1. Introduction
Produced water can come from naturally occurring water sources associated with underground oil and gas formations (connate water), or from production fluids injected into underground formations to enhance hydrocarbon recovery [
1]. Produced water volumes tend to be much larger than the volumes of produced hydrocarbons; in the U.S., the average is about seven to eight barrels of water for every barrel of oil produced [
2]. Produced waters can contain high concentrations of sand and sediment, free and emulsified hydrocarbons, suspended and colloidal solids, dissolved salts, minerals and metals, soluble hydrocarbons (e.g., toxic compounds such as benzene, toluene, ethylbenzene and xylenes (BTEX), polycyclic aromatic hydrocarbons and naphthenic acids), and naturally occurring radioactive materials (NORM) [
3]. Historically, produced water is managed differently depending on the production method. Typical approaches include: (1) direct onsite deep well injection after oil and suspended solids removal (e.g., most conventional U.S. onshore fields), (2) hauling offsite for deep well injection (e.g., most U.S. shale oil/gas fields), (3) treatment to achieve 29 ppm marine discharge standard (e.g., offshore Gulf of Mexico), and (4) treatment to enable direct reuse in enhanced oil production (e.g., steam flooding in California and Alberta heavy oil fields) [
4]. With the more recent ramping up of hydraulic fracturing in the U.S., the cost of hauling for offsite deep well injection has risen dramatically. This, combined with the lack of available fresh water in many oil and gas producing areas (e.g., the Permian basin [
5]), have led industries to consider on-site treatment for permitted discharge, direct reuse, or other beneficial reuse [
2]. A typical produced water treatment train is composed of the following steps: (1) primary treatment to remove suspended solids and free oil, (2) secondary treatment to remove free oil and macroemulsion (i.e., emulsified oil droplets > 1 μm), (3) tertiary treatment to remove microemulsion (i.e., emulsified oil droplets < 1 μm) and some soluble hydrocarbons (HC’s), and (4) advanced treatment, which could involve softening, desilication and/or desalination to reduce hardness, silica, and dissolved substances (salts, metals, soluble HCs, NORM, etc.) [
4].
A critical step in any produced water treatment approach is the removal of free and emulsified hydrocarbons, which typically requires chemical destabilization of emulsified oil droplets stabilized by surface charges and/or steric forces imparted by the sorption of surfactants at the oil/water interface. Mechanisms of particle destabilization include: (1) electrical double layer compression, (2) counter-ion adsorption and charge neutralization, (3) polymer adsorption and inter-particle bridging, and (4) precipitate entrapment and “sweep flocculation” [
6]. As such, both chemical coagulation (CC) and electro-chemical coagulation or “electro-coagulation” (EC) processes have been widely studied for treating wastewaters with high oil and grease (O&G) content, turbidity (or suspended solids), chemical oxygen demand (COD), and total organic carbon (TOC), such as industrial oil-in-water emulsions [
7,
8,
9], olive oil mill wastewater [
10,
11], textile wastewater [
11,
12,
13], green table olive packaging industry wastewater [
14], and restaurant wastewater [
15]. More recently, EC was used to remove antibiotics [
16], heavy metals [
17], perfluorooctanoic acid [
18], and refractory wastewater [
19].
Conventional CC involves rapid mixing of metal salts and/or polymeric coagulants into the water to destabilize charged colloidal materials, slow mixing to enhance aggregation of destabilized particles and/or coalescence of emulsified oil droplets, and separation of particles by sedimentation, flotation, or filtration [
20,
21]. Similarly, EC uses sacrificial metal electrodes (
typ., iron or aluminum) to supply coagulants in situ as water passes through a direct current (DC) electrochemical reactor. In EC, metal ions generated from the anode rapidly hydrolyze to form metal hydroxide precipitates, which ultimately destabilize particles through a combination of charge neutralization and sweep flocculation [
22,
23]. The DC electric field also migrates negatively charged species towards the positively charged sacrificial anode, enhancing interactions with metal ions and metal hydroxides [
24,
25,
26]. Finally, H
2(g) gas bubbles generated at the cathode induce some flocculation and flotation [
23,
27]. An additional benefit of EC is that it does not increase the chloride or sulfate content of the treated water because the anion associated with sacrificial iron or aluminum at the anode is hydroxide, produced at the cathode.
The effectiveness, efficiency, and economics of CC and EC have also been reported for various wastewaters. EC is reportedly more economical than CC for palm oil mill effluent treatment [
28,
29], textile wastewater and [
30], in general, when coagulant demand is small [
27,
31]. These cost analyses mainly focused on the cost of purchasing electricity versus chemicals. However, energy demand and the cost of coagulant chemical production and transportation should also be considered. In addition, the direct comparison of EC and CC is not straight forward. Coagulants are dosed in one discrete event for CC with a fixed settling time, while there is continuous dosing for EC throughout the reactor. As such, other than electricity and chemical costs, retention time plays an important role in the capital cost of the two coagulation methods in terms of storage area and treatment tank size. Particularly when required coagulant dose is high (as for many challenging oily wastewaters), the lack of Cl
− or SO
42− anions in EC can minimize undesirable water quality impairment by coagulant salts.
In this study, we evaluate the effectiveness, efficiency and economics of CC and EC to treat produced water, comprising surfactant-stabilized oil emulsions dispersed in a mineral rich simulated produced water matrix. Influent and effluent turbidity, COD, O&G, and TOC were determined from bench tests. Removals were scaled against the total amount of iron introduced by varying both the chemical coagulant dose and the applied current density and reaction time. Energy consumption and operating costs were calculated based on North American market data and experimentally determined operating conditions.
2. Materials and Methods
2.1. Lab Synthesized Produced Water
Synthetic produced water samples were generated in the laboratory based on previously reported produced water quality data [
32]. Hexadecane and sodium dodecyl sulfate (SDS) (Sigma-Aldrich, St. Louis, Missouri, USA) were combined in a ratio of 9:1 oil-to-surfactant (
w/w) [
33] in the aqueous electrolyte recipe in
Table 1 based on the water quality analysis in
Table 2 (NaCl, CaCl
2, NaHCO
3, Na
2SiO
3, tannic acid obtained from Sigma-Aldrich, St. Louis, Missouri, USA). The SDS concentration was below its critical micelle concentration [
34]. This could have led to less stable oil/water emulsions. However, such oil to surfactant ratio was enough to cause fouling on PSF membranes [
33]. The mixture was stirred using an industrial blender (7011G, Waring, Torrington, CT, USA) at 22,000 rpm for 5 min. The simulated produced water samples were prepared immediately before coagulation experiments.
2.2. Chemical Coagulation Experiments
CC experiments were carried out at room temperature (20 ± 2 °C) using a bench-scale jar testing apparatus (PB-700TM Jar Tester, Phipps and Bird, Richmond, VA, USA). The CC process includes three consecutive stages: (1) rapid mixing (200 rpm, 2 min) of coagulant (FeCl3) and produced water, (2) slow mixing (25 rpm, 30 min) to promote flocculation, and (3) quiescent gravity separation (60 min). Effluent samples were withdrawn and measured immediately after gravity separation from the center of the reactor.
2.3. Electro-Coagulation Experiments
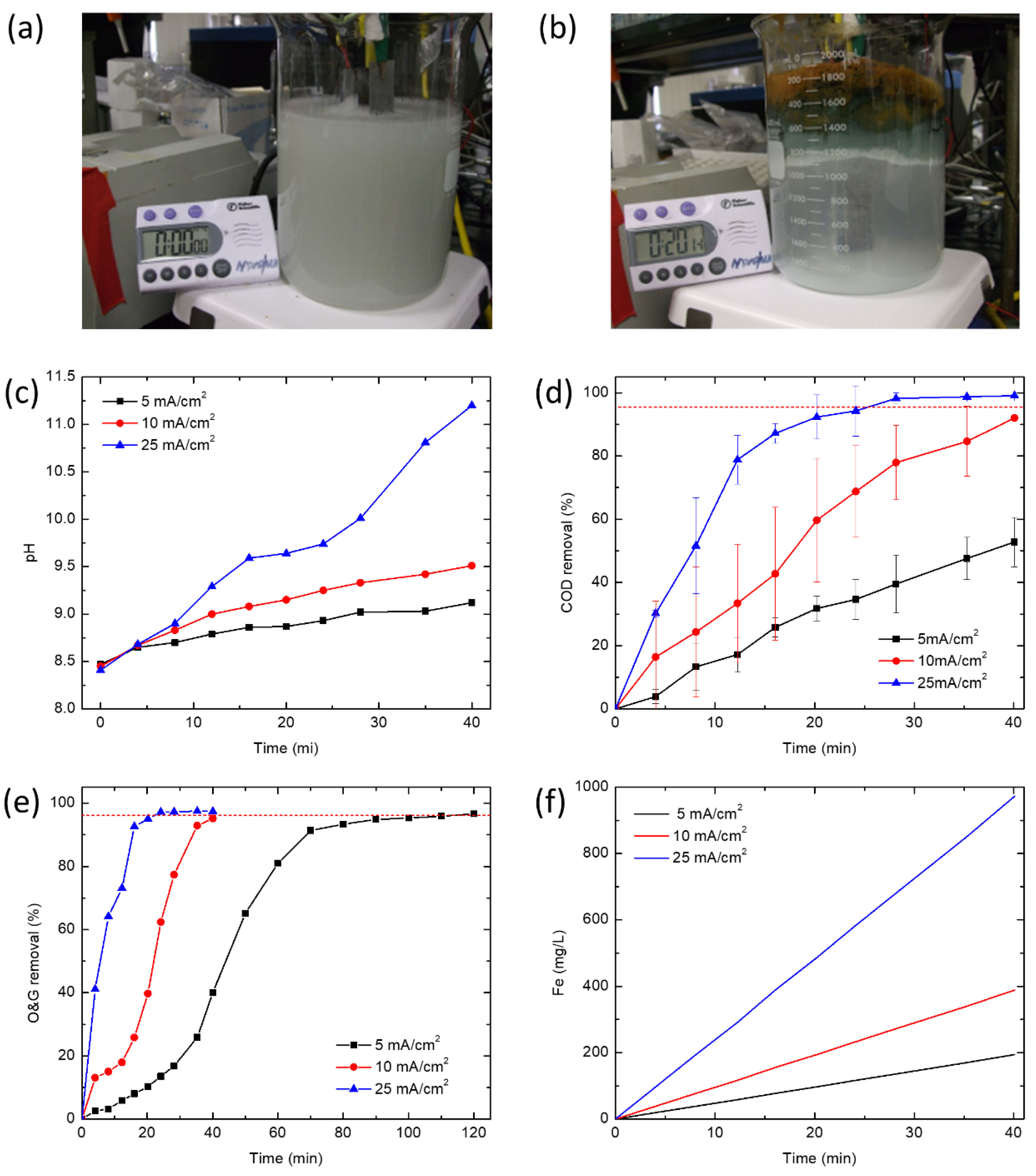
EC experiments were performed in a 1500 mL batch electrolytic cell under galvanostatic conditions at current densities of 5, 10 and 25 mA/cm2. Iron (Fe) plates were used as both the cathode and the sacrificial anode. The total effective electrode area for 4 electrodes was 168 cm2. Four electrodes were connected vertically with a gap of 2 cm and connected to the direct current power supply in monopolar parallel mode. Before each test, the iron electrodes were dipped in 10% HCl solution for 10 min and rinsed by deionized water to remove any residual oxides from prior experiments. Water quality indicators were characterized from samples pipetted at different time intervals from the middle layer of the reactor to account for oil removed by flotation as well as sedimentation. The reactor was stirred before samples were taken to determine the iron concentration.
2.4. Analytical Methods
Turbidity was measured using a turbidimeter (2100AN, Hach Instruments, Loveland, CO, USA). TOC was determined by a TOC analyzer (Apollo 9000, Tekmar Dohrmann, Mason, OH, USA). A calibration curve between O&G (per EPA Method 1664) and TOC was obtained from hexadecane emulsions prepared at different concentrations. O&G values reported for experiments were converted from measured TOC values using the calibration curve. COD analyses were conducted by adding the appropriate volume of sample in COD reagent vial and digesting for 2 h at 150 °C in a COD digestor (DRB200, Hach Instruments, Loveland, CO, USA). The COD was measured by a UV-vis spectrophotometer (LAMBDA 45, PerkinElmer, MA, USA) at 620 nm.
2.5. Iron Concentration Calculation
Theoretical iron concentration values were calculated using Faraday’s law, which relates current and time to the total amount of iron ions generated. It was assumed there was no loss of efficiency due to water splitting, heating, or other side reactions, and that all dissolved iron was initially produced as Fe
2+ ions,
where [Fe] is the molar iron concentration, I is the current (A), t is time (s), M
W is the molecular weight of iron (55.845 g/mol), F is Faraday’s constant (96,486 C/mol), Q is the effluent volume (L), and Z is the charge of Fe
2+.
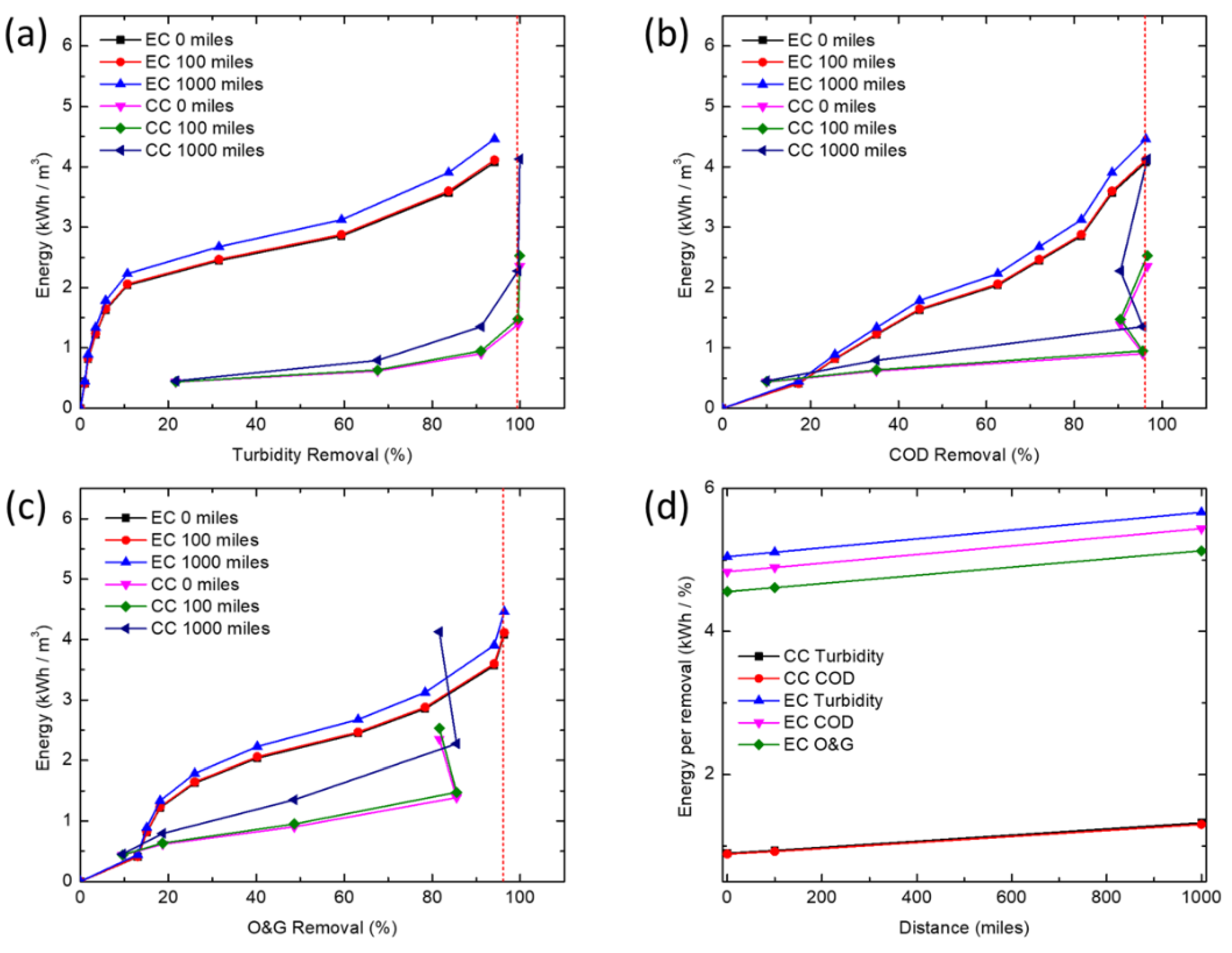
2.6. Energy Demand Calculations
The power needed for rapid mixing and flocculation in conventional CC, the power of mixing P (W) is determined from,
where N
p is a dimensionless power number (N
p = 4, when the Reynolds number is larger than 10,000), ρ is the fluid density (kg/m
3); N is the rotation speed (rpm), and D is the diameter of the impeller (m). For the energy consumption associated with transportation (e.g., moving chemicals), several assumptions were made in this study: (1) the chemical coagulant during transportation was FeCl
3 in solid form; and (2) the transportation distance varied from 0 to 1000 miles. Values of the energy consumption in each process of CC were calculated based on experimental and literature data in
Table 3. Energy demand by CC was calculated as follows:
where CC
pEnergy represents the energy demandfor FeCl
3 production, CC
TEnergy represents the energy demand for FeCl
3 transportation, and CC
MEnergy represents the energy demand for mixing FeCl
3.
The energy consumption per unit volume of water treated by EC includes direct electrical energy input plus the energy cost associated with the production and transportation of sacrificial iron electrode plates. The electric energy input in the EC process is calculated by the following equation:
where U is the applied voltage (V), I is the current (A), t is reaction time (h), and Q is the volume of produced water (m
3). To calculate the energy consumption associated with the transportation of iron plates, the transportation distance was varied from 0 to 1000 miles, and values of the energy consumption were calculated based on experimental and literature data (
Table 4). The energy demand of EC was calculated using the following equation:
where EC
PEnergy represents the energy demand for iron sheet production, EC
TEnergy represents the energy demand for iron sheet transportation, and EC
DEnergy represents the energy demand for iron dissolution.
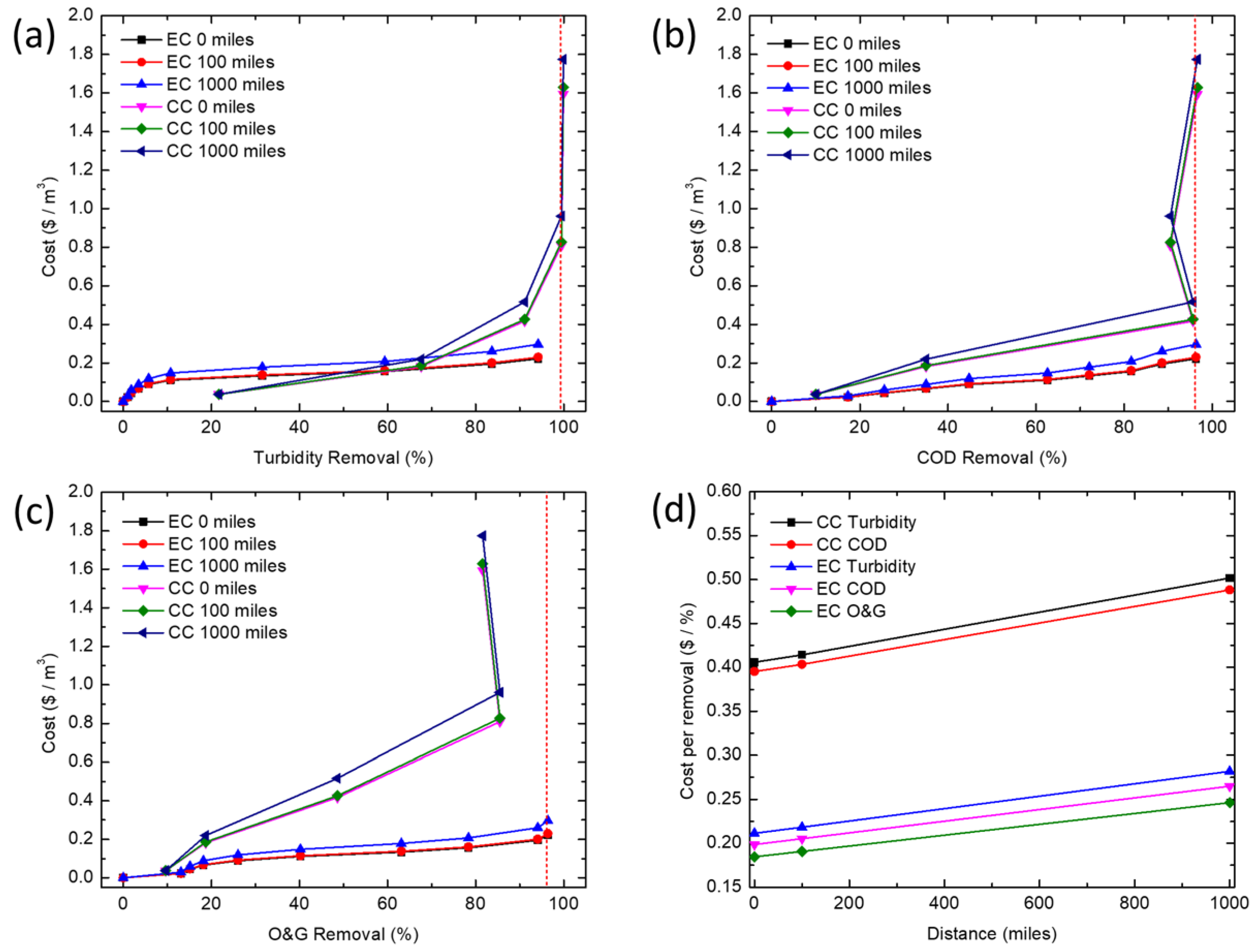
2.7. Operating Cost Calculations
The operating cost per unit volume of produced water treated by CC includes the cost of electricity used for mixing (rapid and slow mixing), and the cost of purchasing and moving the chemical coagulant. The cost of electricity in mixing is calculated based on the mixing energy consumption and the electricity cost. The price of purchasing and moving a chemical coagulant is estimated based on market prices (
Table 5). The operating cost per unit volume of produced water sample treated by EC includes the cost of electricity used to power (i.e., dissolve the iron) the process, and the cost of purchasing and transporting the iron electrodes. The cost of electricity was calculated based on the electrical energy consumption and the electricity cost. The price of purchasing and transporting the iron electrodes was estimated based on market prices (
Table 5). The costs of chemicals and EC were calculated using Equations (6) and (7), respectively:
where CC
TCost represents the cost for FeCl
3 transportation, CC
PCost represents the price of FeCl
3, and CC
ECost represents the electricity cost for FeCl
3 mixing.
where EC
TCost represents the cost for sheet iron transportation, EC
PPCost represents the cost for pig iron, EC
SPCost represents the price of electricity for sheet iron production, and EC
ECost represents the electricity price for iron dissolution. Additional equations of each component are given in
Table 6.
4. Conclusions
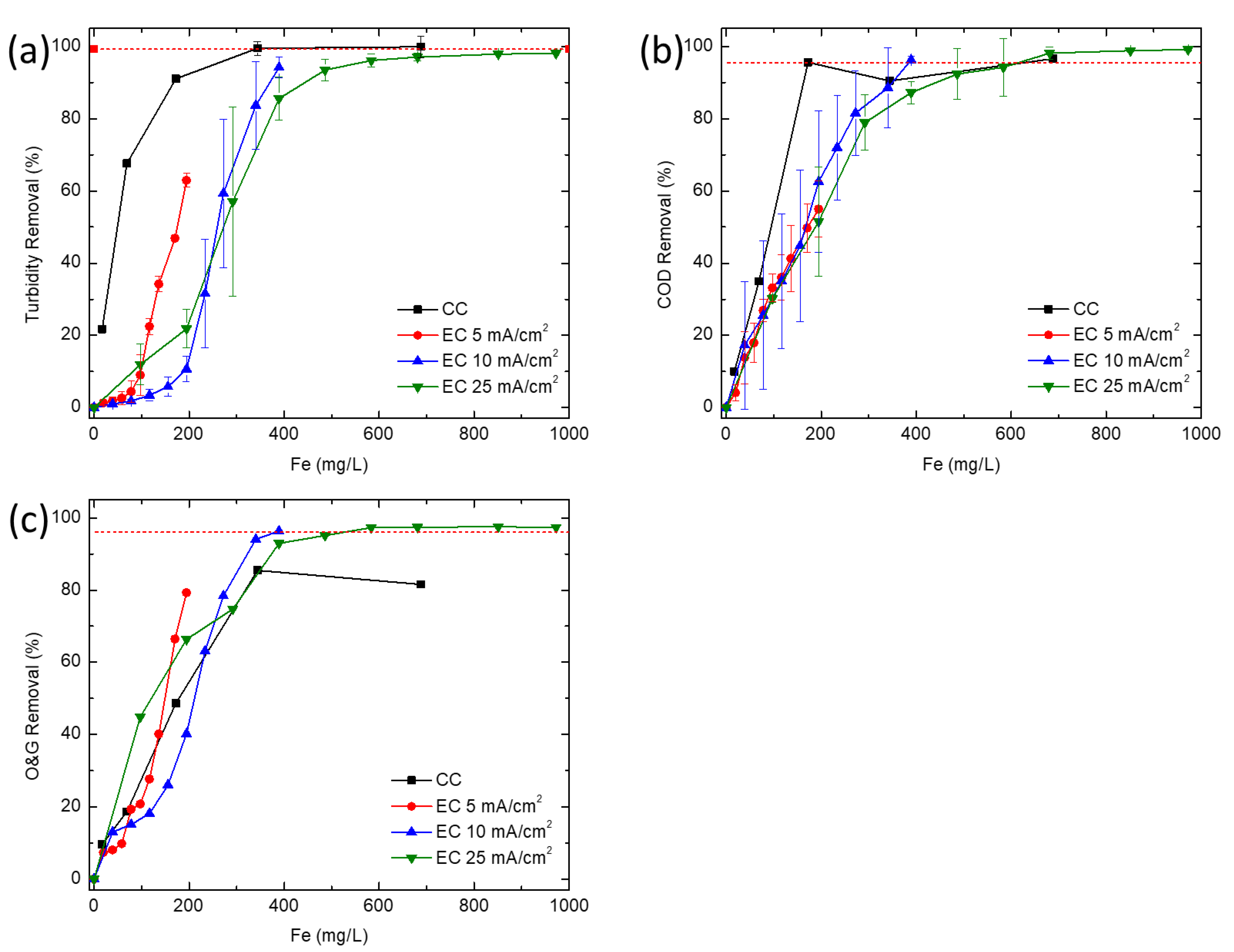
In this study, CC and EC were compared for the removal of turbidity, COD and O&G from a simulated synthetic produced water. CC was able to achieve high COD and turbidity removals at much lower concentrations of iron than EC, but is unable to effectively remove enough O&G to meet offshore discharge limits, even at very high coagulant doses (>345 mg/L). At higher iron concentrations, EC removed COD and O&G adequately to meet typical discharge standards, whereas CC would require additional treatment (e.g., flotation or filtration) to meet most O&G discharge limits. Coagulant overdose leading to poorer coagulation performance seems to only affect CC. This points to the need for extensive testing before an optimum dosage of chemical coagulant can be established, thus further increasing operational costs. While CC is a more established and proven technology, EC offers certain advantages for resource extraction industries such as oil and gas. In particular, oil production is increasingly being explored in remote locations (e.g., offshore, Gulf of Mexico, Athabasca oil sands, Alaskan North Slope, Central Australia, etc.) far from major chemical production centers, so supplying water treatment chemicals could pose significant logistics challenges, energy demand, and high cost for produced water treatment. Additionally, EC offers the opportunity for more compact systems while minimizing onsite chemical storage. Processes such as EC, where water treatment chemistry is generated in situ and onsite, may reduce the energy demand and operating costs associated with meeting existing and future produced water treatment requirements.