Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
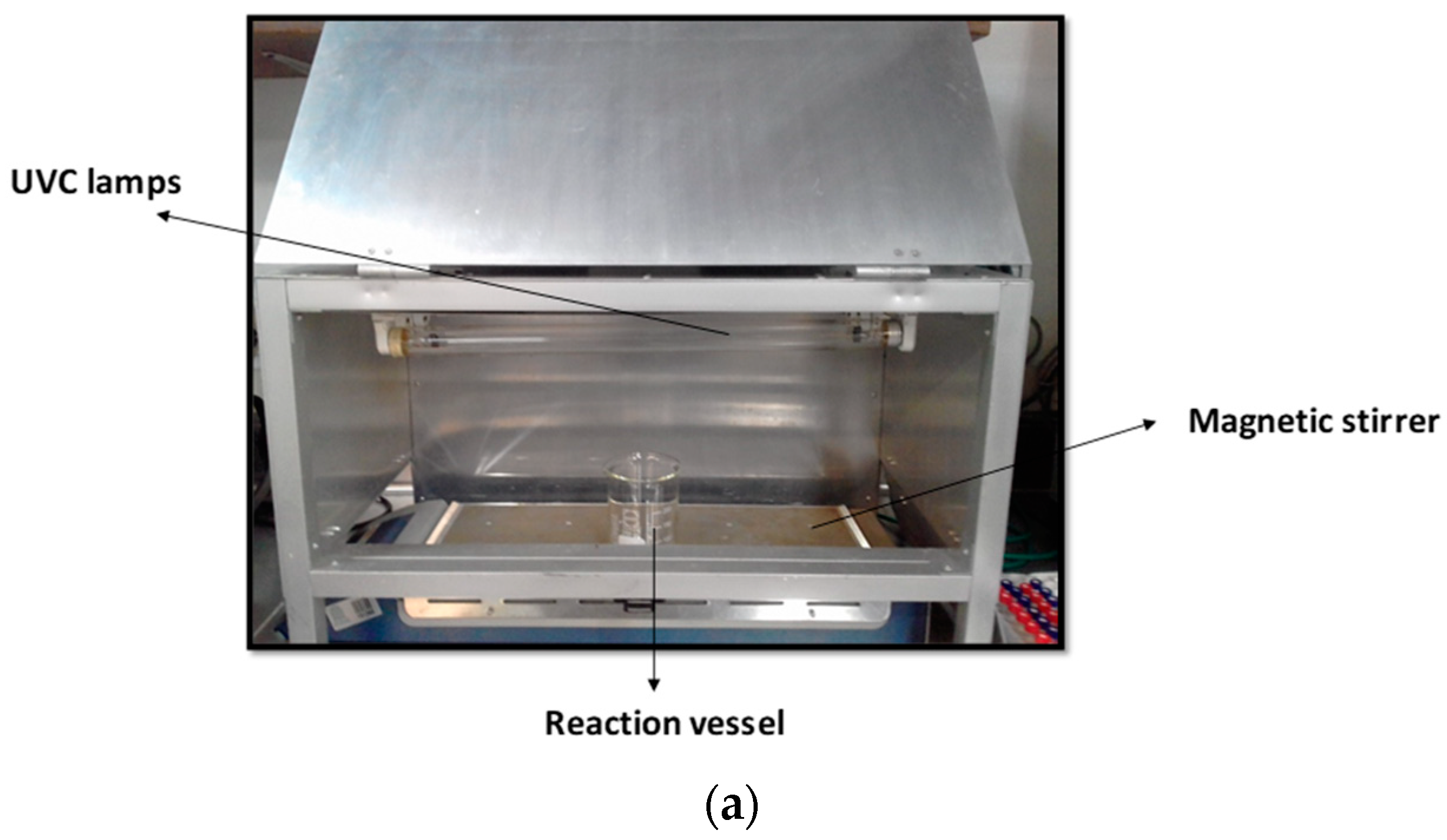
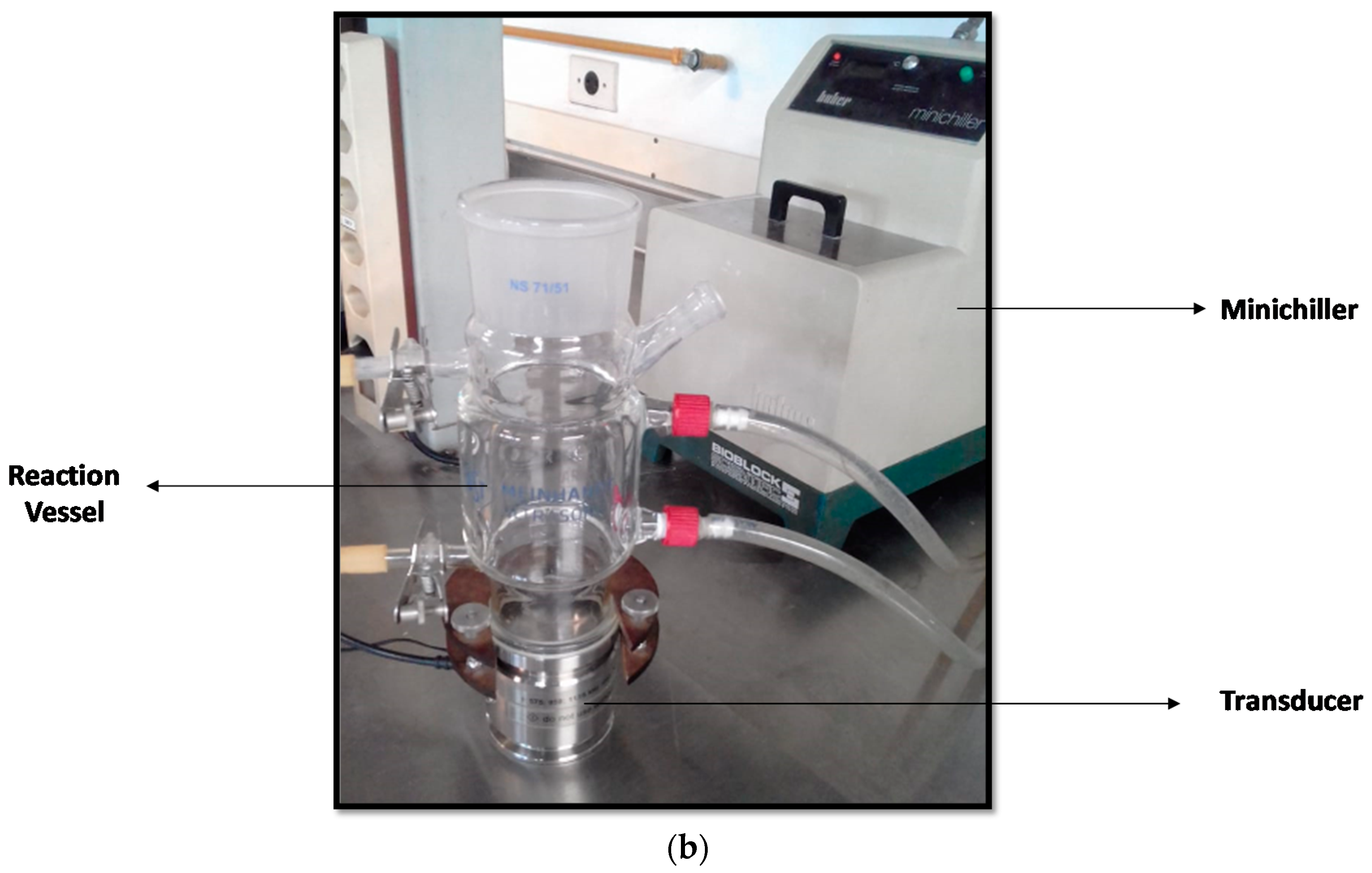
2.2. Reaction Systems
2.3. Analyses
2.3.1. Chromatographic Analyses
2.3.2. Oxidizing Species Accumulation
2.3.3. Mineralization Determinations
2.3.4. Phytotoxicity Tests
2.3.5. Computational Analyses
3. Results
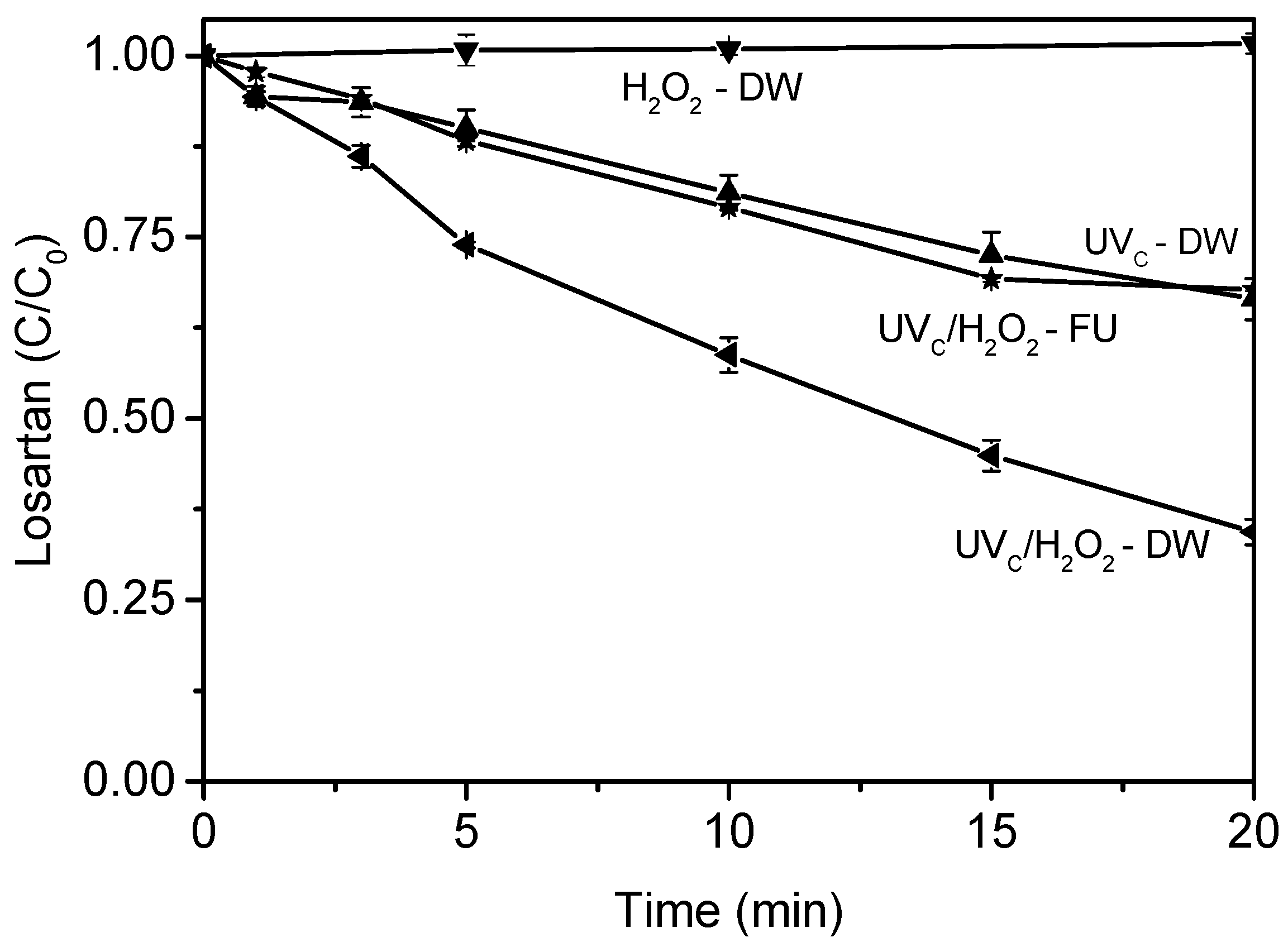
3.1. Treatment of Fresh Urine Loaded with Losartan
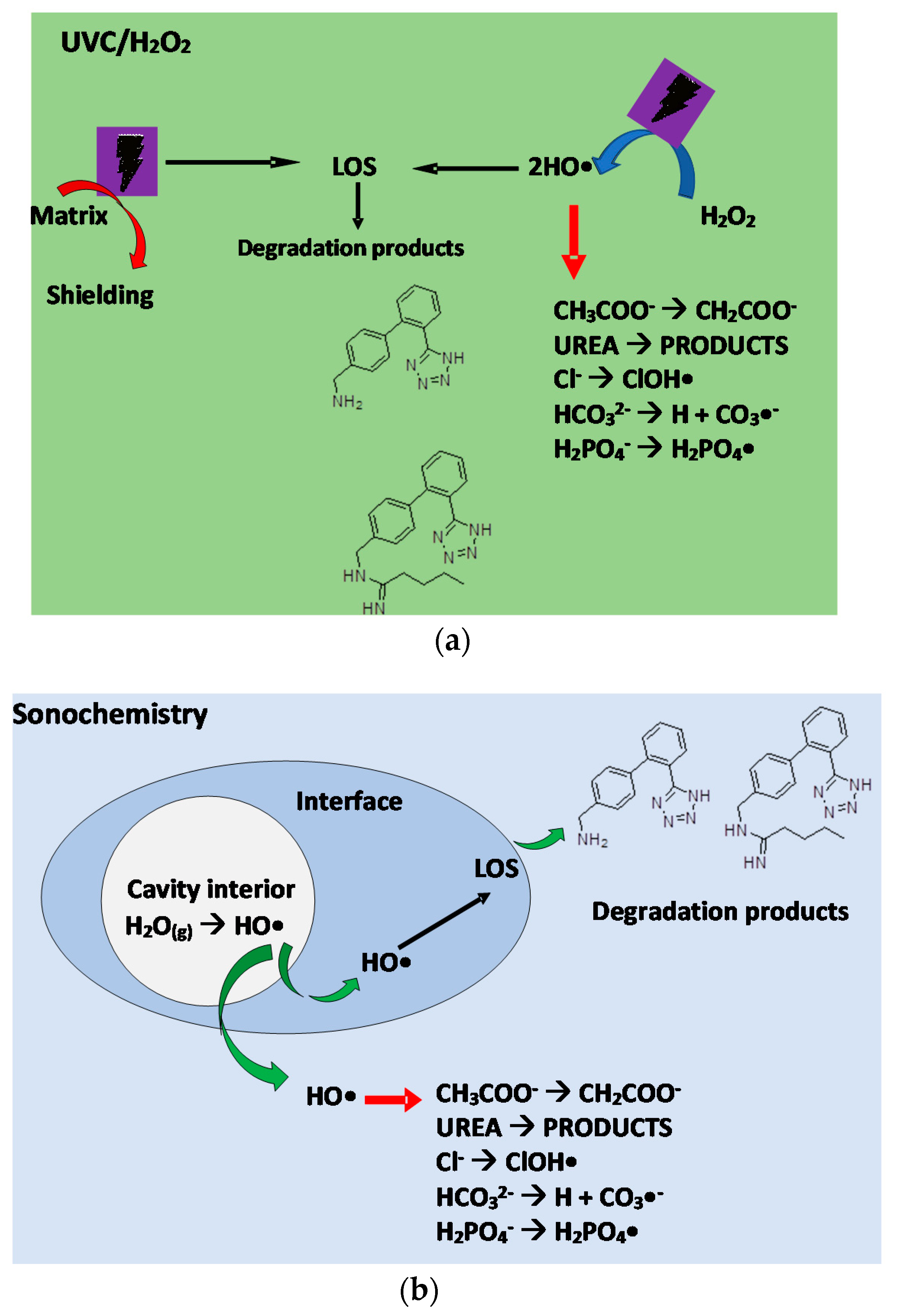
3.2. Degradation Routes of Losartan (LOS) in Different AOPs
3.2.1. Action Routes of the UVC/H2O2 Process
3.2.2. Degradation Routes Involved in the Sonochemical Treatment
3.3. Analysis of Losartan Susceptibility to Attacks by Radical Species
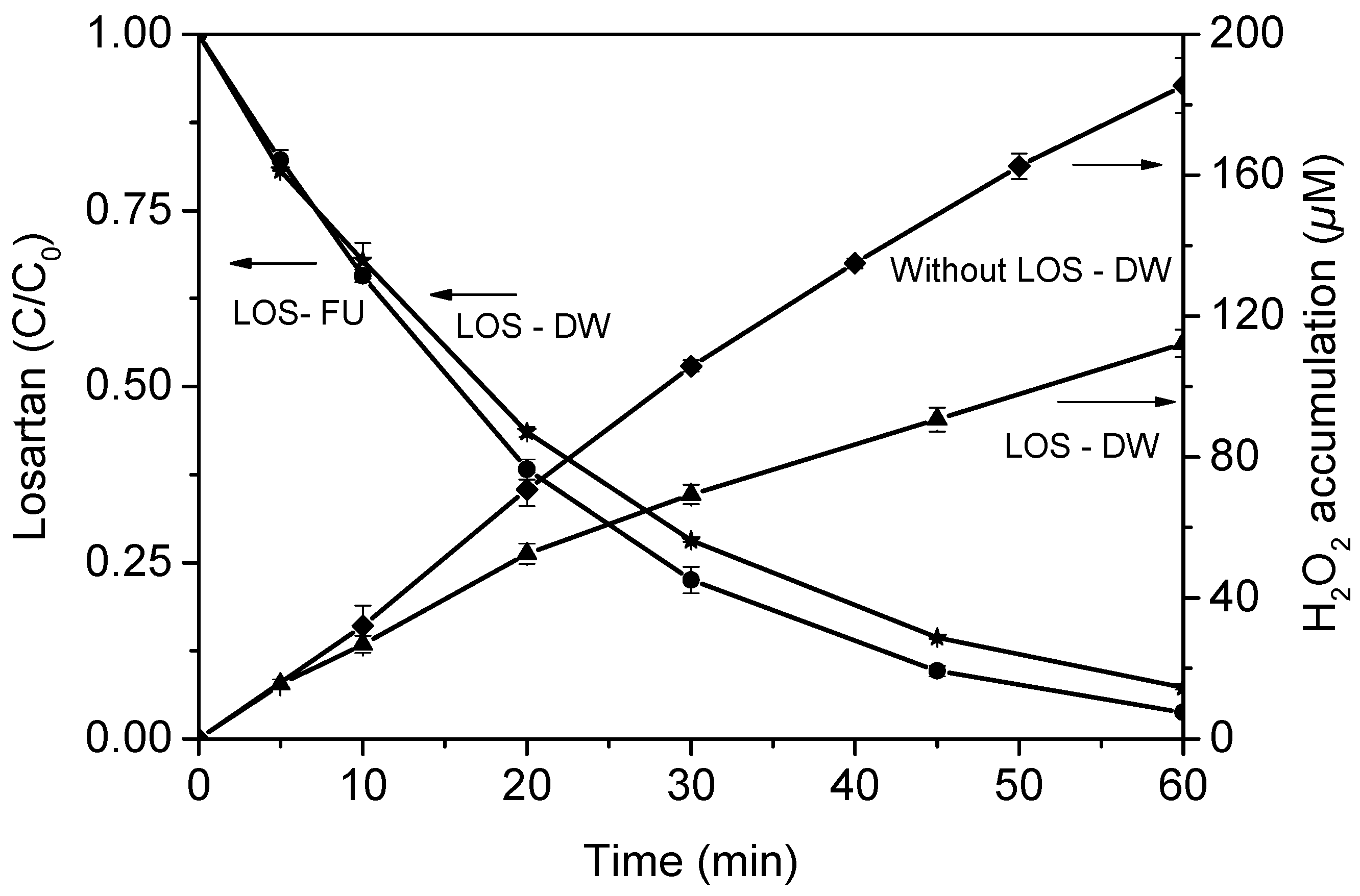
3.4. Mineralization and Toxicity Evolution in Distilled Water
4. Discussion
4.1. Treatment of Fresh Urine Loaded with Losartan
4.2. Degradation Routes of Losartan (LOS) in the Different AOPs
4.2.1. UVC/H2O2 Process
4.2.2. Ultrasound Process
4.2.3. Understanding the Interference of Urine Matrix
4.3. Analysis of Losartan Susceptibility to Attacks by Radical Species
4.4. Mineralization and Toxicity Evolution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, Q.; Burt, V.L.; Dillon, C.F.; Yoon, S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: The National Health And Nutrition Examination Survey, 2001 to 2010. Circulation 2012, 126, 2105–2114. [Google Scholar] [CrossRef]
- Israili, Z.H. Clinical pharmacokinetics of angiotensin II (AT 1) receptor blockers in hypertension. J. Hum. Hypertens. 2000, 14 (Suppl. 1), S73–S87. [Google Scholar] [CrossRef]
- Gurke, R.; Rößler, M.; Marx, C.; Diamond, S.; Schubert, S.; Oertel, R.; Fauler, J. Science of the Total Environment Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant. Sci. Total Environ. 2015, 532, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Dulova, N. UV-assisted chemical oxidation of antihypertensive losartan in water. J. Environ. Manag. 2020, 261, 110170. [Google Scholar] [CrossRef]
- Osorio, V.; Larrañaga, A.; Aceña, J.; Pérez, S.; Barceló, D. Science of the Total Environment Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers. Sci. Total Environ. 2016, 540, 267–277. [Google Scholar] [CrossRef]
- Sanzi, F.; Souza, S.; Lopes, L.; Emanoel, J.; Hermes, F.; Alves, L.; Gonçalves, L.; Rodrigues, C.; Barbosa, B.; Moledo, D.; et al. Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). Sci. Total Environ. 2018, 637–638, 1363–1371. [Google Scholar] [CrossRef]
- Bayer, A.; Asner, R.; Schüssler, W.; Kopf, W.; Weiß, K.; Sengl, M.; Letzel, M. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ. Sci. Pollut. Res. 2014, 21, 10830–10839. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, N.; Deng, Y.; Chu, W.; Rong, W.; Zhou, S. Factors affecting ultraviolet irradiation/hydrogen peroxide (UV/H2O2) degradation of mixed N-nitrosamines in water. J. Hazard. Mater. 2012, 231–232, 43–48. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Y.; Huang, C.-H.; Zhao, L.; Sun, P. Kinetics and modeling of sulfonamide antibiotic degradation in wastewater and human urine by UV/H2O2 and UV/PDS. Water Res. 2016, 103, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Hendaoui, I.; Jovic, M.; Grandjean, D.; De Alencastro, L.F.; Girault, H.; Pulgarin, C. Solar photo-Fenton and UV/H2O2 processes against the antidepressant Venlafaxine in urban wastewaters and human urine. Intermediates formation and biodegradability assessment. Chem. Eng. J. 2017, 308, 492–504. [Google Scholar] [CrossRef]
- Giannakis, S.; Jovic, M.; Gasilova, N.; Pastor Gelabert, M.; Schindelholz, S.; Furbringer, J.-M.M.; Girault, H.; Pulgarin, C. Iohexol degradation in wastewater and urine by UV-based Advanced Oxidation Processes (AOPs): Process modeling and by-products identification. J. Environ. Manag. 2017, 195, 174–185. [Google Scholar] [CrossRef]
- Singla, J.; Verma, A.; Sangal, V.K. Parametric optimization for the treatment of human urine metabolite, creatinine using electro-oxidation. J. Electroanal. Chem. 2018, 809, 136–146. [Google Scholar] [CrossRef]
- Giannakis, S.; Androulaki, B.; Comninellis, C.; Pulgarin, C. Wastewater and urine treatment by UVC-based advanced oxidation processes: Implications from the interactions of bacteria, viruses, and chemical contaminants. Chem. Eng. J. 2018, 343, 270–282. [Google Scholar] [CrossRef]
- Yin, K.; He, Q.; Liu, C.; Deng, Y.; Wei, Y.; Chen, S.; Liu, T.; Luo, S. Prednisolone degradation by UV/chlorine process: Influence factors, transformation products and mechanism. Chemosphere 2018, 212, 56–66. [Google Scholar] [CrossRef]
- Luo, J.; Liu, T.; Zhang, D.; Yin, K.; Wang, D.; Zhang, W.; Liu, C.; Yang, C.; Wei, Y.; Wang, L.; et al. The individual and Co-exposure degradation of benzophenone derivatives by UV/H2O2 and UV/PDS in different water matrices. Water Res. 2019, 159, 102–110. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, D.M.; Serna-Galvis, E.A.; Ferraro, F.; Torres-Palma, R.A. Degradation of the emerging concern pollutant ampicillin in aqueous media by sonochemical advanced oxidation processes—Parameters effect, removal of antimicrobial activity and pollutant treatment in hydrolyzed urine. J. Environ. Manage. 2020, 261, 110224. [Google Scholar] [CrossRef]
- Lacasa, E.; Herraiz, M. The role of anode material in the selective oxidation of 2 penicillin G in urine. Chemelectrochem 2019, 6, 1376–1380. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Removal of pharmaceuticals from the urine of polymedicated patients: A first approach. Chem. Eng. J. 2018, 331, 606–614. [Google Scholar] [CrossRef]
- Schmidt, B.; Schieffer, B. Angiotensin II AT1 Receptor Antagonists. Clinical Implications of Active Metabolites. J. Med. Chem. 2003, 46, 2261–2270. [Google Scholar] [CrossRef]
- Amstutz, V.; Katsaounis, A.; Kapalka, A.; Comninellis, C.; Udert, K.M. Effects of carbonate on the electrolytic removal of ammonia and urea from urine with thermally prepared IrO2 electrodes. J. Appl. Electrochem. 2012, 42, 787–795. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Giraldo, A.L.; Flórez, O.A.; Torres-Palma, R.A. Comparison of route, mechanism and extent of treatment for the degradation of a β-lactam antibiotic by TiO2 photocatalysis, sonochemistry, electrochemistry and the photo-Fenton system. Chem. Eng. J. 2016, 284, 953–962. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Isaza-Pineda, L.; Moncayo-Lasso, A.; Hernández, F.; Ibáñez, M.; Torres-Palma, R.A. Comparative degradation of two highly consumed antihypertensives in water by sonochemical process. Determination of the reaction zone, primary degradation products and theoretical calculations on the oxidative process. Ultrason. Sonochem. 2019, 58, 104635. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Giraldo-Aguirre, A.L.; Torres-Palma, R.A. Sonochemical degradation of the pharmaceutical fluoxetine: Effect of parameters, organic and inorganic additives and combination with a biological system. Sci. Total Environ. 2015, 524–525, 354–360. [Google Scholar] [CrossRef]
- Hoekstra, N.J.; Bosker, T.; Lantinga, E.A. Effects of cattle dung from farms with different feeding strategies on germination and initial root growth of cress (Lepidium sativum L.). Agric. Ecosyst. Environ. 2002, 93, 189–196. [Google Scholar] [CrossRef]
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Li, G.; Song, W.; Cooper, W.J.; Nie, X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Gurkan, Y.Y.; Turkten, N.; Hatipoglu, A.; Cinar, Z. Photocatalytic degradation of cefazolin over N-doped TiO2 under UV and sunlight irradiation: Prediction of the reaction paths via conceptual DFT. Chem. Eng. J. 2012, 184, 113–124. [Google Scholar] [CrossRef]
- Li, L.; Wei, D.; Wei, G.; Du, Y. Transformation of cefazolin during chlorination process: Products, mechanism and genotoxicity assessment. J. Hazard. Mater. 2013, 262, 48–54. [Google Scholar] [CrossRef]
- Clara, M.; Starling, V.M.; Souza, P.P.; Le, A.; Amorim, C.C.; Criquet, J. Intensification of UV-C treatment to remove emerging contaminants by UV-C/H2O2 and UV-C/S2O82−: Susceptibility to photolysis and investigation of acute toxicity UV-C. Chem. Eng. J. 2019, 376, 120856. [Google Scholar] [CrossRef]
- Gerrity, D.; Lee, Y.; Von Gunten, U. Prediction of Trace Organic Contaminant Abatement with UV/H2O2: Development and Validation of Semi-Empirical Models for Municipal Wastewater Effluents. Environ. Sci. Water Res. Technol. 2016, 2, 460–473. [Google Scholar] [CrossRef]
- Challis, J.K.; Hanson, M.L.; Friesen, K.J.; Wong, C.S. A critical assessment of the photodegradation of pharmaceuticals in aquatic environments: Defining our current understanding and identifying knowledge gaps. Environ. Sci. Process. Impacts 2014, 16, 672–696. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Sonochemistry: Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715. [Google Scholar] [CrossRef]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in landfill groundwater: Environmental matrix effects. Environ. Sci. Technol. Ronmental. 2008, 42, 8057–8063. [Google Scholar] [CrossRef]
- Jiang, Y.; Pétrier, C.; David Waite, T. Kinetics and mechanisms of ultrasonic degradation of volatile chlorinated aromatics in aqueous solutions. Ultrason. Sonochem. 2002, 9, 317–323. [Google Scholar] [CrossRef]
- Fernandez, N.A.; Rodriguez-freire, L.; Keswani, M.; Sierra-alvarez, R. Degradation of perfluoroalkyl and polyfluoroalkyl. Environ. Sci. 2016, 975–983. [Google Scholar] [CrossRef]
- Yasman, Y.; Bulatov, V.; Gridin, V.V.; Agur, S.; Galil, N.; Armon, R.; Schechter, I. A new sono-electrochemical method for enhanced detoxification of hydrophilic chloroorganic pollutants in water. Ultrason. Sonochem. 2004, 11, 365–372. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Montoya-Rodríguez, D.M.; Isaza-Pineda, L.; Ibáñez, M.; Hernández, F.; Moncayo-Lasso, A.; Torres-Palma, R.A. Sonochemical degradation of antibiotics from representative classes-Considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason. Sonochem. 2018, 50, 157–165. [Google Scholar] [CrossRef]
- Tran, N.; Drogui, P.; Brar, S.K. Sonochemical techniques to degrade pharmaceutical organic pollutants. Environ. Chem. Lett. 2015, 13, 251–268. [Google Scholar] [CrossRef]
- Lian, L.; Yao, B.; Hou, S.; Fang, J.; Yan, S.; Song, W. Kinetic Study of Hydroxyl and Sulfate Radical-Mediated Oxidation of Pharmaceuticals in Wastewater Effluents. Environ. Sci. Technol. 2017, 51, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electron, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O in Aqueous Solution). J. Phys. Chem. Ref. Data 1988, 513. [Google Scholar] [CrossRef]
- Minakata, D.; Song, W.; Crittenden, J. Reactivity of Aqueous Phase Hydroxyl Radical with Halogenated Carboxylate Anions: Experimental and Theoretical Studies. Environ. Sci. Technol. 2011, 45, 6057–6065. [Google Scholar] [CrossRef]
- Toth, J.E.; Rickman, K.A.; Venter, A.R.; Kiddle, J.J.; Mezyk, S.P. Reaction kinetics and efficiencies for the hydroxyl and sulfate radical based oxidation of artificial sweeteners in water. J. Phys. Chem. A. 2012, 116, 9819–9824. [Google Scholar] [CrossRef]
- Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Silva-Agredo, J.; Ávila-Torres, Y.; Torres-Palma, R.A. Dataset on the degradation of losartan by TiO2-photocatalysis and UVC/persulfate processes. Data Br. 2020, 31. [Google Scholar] [CrossRef]
- Singla, R.; Grieser, F.; Ashokkumar, M. The mechanism of sonochemical degradation of a cationic surfactant in aqueous solution. Ultrason. Sonochem. 2011, 18, 484–488. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef]
- Adams, E. Ecotoxicity and Genotoxicity Evaluation of Losartan Potassium after UVC Photolysis and UV/H2O2 Process; Universidade Tecnológica Federal Do Paraná: Curitiba, Brazil, 2019. [Google Scholar]
- Emery, R.J.; Papadaki, M.; Freitas dos Santos, L.M.; Mantzavinos, D. Extent of sonochemical degradation and change of toxicity of a pharmaceutical precursor (triphenylphosphine oxide) in water as a function of treatment conditions. Environ. Int. 2005, 31, 207–211. [Google Scholar] [CrossRef]
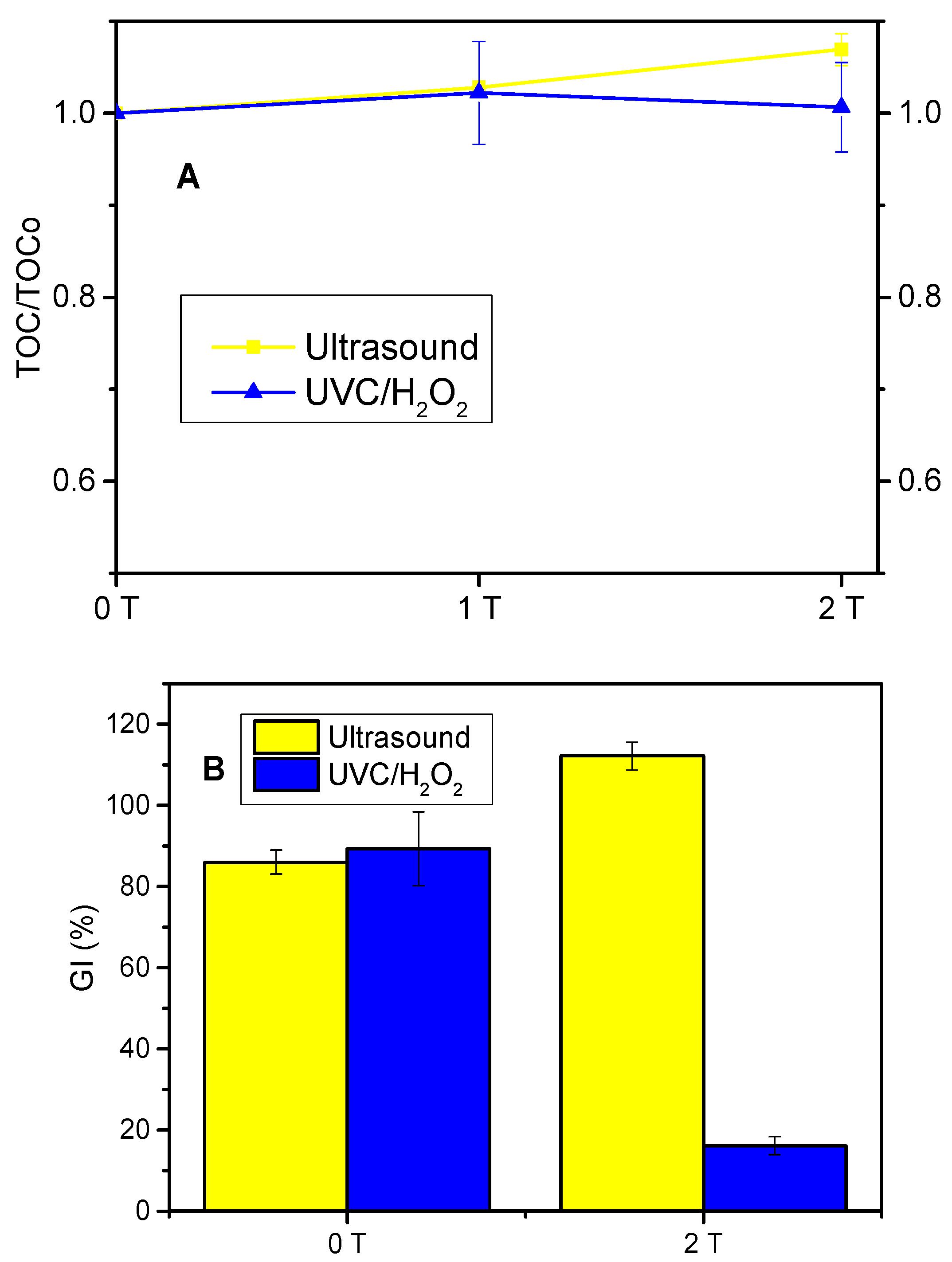
| Compound | Concentration (M) |
|---|---|
| Urea | 0.2664 |
| NaCH3COO | 0.1250 |
| Na2SO4 | 0.01619 |
| NH4Cl | 0.03365 |
| NaH2PO4 | 0.02417 |
| KCl | 0.05634 |
| MgCl2 | 0.003886 |
| CaCl2 | 0.004595 |
| NaOH | 0.00300 |
| pH: 6.1 | |
| AOP | kDW (R2) | kFU (R2) | Rk = kFU/kDW |
|---|---|---|---|
| Sonochemistry | 0.0549 (0.9972) | 0.0437 (0.9975) | 0.796 |
| UVC/H2O2 | 0.0532 (0.9987) | 0.0245 (0.9981) | 0.461 |
| Structure and Numeration | Atoms | Fukui Function Indices | ||
|---|---|---|---|---|
| f − | f+ | f ave | ||
 | 1 C | 0.045 | 0.054 | 0.049 |
| 2 C | −0.027 | 0.005 | −0.011 | |
| 3 C | 0.066 | −0.022 | 0.022 | |
| 4 C | 0.006 | 0.004 | 0.005 | |
| 5 C | 0.055 | −0.015 | 0.020 | |
| 6 C | 0.004 | −0.007 | −0.002 | |
| 7 C | −0.103 | −0.012 | −0.058 | |
| 8 C | −0.100 | −0.031 | −0.066 | |
| 10 C | −0.112 | 0.160 | 0.024 | |
| 11 C | −0.094 | −0.088 | −0.091 | |
| 12 C | 0.160 | −0.104 | 0.028 | |
| 13 C | −0.050 | −0.045 | −0.048 | |
| 14 C | −0.660 | 0.117 | −0.272 | |
| 15 C | 0.402 | 0.126 | 0.264 | |
| 16 C | 0.007 | 0.009 | 0.008 | |
| 17 C | 0.000 | −0.044 | −0.022 | |
| 18 C | 1.841 | 1.119 | 1.480 | |
| 19 C | −0.661 | −0.739 | −0.700 | |
| 21 C | 0.216 | −0.116 | 0.050 | |
| 22 C | 0.008 | −0.141 | −0.067 | |
| 1 N | 0.053 | −0.005 | 0.024 | |
| 2 N | −0.043 | 0.003 | −0.020 | |
| 3 N | 0.016 | 0.005 | 0.011 | |
| 4 N | 0.022 | 0.000 | 0.011 | |
| 5 N | −0.258 | 0.044 | −0.107 | |
| 6 N | −0.066 | 0.179 | 0.057 | |
| Cl | 0.061 | 0.058 | 0.060 | |
| O | 0.061 | 0.000 | 0.031 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Ávila-Torres, Y.; Torres-Palma, R.A. Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water 2020, 12, 3398. https://doi.org/10.3390/w12123398
Guateque-Londoño JF, Serna-Galvis EA, Ávila-Torres Y, Torres-Palma RA. Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water. 2020; 12(12):3398. https://doi.org/10.3390/w12123398
Chicago/Turabian StyleGuateque-Londoño, John F., Efraím A. Serna-Galvis, Yenny Ávila-Torres, and Ricardo A. Torres-Palma. 2020. "Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes" Water 12, no. 12: 3398. https://doi.org/10.3390/w12123398
APA StyleGuateque-Londoño, J. F., Serna-Galvis, E. A., Ávila-Torres, Y., & Torres-Palma, R. A. (2020). Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water, 12(12), 3398. https://doi.org/10.3390/w12123398





