.11 | 0.71 | −0.22 | 0.95 |
| pH | 0.14 | 0.07 | 0.07 | 0.21 |
| EC | 0.27 | <0.01 | 0.32 | <0.01 |
| SOC | 0.27 | <0.05 | 0.15 | 0.15 |
| TSN | 0.35 | <0.01 | 0.16 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, F.; Qi, X.; Li, P.; Qiao, D.; Wang, J.; Du, Z.; She, Y.; Guo, W.; Lu, H. Depression of Groundwater Table and Reduced Nitrogen Application Jointly Regulate the Bacterial Composition of nirS-Type and nirK-Type Genes in Agricultural Soil. Water 2020, 12, 3459. https://doi.org/10.3390/w12123459
Bai F, Qi X, Li P, Qiao D, Wang J, Du Z, She Y, Guo W, Lu H. Depression of Groundwater Table and Reduced Nitrogen Application Jointly Regulate the Bacterial Composition of nirS-Type and nirK-Type Genes in Agricultural Soil. Water. 2020; 12(12):3459. https://doi.org/10.3390/w12123459
Chicago/Turabian StyleBai, Fangfang, Xuebin Qi, Ping Li, Dongmei Qiao, Jianming Wang, Zhenjie Du, Yingjun She, Wei Guo, and Hongfei Lu. 2020. "Depression of Groundwater Table and Reduced Nitrogen Application Jointly Regulate the Bacterial Composition of nirS-Type and nirK-Type Genes in Agricultural Soil" Water 12, no. 12: 3459. https://doi.org/10.3390/w12123459
APA StyleBai, F., Qi, X., Li, P., Qiao, D., Wang, J., Du, Z., She, Y., Guo, W., & Lu, H. (2020). Depression of Groundwater Table and Reduced Nitrogen Application Jointly Regulate the Bacterial Composition of nirS-Type and nirK-Type Genes in Agricultural Soil. Water, 12(12), 3459. https://doi.org/10.3390/w12123459









