Distribution of Toxic Cyanobacteria in Volcanic Lakes of the Azores Islands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collection and Environmental Variables
2.3. Phytoplankton Analysis
2.4. DNA Extraction and PCR Amplification
2.5. Statistical Analysis
3. Results
3.1. Environmental Variables
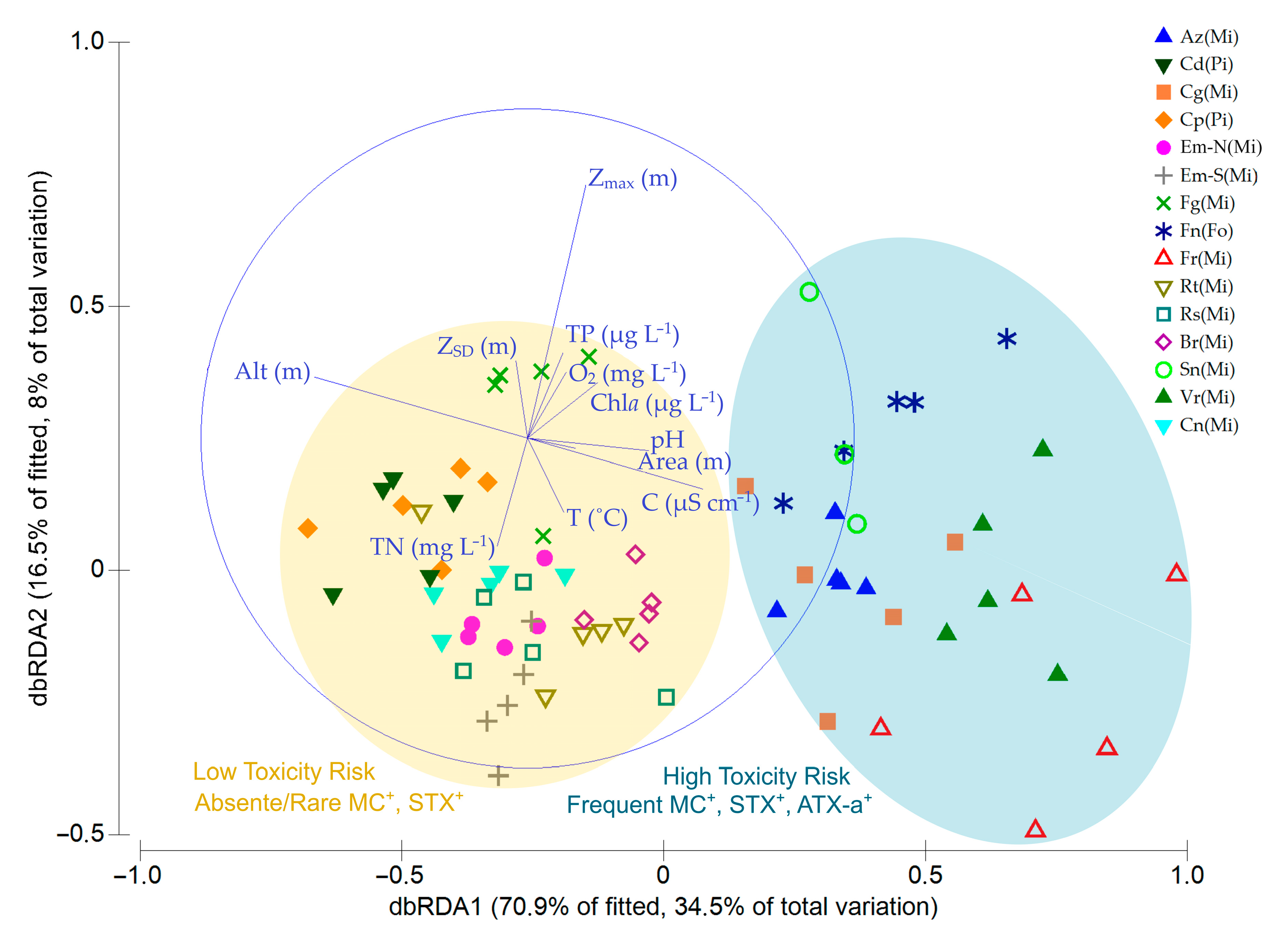
3.2. Cyanobacteria and Environmental Drivers
3.3. Distribution of Cyanotoxin-Producing Genes
4. Discussion
4.1. Toxigenic Cyanobacteria
4.2. Environmental Drivers of Cyanobacteria Occurrence and Cyanotoxins Production Potential
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Townhill, B.L.; Tinker, J.; Jones, M.; Pitois, S.; Creach, V.; Simpson, S.D.; Dye, S.; Bear, E.; Pinnegar, J.K. Harmful algal blooms and climate change: Exploring future distribution changes. ICES J. Mar. Sci. 2018, 75, 1882–1893. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H. Mitigating toxic planktonic cyanobacterial blooms in aquatic ecosystems facing increasing anthropogenic and climatic pressures. Toxins 2018, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, V. Global changes and the new challenges in the research on cyanotoxin risk evaluation. Limnetica 2015, 34, 149–158. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; Domis, L.D.S.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Mantzouki, E.; Visser, P.M.; Bormans, M.; Ibelings, B.W. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquat. Ecol. 2016, 50, 333–350. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms Like It Hot. Climate 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The diversity of cyanobacterial toxins on structural characterization, distribution and identification: A systematic review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Liu, Y.-L.; Conklin, A.; Westrick, J.; Weavers, L.K.; Dionysiou, D.D.; Lenhart, J.J.; Mouser, P.J.; Szlag, D.; Walker, H.W. Toxic cyanobacteria and drinking water: Impacts, detection, and treatment. Harmful Algae 2016, 54, 174–193. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; Chorus, I., Bartram, J., Eds.; F & FN Spon, WHO: London, UK, 1999; pp. 41–111. [Google Scholar]
- Ramos, V.; Moreira, C.; Mankiewicz-Boczek, J.; Vasconcelos, V. Application of molecular tools in monitoring cyanobacteria and their potential toxin production. In Molecular Tools for the Detection and Quantification of Toxigenic Cyanobacteria; Kurmayer, R., Sivonen, K., Wilmotte, A., Salmaso, N., Eds.; John Wiley and Sons Ltd.: West Sussex, UK, 2017. [Google Scholar]
- Codd, G.A.; Meriluoto, J.; Metcalf, J.S. Introduction: Cyanobacteria, Cyanotoxins, Their Human Impact, and Risk Management. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Codd, G.A., Meriluoto, J., Metcalf, J.S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 1–8. [Google Scholar]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Cirés, S.; Casero, M.C.; Quesada, A. Toxicity at the edge of life: A review on cyanobacterial toxins from extreme environments. Mar. Drugs 2017, 15, 233. [Google Scholar] [CrossRef]
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and toxic: Temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 2018, 610–611, 786–795. [Google Scholar] [CrossRef]
- Omidi, A.; Esterhuizen-Londt, M.; Pflugmacher, S. Still challenging: The ecological function of the cyanobacterial toxin microcystin—What we know so far. Toxin Rev. 2018, 37, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- Renaud, S.L.; Pick, F.R.; Fortin, N. Effect of Light Intensity on the Relative Dominance of Toxigenic and Nontoxigenic Strains of Microcystis aeruginosa. Appl. Environ. Microbiol. 2011, 77, 7016–7022. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.A.; Smith, F.M.J.; Heath, M.W.; Palfroy, T.; Gaw, S.; Young, R.G.; Ryan, K.G. Within-mat variability in anatoxin-a and homoanatoxin-a production among benthic Phormidium (cyanobacteria) strains. Toxins 2012, 4, 900–912. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, G.; Molitor, C.; Philmus, B.; Kurmayer, R. Nontoxic Strains of Cyanobacteria are the Result of Major Gene Deletion Events Induced by a Transposable Element. Mol. Biol. Evol. 2008, 25, 1695–1704. [Google Scholar] [CrossRef]
- Fathalli, A.; Jenhani, A.B.R.; Moreira, C.; Welker, M.; Romdhane, M.; Antunes, A.; Vasconcelos, V. Molecular and phylogenetic characterization of potentially toxic cyanobacteria in Tunisian freshwaters. Syst. Appl. Microbiol. 2011, 34, 303–310. [Google Scholar] [CrossRef]
- Feio, M.J.; Filipe, A.F.; Garcia-Raventós, A.; Ardura, A.; Calapez, A.R.; Pujante, A.M.; Mortágua, A.; Múrria, C.; Diaz-de-Quijano, D.; Martins, F.M.; et al. Advances in the use of molecular tools in ecological and biodiversity assessment of aquatic ecosystems. Limnetica 2020, 39, 419–440. [Google Scholar] [CrossRef]
- Moreira, C.; Gomes, C.; Vasconcelos, V.; Antunes, A. Cyanotoxins occurrence in Portugal: A new report on their recent multiplication. Toxins 2020, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Moreira, C.; Ramos, V.; Azevedo, J.; Vasconcelos, V. Methods to detect cyanobacteria and their toxins in the environment. Appl. Microbiol. Biotechnol. 2014, 98, 8073–8082. [Google Scholar] [CrossRef]
- Santos, M.C.R.; Muelle, H.; Pacheco, D.M.D. Cyanobacteria and microcystins in lake Furnas (S. Miguel island-Azores). Limnetica 2012, 31, 107–118. [Google Scholar] [CrossRef]
- Cruz, J.V.; Pacheco, D.; Porteiro, J.; Cymbron, R.; Mendes, S.; Malcata, A.; Andrade, C. Sete Cidades and Furnas lake eutrophication (São Miguel, Azores): Analysis of long-term monitoring data and remediation measures. Sci. Total Environ. 2015, 520, 168–186. [Google Scholar] [CrossRef]
- Vasconcelos, V.M. Eutrophication, toxic cyanobacteria and cyanotoxins: When ecosystems cry for help. Limnetica 2006, 25, 425–432. [Google Scholar] [CrossRef]
- Watson, S.B.; Miller, C.; Arhonditsis, G.; Boyer, G.L.; Carmichael, W.; Charlton, M.N.; Confesor, R.; Depew, D.C.; Höök, T.O.; Ludsin, S.A.; et al. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae 2016, 56, 44–66. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L.; Miller, T.R. Variable cyanobacterial toxin and metabolite profiles across six eutrophic lakes of differing physiochemical characteristics. Toxins 2017, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, R.; Luz, R.; Vasconcelos, V.; Fonseca, A.; Gonçalves, V. A Critical Review of Cyanobacteria Distribution and Cyanotoxins Occurrence in Atlantic Ocean Islands. Cryptogam. Algol. 2020, 41, 73–89. [Google Scholar] [CrossRef]
- Santos, M.C.R.; Pacheco, D.M.M.; Santana, F.; Muelle, H. Cyanobacteria blooms in Sete-Cidades lake—(S. Miguel Island-Azores). Arch. Hydrobiol. Suppl. Algol. Stud. 2005, 117, 393–406. [Google Scholar] [CrossRef]
- Archer, W. Notes on some collections made from Furnas Lake, Azores, containing algae and a few other organisms. Bot. J. Linn. Soc. 1874, 14, 328–340. [Google Scholar] [CrossRef]
- Moseley, H.N. Notes on Fresh-water Algae obtained at the Boiling Springs at Furnas, St. Michael’s, Azores, and their neighbourhood. Bot. J. Linn. Soc. 1874, 14, 321–325. [Google Scholar] [CrossRef]
- Bohlin, K. Étude sur la flore algologique d’eau douce des Açores. K. Sven. Vetensk. Akad. Handl. 1901, 27, 1–85. [Google Scholar]
- Moreira, C.; Martins, A.; Moreira, C.; Vasconcelos, V. Toxigenic cyanobacteria in volcanic lakes and hot springs of a North Atlantic island (S. Miguel, Azores, Portugal). Fresenius Environ. Bull. 2011, 20, 420–426. [Google Scholar]
- Mantzouki, E.; Campbell, J.; van Loon, E.; Visser, P.; Konstantinou, I.; Antoniou, M.; Giuliani, G.; Machado-Vieira, D.; Gurjão de Oliveira, A.; Maronić, D.Š.; et al. A European Multi Lake Survey dataset of environmental variables, phytoplankton pigments and cyanotoxins. Sci. Data 2018, 5, 180226. [Google Scholar] [CrossRef] [Green Version]
- Ledreux, A.; Thomazeau, S.; Catherine, A.; Duval, C.; Yéprémian, C.; Marie, A.; Bernard, C. Evidence for saxitoxins production by the cyanobacterium Aphanizomenon gracile in a French recreational water body. Harmful Algae 2010, 10, 88–97. [Google Scholar] [CrossRef]
- Park, H.-D.; Watanabe, M.F.; Harada, K.-I.; Nagai, H.; Suzuki, M.; Watanabe, M.; Hayashi, H. Hepatotoxin (microcystin) and neurotoxin (anatoxin-a) contained in natural blooms and strains of cyanobacteria from Japanese freshwaters. Nat. Toxins 1993, 1, 353–360. [Google Scholar] [CrossRef]
- Li, R.; Carmichael, W.W.; Brittain, S.; Eaglesham, G.K.; Shaw, G.R.; Liu, Y.; Watanabe, M.M. First Report of the Cyanotoxins Cylindrospermopsin and Deoxycylindrospermopsin from Raphidiopsis curvata (Cyanobacteria). J. Phycol. 2001, 37, 1121–1126. [Google Scholar] [CrossRef]
- Luz, R.; Cordeiro, R.; Vilaverde, J.; Raposeiro, P.; Fonseca, A.; Gonçalves, V. Cyanobacteria from freshwater lakes in the Azores archipelago, Portugal: Data from long term phytoplankton monitoring. Biodivers. Data J. 2020, 8, e51928. [Google Scholar] [CrossRef]
- Hernández, A.; Kutiel, H.; Trigo, R.M.; Valente, M.A.; Sigró, J.; Cropper, T.; Santo, F.E. New Azores archipelago daily precipitation dataset and its links with large-scale modes of climate variability. Int. J. Climatol. 2016, 36, 4439–4454. [Google Scholar] [CrossRef]
- Porteiro, J.M.M. Lagoas dos Açores. Elementos de Suporte ao Planeamento Integrado. Ph.D. Thesis, University of the Azores, Ponta Delgada, Portugal, 2000. [Google Scholar]
- Vázquez-Loureiro, D.; Gonçalves, V.; Sáez, A.; Hernández, A.; Raposeiro, P.M.; Giralt, S.; Rubio-Inglés, M.J.; Rull, V.; Bao, R. Diatom-inferred ecological responses of an oceanic lake system to volcanism and anthropogenic perturbations since 1290 CE. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 534, 109285. [Google Scholar] [CrossRef]
- Raposeiro, P.M.; Rubio, M.J.; González, A.; Hernández, A.; Sánchez-López, G.; Vázquez-Loureiro, D.; Rull, V.; Bao, R.; Costa, A.C.; Gonçalves, V.; et al. Impact of the historical introduction of exotic fishes on the chironomid community of Lake Azul (Azores Islands). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 466, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.L.; Raposeiro, P.M.; Costa, A.C.; Bao, R.; Giralt, S.; Gonçalves, V. Biogeography and lake morphometry drive diatom and chironomid assemblages’ composition in lacustrine surface sediments of oceanic islands. Hydrobiologia 2014, 730, 93–112. [Google Scholar] [CrossRef] [Green Version]
- IPQ. Qualidade da Água. Doseamento da Clorofila a e dos Feopigmentos por Espectrofotometria de Absorção Molecular. Método de Extracção com Acetona. NP 4327/1996; Instituto Português da Qualidade (IPQ): Monte da Caparica, Portugal, 1997. [Google Scholar]
- Lorenzen, C. Determination of chlorophyll and pheopigments: Spectrophotometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, Teil 1/Part 1: Chroococcales; Spektrum Akademischer Verlag: Berlin, Germany, 2008; ISBN 978-3-8274-2111-1. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil/Part 2: Oscillatoriales; Spektrum Akademischer Verlag: Münche, Germany, 2005; ISBN 978-3-8274-1914-9. [Google Scholar]
- Komárek, J. Cyanoprokaryota 3. Teil/Part 3: Heterocytous Genera; Spektrum Akademischer Verlag: Berlin, Germany, 2013; ISBN 978-3-8274-0932-4. [Google Scholar]
- Komárek, J.; Kastovsky, J.; Mares, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 23 July 2019).
- Hisbergues, M.; Christiansen, G.; Rouhiainen, L.; Sivonen, K.; Borner, T. PCR-based identification of microcystin-producing genotypes of different cyanobacterial genera. Arch. Microbiol. 2003, 180, 402–410. [Google Scholar] [CrossRef]
- Ouahid, Y.; Pérez-Silva, G.; Del Campo, F.F. Identification of potentially toxic environmental Microcystis by individual and multiple PCR amplification of specific microcystin synthetase gene regions. Environ. Toxicol. 2005, 20, 235–242. [Google Scholar] [CrossRef]
- Neilan, B.A.; Jacobs, D.; Del Dot, T.; Blackall, L.L.; Hawkins, P.R.; Cox, P.T.; Goodman, A.E. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int. J. Syst. Bacteriol. 1997, 47, 693–697. [Google Scholar] [CrossRef]
- Rantala-Ylinen, A.; Känä, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl. Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef] [Green Version]
- Schembri, M.A.; Neilan, B.A.; Saint, C.P. Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. 2001, 16, 413–421. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E; Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
- Clarke, K.R.; Green, R.H. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E; Plymouth Marine Laboratory: Plymouth, UK, 2008. [Google Scholar]
- OECD. Eutrophication of Waters. Monitoring, Assessment and Control; Organisation for Economic Co-Operation and Development: Paris, France, 1982. [Google Scholar]
- AHA-DRA. Plano de Gestão da Região Hidrográfica dos Açores–RH9. Versão para Consulta Pública (Azores River Basin Management Plan). Ponta Delgada, Azores, Portugal. 2015. Available online: https://siaia.apambiente.pt/AAEstrategica/DA_AAE676.pdf (accessed on 1 December 2020).
- Bernard, C.; Ballot, A.; Thomazeau, S.; Maloufi, S.; Furey, A.; Mankiewicz-Boczek, J.; Pawlik-Skowrońska, B.; Capelli, C.; Salmaso, N. Appendix 2: Cyanobacteria associated with the production of cyanotoxins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 501–525. [Google Scholar]
- Bober, B.; Bialczyk, J. Determination of the toxicity of the freshwater cyanobacterium Woronichinia naegeliana (Unger) Elenkin. J. Appl. Phycol. 2017, 29, 1355–1362. [Google Scholar] [CrossRef] [Green Version]
- Christophoridis, C.; Zervou, S.-K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and diversity of cyanotoxins in Greek lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef] [Green Version]
- Sivonen, K.; Himberg, K.; Luukkainen, R.; Niemelä, S.I.; Poon, G.K.; Codd, G.A. Preliminary characterization of neurotoxic cyanobacteria blooms and strains from Finland. Toxic. Assess. 1989, 4, 339–352. [Google Scholar] [CrossRef]
- Vasconcelos, V.M.; Sivonen, K.; Evans, W.R.; Carmichael, W.W.; Namikoshi, M. Hepatotoxic microcystin diversity in cyanobacterial blooms collected in portuguese freshwaters. Water Res. 1996, 30, 2377–2384. [Google Scholar] [CrossRef]
- Menezes, C.; Churro, C.; Dias, E. Risk Levels of Toxic Cyanobacteria in Portuguese Recreational Freshwaters. Toxins 2017, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Osswald, J.; Rellán, S.; Gago-Martinez, A.; Vasconcelos, V. Production of anatoxin-a by cyanobacterial strains isolated from Portuguese fresh water systems. Ecotoxicology 2009, 18, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in Northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Casero, M.C.; Ballot, A.; Agha, R.; Quesada, A.; Cirés, S. Characterization of saxitoxin production and release and phylogeny of sxt genes in paralytic shellfish poisoning toxin-producing Aphanizomenon gracile. Harmful Algae 2014, 37, 28–37. [Google Scholar] [CrossRef]
- Joung, S.-H.; Oh, H.-M.; Ko, S.-R.; Ahn, C.-Y. Correlations between environmental factors and toxic and non-toxic Microcystis dynamics during bloom in Daechung Reservoir, Korea. Harmful Algae 2011, 10, 188–193. [Google Scholar] [CrossRef]
- Gobler, C.J.; Burkholder, J.M.; Davis, T.W.; Harke, M.J.; Johengen, T.; Stow, C.A.; Van de Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef]
- Geada, P.; Pereira, R.N.; Vasconcelos, V.; Vicente, A.A.; Fernandes, B.D. Assessment of synergistic interactions between environmental factors on Microcystis aeruginosa growth and microcystin production. Algal Res. 2017, 27, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Beversdorf, L.J.; Miller, T.R.; McMahon, K.D. The Role of Nitrogen Fixation in Cyanobacterial Bloom Toxicity in a Temperate, Eutrophic Lake. PLoS ONE 2013, 8, e56103. [Google Scholar] [CrossRef] [Green Version]
- Pearson, L.; Neilan, B. The molecular genetics of cyanobacterial toxicity as a basis for monitoring water quality and public health risk. Curr. Opin. Biotechnol. 2008, 19, 281–288. [Google Scholar] [CrossRef]
- Borges, H.L.F.; Branco, L.H.Z.; Martins, M.D.; Lima, C.S.; Barbosa, P.T.; Lira, G.A.S.; Bittencourt-Oliveira, M.C.; Molica, R.J.R. Cyanotoxin production and phylogeny of benthic cyanobacterial strains isolated from the northeast of Brazil. Harmful Algae 2015, 43, 46–57. [Google Scholar] [CrossRef]
- Pereira, P.; Onodera, H.; Andrinolo, D.; Franca, S.; Araújo, F.; Lagos, N.; Oshima, Y. Paralytic shellfish toxins in the freshwater cyanobacterium Aphanizomenon flos-aquae, isolated from Montargil reservoir, Portugal. Toxicon 2000, 38, 1689–1702. [Google Scholar] [CrossRef]
- Rücker, J.; Stüken, A.; Nixdorf, B.; Fastner, J.; Chorus, I.; Wiedner, C. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 2007, 50, 800–809. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S.; Hadas, O.; Teltsch, B.; Porat, R.; Sukenik, A. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from lake Kinneret, Israel. J. Phycol. 1997, 33, 613–616. [Google Scholar] [CrossRef]
- Quesada, A.; Moreno, E.; Carrasco, D.; Paniagua, T.; Wormer, L.; De Hoyos, C.; Sukenik, A. Toxicity of Aphanizomenon ovalisporum (Cyanobacteria) in a Spanish water reservoir. Eur. J. Phycol. 2006, 41, 39–45. [Google Scholar] [CrossRef]
- Ohtani, I.; Moore, R.E.; Runnegar, M.T.C. Cylindrospermopsin: A potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 1992, 114, 7941–7942. [Google Scholar] [CrossRef]
- INOVA. Análise das Águas das Lagoas da Região Autónoma dos Açores; INOVA: Ponta Delgada, Portugal, 1996. [Google Scholar]
- Moreira, C.; Mendes, R.; Azevedo, J.; Vasconcelos, V.; Antunes, A. First occurrence of cylindrospermopsin in Portugal: A contribution to its continuous global dispersal. Toxicon 2017, 130, 87–90. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; Van Donk, E.; Hansson, L.-A.; Jeppesenk, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Robarts, R.D.; Zohary, T. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. N. Zeal. J. Mar. Freshw. Res. 1987, 21, 391–399. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; Official Journal 22 December 2000 L 327/1; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Cruz, J.V.; Antunes, P.; Amaral, C.; França, Z.; Nunes, J.C. Volcanic lakes of the Azores archipelago (Portugal): Geological setting and geochemical characterization. J. Volcanol. Geotherm. Res. 2006, 135–157. [Google Scholar] [CrossRef]
- Richardson, J.; Miller, C.; Maberly, S.C.; Taylor, P.; Globevnik, L.; Hunter, P.; Jeppesen, E.; Mischke, U.; Moe, S.J.; Pasztaleniec, A.; et al. Effects of multiple stressors on cyanobacteria abundance vary with lake type. Glob. Chang. Biol. 2018, 24. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.A.; Maier, M.Y.; Puddick, J.; Pochon, X.; Zaiko, A.; Dietrich, D.R.; Hamilton, D.P. Trophic state and geographic gradients influence planktonic cyanobacterial diversity and distribution in New Zealand lakes. FEMS Microbiol. Ecol. 2017, 93, fiw234. [Google Scholar] [CrossRef]
- Gallina, N.; Salmaso, N.; Morabito, G.; Beniston, M. Phytoplankton configuration in six deep lakes in the peri-Alpine region: Are the key drivers related to eutrophication and climate? Aquat. Ecol. 2013, 47, 177–193. [Google Scholar] [CrossRef] [Green Version]
- Bukowska, A.; Kaliński, T.; Koper, M.; Kostrzewska-Szlakowska, I.; Kwiatowski, J.; Mazur-Marzec, H.; Jasser, I. Predicting blooms of toxic cyanobacteria in eutrophic lakes with diverse cyanobacterial communities. Sci. Rep. 2017, 7, 8342. [Google Scholar] [CrossRef] [Green Version]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Petersen, A.C. The distribution of planktonic Cyanobacteria in Irish lakes in relation to their trophic states. Hydrobiologia 2000, 91–99. [Google Scholar] [CrossRef]
- Whitton, B.; Potts, M. Introduction to the Cyanobacteria. In The Ecology of Cyanobacteria II—Their Diversity in Time and Space; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. ISBN 978-94-007-3854-6. [Google Scholar]
- Hamilton, D.P.; Salmaso, N.; Paerl, H.W. Mitigating harmful cyanobacterial blooms: Strategies for control of nitrogen and phosphorus loads. Aquat. Ecol. 2016, 50, 351–366. [Google Scholar] [CrossRef]
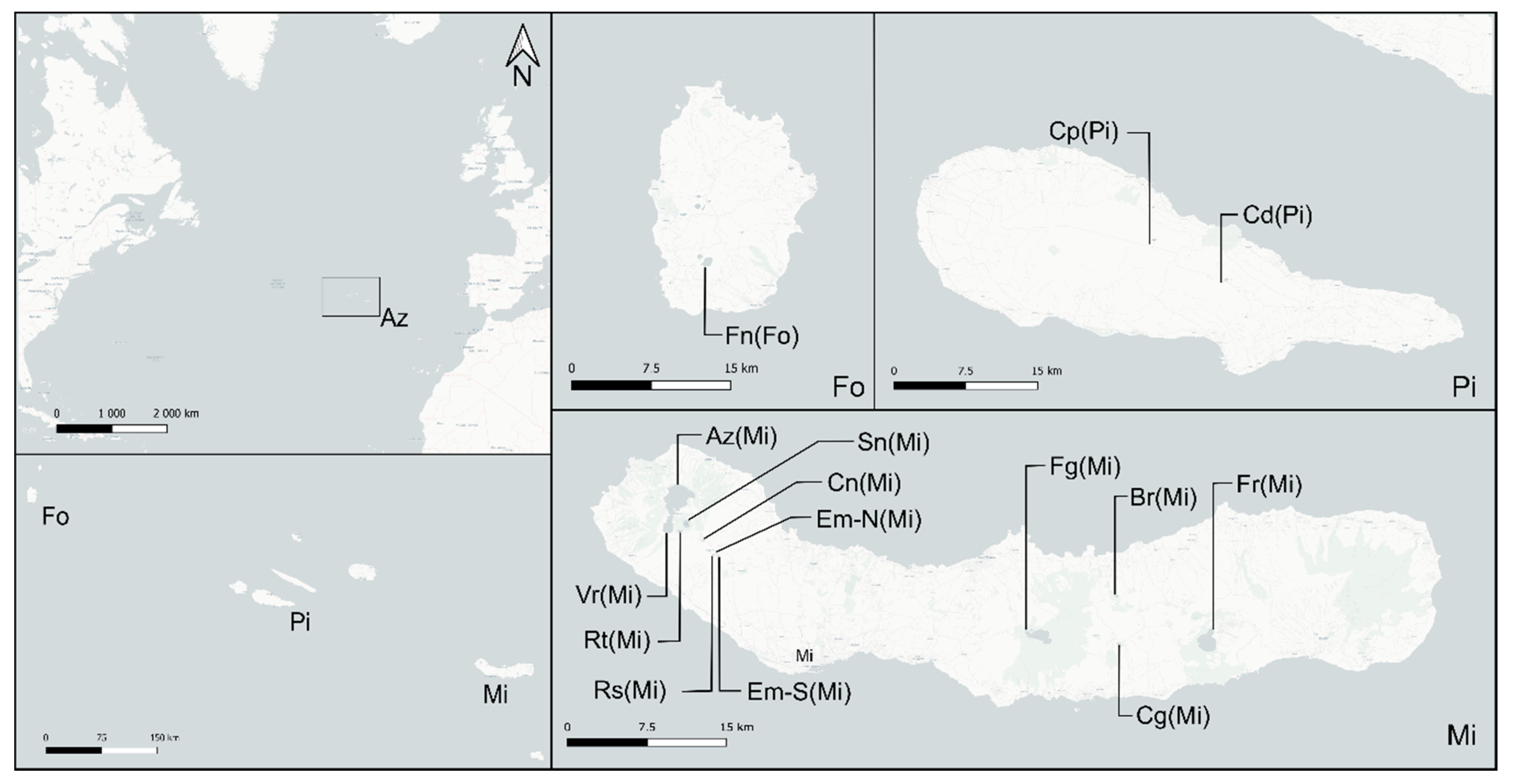


| Lake Name | Lake | Hydromorphological Characteristics | Physicochemical Variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lat (UTM) | Lon (UTM) | Alt (m) | Area (km2) | Zmax (m) | ZSD (m) | T (°C) | pH | O2 (mg L−1) | C (µS cm−1) | TN (mg L−1) | TP (µg L−1) | Chla (µg L−1) | ||
| Azul | Az(Mi) | 4,192,417.68 | 608,243.52 | 260 | 3.587 | 25.4 | 3.2 ± 0.7 | 19.0 ± 3.7 | 7.5 ± 0.2 | 9.3 ± 0.8 | 102 ± 5 | 1.1 ± 0.1 | 20.6 ± 7.4 | 2.8 ± 1.3 |
| São Brás | Br(Mi) | 4,184,056.94 | 640,013.62 | 610 | 0.058 | 2.0 | 0.5 ± 0.4 | 16.2 ± 3.5 | 6.7 ± 0.4 | 9.7 ± 0.6 | 38 ± 4 | 0.9 ± 0.3 | 109.4 ± 47.3 | 24.4 ± 14.5 |
| Caiado | Cd(Pi) | 4,257,155.56 | 390,879.19 | 810 | 0.055 | 4.7 | 2.7 ± 0.4 | 14.2 ± 3.0 | 6.6 ± 0.3 | 9.9 ± 0.9 | 30 ± 3 | 0.5 ± 0.3 | 17.8 ± 7.0 | 2.0 ± 0.9 |
| Congro | Cg(Mi) | 4,179,982.69 | 640,241.90 | 420 | 0.037 | 17.6 | 2.4 ± 0.7 | 18.0 ± 4.1 | 8.2 ± 1.0 | 9.4 ± 1.6 | 98 ± 3 | 1.0 ± 0.2 | 17.6 ± 9.2 | 12.5 ± 7.4 |
| Canário | Cn(Mi) | 4,188,336.28 | 609,147.63 | 750 | 0.018 | 2.7 | 1.0 ± 0.2 | 16.6 ± 4.8 | 7.0 ± 0.8 | 8.9 ± 0.9 | 38 ± 4 | 0.4 ± 0.1 | 37.4 ± 12.6 | 8.1 ± 3.7 |
| Capitão | Cp(Pi) | 4,260,771.00 | 384,929.00 | 790 | 0.027 | 4.3 | 0.9 ± 0.1 | 14.4 ± 2.8 | 6.0 ± 0.9 | 10.5 ± 1.2 | 35 ± 7 | 0.6 ± 0.3 | 45.0 ± 15.6 | 23.3 ± 15.3 |
| Empadadas-N | Em-N(Mi) | 4,187,226.07 | 610,176.10 | 740 | 0.018 | 2.7 | 1.8 ± 0.4 | 16.8 ± 5.0 | 7.2 ± 0.5 | 9.1 ± 0.7 | 39 ± 4 | 0.5 ± 0.3 | 19.4 ± 6.2 | 5.8 ± 3.4 |
| Empadadas-S | Em-S(Mi) | 4,187,091.70 | 610,274.94 | 750 | 0.005 | 2.7 | 2.0 ± 0.5 | 15.6 ± 3.4 | 7.6 ± 0.4 | 9.0 ± 0.7 | 51 ± 6 | 1.1 ± 0.5 | 13.8 ± 1.8 | 4.5 ± 2.7 |
| Fogo | Fg(Mi) | 4,180,740.35 | 633,514.96 | 574 | 1.437 | 26.6 | 3.1 ± 0.4 | 15.8 ± 3.0 | 7.5 ± 0.4 | 9.1 ± 1.2 | 48 ± 2 | 0.7 ± 0.2 | 17.6 ± 2.6 | 4.0 ± 2.1 |
| Funda | Fn(Fo) | 4,363,277.32 | 653,537.56 | 360 | 0.355 | 33.8 | 1.8 ± 1.1 | 17.0 ± 2.8 | 7.9 ± 0.7 | 10.2 ± 1.5 | 120 ± 0 | 0.5 ± 0.2 | 25.1 ± 5.3 | 19.6 ± 14.8 |
| Furnas | Fr(Mi) | 4,180,143.86 | 647,150.83 | 280 | 1.926 | 12.0 | 0.8 ± 0.3 | 18.2 ± 3.7 | 8.0 ± 0.5 | 10.1 ± 1.3 | 148 ± 13 | 1.2 ± 1.0 | 59.4 ± 25.1 | 25.0 ± 14.6 |
| Rasa das Sete Cidades | Rt(Mi) | 4,189,125.49 | 607,359.61 | 545 | 0.039 | 3.9 | 3.8 ± 0.3 | 16.6 ± 4.2 | 6.1 ± 0.6 | 9.3 ± 0.6 | 48 ± 3 | 0.5 ± 0.3 | 21.0 ± 19.0 | 1.6 ± 0.7 |
| Rasa da Serra Devassa | Rs(Mi) | 4,187,131.44 | 609,876.37 | 765 | 0.033 | 0.4 | 0.6 ± 0.4 | 15.2 ± 4.0 | 7.0 ± 0.6 | 9.1 ± 0.6 | 40 ± 5 | 0.4 ± 0.2 | 20.0 ± 12.2 | 2.3 ± 1.2 |
| Santiago | Sn(Mi) | 4,189,551.18 | 607,989.45 | 360 | 0.254 | 26.0 | 2.6 ± 0.8 | 18.7 ± 4.0 | 7.5 ± 0.5 | 8.5 ± 0.1 | 123 ± 6 | 0.4 ± 0.0 | 17.0 ± 4.4 | 9.9 ± 6.0 |
| Verde | Vr(Mi) | 4,189,071.90 | 606,553.94 | 260 | 0.856 | 22.4 | 3.3 ± 3.2 | 18.0 ± 3.7 | 8.2 ± 1.0 | 9.6 ± 1.4 | 126 ± 5 | 0.5 ± 0.2 | 26.8 ± 17.8 | 17.7 ± 14.9 |
| Variable | Cyanobacteria Species | Cyanotoxin-Producing Genes | ||||||
|---|---|---|---|---|---|---|---|---|
| SS (Trace) | Pseudo-F | p | Prop | SS (Trace) | Pseudo-F | p | Prop | |
| Zmax (m) | 34,170.0 | 9.48 | 0.001 | 0.118 | 5.97 | 13.89 | 0.001 | 0.0164 |
| Alt (m) | 31,393.0 | 8.62 | 0.001 | 0.108 | 10.96 | 30.40 | 0.001 | 0.300 |
| C (µS cm−1) | 29,600.0 | 8.07 | 0.001 | 0.102 | 10.97 | 30.43 | 0.001 | 0.300 |
| Area (Km2) | 21,175.0 | 5.59 | 0.001 | 0.073 | 2.58 | 5.39 | 0.003 | 0.071 |
| pH | 16,805.0 | 4.37 | 0.001 | 0.058 | 5.72 | 13.18 | 0.001 | 0.157 |
| TP (µg L−1) | 10,648.0 | 2.71 | 0.006 | 0.037 | 1.59 | 3.23 | 0.025 | 0.044 |
| T (°C) | 9490.4 | 2.4 | 0.016 | 0.033 | 1.2 | 2.42 | 0.073 | 0.033 |
| Chla (µg L−1) | 9279.2 | 2.35 | 0.019 | 0.032 | 2.15 | 4.44 | 0.007 | 0.059 |
| ZSD (m) | 6824.9 | 1.71 | 0.085 | 0.024 | 0.44 | 0.88 | 0.430 | 0.022 |
| O2 (mg L−1) | 3259.7 | 0.81 | 0.603 | 0.011 | 0.64 | 1.26 | 0.282 | 0.017 |
| TN (mg L−1) | 1844.8 | 0.45 | 0.875 | 0.006 | 0.54 | 1.06 | 0.371 | 0.015 |
| October 2015 | February 2016 | April 2016 | August 2016 | November 2016 | ||
|---|---|---|---|---|---|---|
| Az(Mi) | Cyanotoxins genes * | MC, STX | STX | MC, STX | ||
| Abundance (cells mL−1) | 15,944 | 12,772 | 52,286 | 230,568 | 26,482 | |
| Biomass (µg L−1) | 2334.87 | 1571.97 | 5223.30 | 6231.13 | 3961.86 | |
| Dominant Species | Plli, Maer | Plli, Wnae, Maer, Mflo, Dpla | Plli, Syne, Mflo, Dpla | Psli | Ckue, Plli, Dpla | |
| Br(Mi) | Cyanotoxins genes * | MC | STX | |||
| Abundance (cells mL−1) | 4635 | 8611 | 200,670 | 812 | 48,477 | |
| Biomass (µg L−1) | 125.15 | 867.40 | 13,351.60 | 59.43 | 1819.47 | |
| Dominant Species | Psli | Plli, Doli | Psli, Doli | Psli, Doli | Psli, Doli | |
| Cyanotoxins genes * | ||||||
| Cd(Pi) | Abundance (cells mL−1) | 9 | 45 | 238 | 235 | 3 |
| Biomass (µg L−1) | 0.62 | 6.59 | 10.90 | 10.92 | 0.21 | |
| Dominant Species | Doli | Plli, Doli | Wnae, Doli | Wnae | Doli | |
| Cg(Mi) | Cyanotoxins genes * | MC | MC | MC, STX | MC | MC, STX |
| Abundance (cells mL−1) | 292 | 13,689 | 577 | 6360 | 474 | |
| Biomass (µg L−1) | 8.53 | 2332.32 | 40.79 | 205.87 | 38.61 | |
| Dominant Species | Mflo, Oten, Dpla | Maer, Mflos, Dpla | Psli, Mflo, Dpla | Wnae, Mflo | Wnae, Dpla | |
| Cn(Mi) | Cyanotoxins genes * | |||||
| Abundance (cells mL−1) | 0 | 226 | 0 | 0 | 914 | |
| Biomass (µg L−1) | 0.00 | 6.10 | 0.00 | 0.00 | 138.93 | |
| Dominant Species | Psli | Plli | ||||
| Cp(Pi) | Cyanotoxins genes * | STX | ||||
| Abundance (cells mL−1) | 104,020 | 100,169 | 10,125 | 258,102 | 1130 | |
| Biomass (µg L−1) | 5329.63 | 6910.65 | 691.44 | 29,215.45 | 83.93 | |
| Dominant Species | Psli, Doli | Doli. | Doli | Doli | Psli, Oten | |
| Em-N(Mi) | Cyanotoxins genes * | |||||
| Abundance (cells mL−1) | 329 | 0 | 268 | 0 | 456 | |
| Biomass (µg L−1) | 8.88 | 0.00 | 27.93 | 0.00 | 16.45 | |
| Dominant Species | Psli | Doli, Dpla | Psli, Wnae | |||
| Em-S(Mi) | Cyanotoxins genes * | |||||
| Abundance (cells mL−1) | 97 | 24 | 2768 | 1811 | 230 | |
| Biomass (µg L−1) | 9.36 | 1.56 | 124.97 | 90.13 | 10.35 | |
| Dominant Species | Doli, Dpla | Cmin | Wnae | Psli, Wnae, Agra | Wnae | |
| Fg(Mi) | Cyanotoxins genes * | STX | STX | |||
| Abundance (cells mL−1) | 282,656 | 4572 | 3182 | 73,514 | 46,632 | |
| Biomass (µg L−1) | 42,963.71 | 694.94 | 477.61 | 11,174.13 | 7088.06 | |
| Dominant Species | Plli | Plli | Plli | Plli | Plli | |
| Fn(Fo) | Cyanotoxins genes * | STX, ATX-a | STX | ATX-a | STX, ATX-a | STX |
| Abundance (cells mL−1) | 89,471 | 23,415 | 25,835 | 14,331 | 76,323 | |
| Biomass (µg L−1) | 7713.89 | 2512.88 | 3902.39 | 803.95 | 5722.07 | |
| Dominant Species | Plli, Agra | Plli, Agra | Plli | Mflo, Dspi | Agra | |
| Fr(Mi) | Cyanotoxins genes * | MC, ATX-a | MC | MC, ATX-a | MC, ATX-a | MC, STX, ATX-a |
| Abundance (cells mL−1) | 451,520 | 40,027 | 32,841 | 483,184 | 486,886 | |
| Biomass (µg L−1) | 6796.31 | 821.51 | 775.22 | 7261.64 | 7841.75 | |
| Dominant Species | Mflo | Ckue, Plli, Maer, Mflo | Syne, Dspi | Ckue, Mflo | Ckue, Maer, Mflo | |
| Rs(Mi) | Cyanotoxins genes * | STX | ||||
| Abundance (cells mL−1) | 1159 | 128 | 971 | 461 | 0 | |
| Biomass (µg L−1) | 32.11 | 8.83 | 47.66 | 20.75 | 0.00 | |
| Dominant Species | Psli | Doli | Wnae, Doli | Wnae | ||
| Rt(Mi) | Cyanotoxins genes * | MC | ||||
| Abundance (cells mL−1) | 11,259 | 9143 | 245 | 13,027 | 11,113 | |
| Biomass (µg L−1) | 724.86 | 594.30 | 15.93 | 846.76 | 720.95 | |
| Dominant Species | Psli, Cmin | Cmin | Cmin | Cmin | Cmin | |
| Sn(Mi) | Cyanotoxins genes * | STX, ATX-a | ** | ** | STX | |
| Abundance (cells mL−1) | 25,099 | ** | ** | 13,011 | 107,668 | |
| Biomass (µg L −1) | 1657.79 | ** | ** | 368.50 | 7742.28 | |
| Dominant Species | Psli, Mflo, Agra | ** | ** | Ckue, Maer, Mflo | Mflo, Agra | |
| Vr(Mi) | Cyanotoxins genes * | MC, STX | MC, STX | MC, STX | MC, STX | MC, STX |
| Abundance (cells mL−1) | 2148 | 1901 | 239,905 | 23,878 | 53,100 | |
| Biomass (µg L−1) | 57.21 | 139.58 | 27,168.49 | 536.99 | 3057.41 | |
| Dominant Species | Mflo, Dpla | Ckue, Plli, Snow, Wnae, Mflos, Agra | Syne, Mflo, Dpla | Ckue, Plli, Syne, Mflo | Ckue, Plli, Maer, Mflo, Doli |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, R.; Luz, R.; Vilaverde, J.; Vasconcelos, V.; Fonseca, A.; Gonçalves, V. Distribution of Toxic Cyanobacteria in Volcanic Lakes of the Azores Islands. Water 2020, 12, 3385. https://doi.org/10.3390/w12123385
Cordeiro R, Luz R, Vilaverde J, Vasconcelos V, Fonseca A, Gonçalves V. Distribution of Toxic Cyanobacteria in Volcanic Lakes of the Azores Islands. Water. 2020; 12(12):3385. https://doi.org/10.3390/w12123385
Chicago/Turabian StyleCordeiro, Rita, Rúben Luz, Joana Vilaverde, Vitor Vasconcelos, Amélia Fonseca, and Vítor Gonçalves. 2020. "Distribution of Toxic Cyanobacteria in Volcanic Lakes of the Azores Islands" Water 12, no. 12: 3385. https://doi.org/10.3390/w12123385
APA StyleCordeiro, R., Luz, R., Vilaverde, J., Vasconcelos, V., Fonseca, A., & Gonçalves, V. (2020). Distribution of Toxic Cyanobacteria in Volcanic Lakes of the Azores Islands. Water, 12(12), 3385. https://doi.org/10.3390/w12123385









