Isolation of Efficient Metal-Binding Bacteria from Boreal Peat Soils and Development of Microbial Biosorbents for Improved Nickel Scavenging
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Microorganisms from Peat Soil Samples with Emphasis on Actinobacteria
2.2. Identification of the Isolates by 16S rDNA Sequencing
2.3. Isolation of Pure Strains from Mixed Cultures
2.4. Cultivation of the Obtained Isolates in Media Containing Various Carbon and Nitrogen Sources
2.5. Siderophore Production
2.5.1. Preparation of CAS-Fe Reagents
2.5.2. CAS Assay with Spent Media
2.6. Immobilization on Sphagnum Peat Moss
2.7. Nickel Biosorption
3. Results
3.1. Identification of the Obtained Isolates
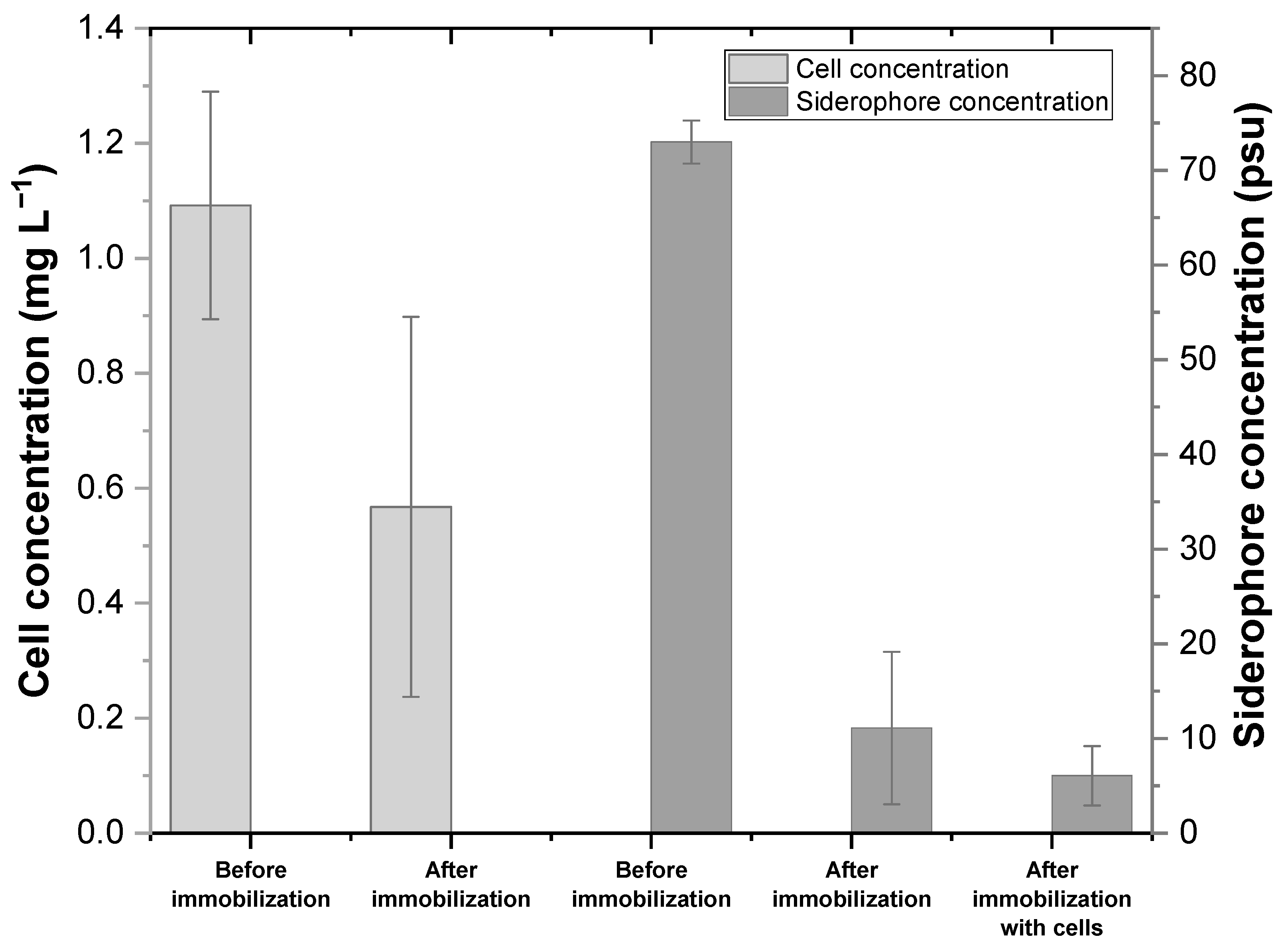
3.2. Evaluation of Siderophore Production and Immobilization Efficiency
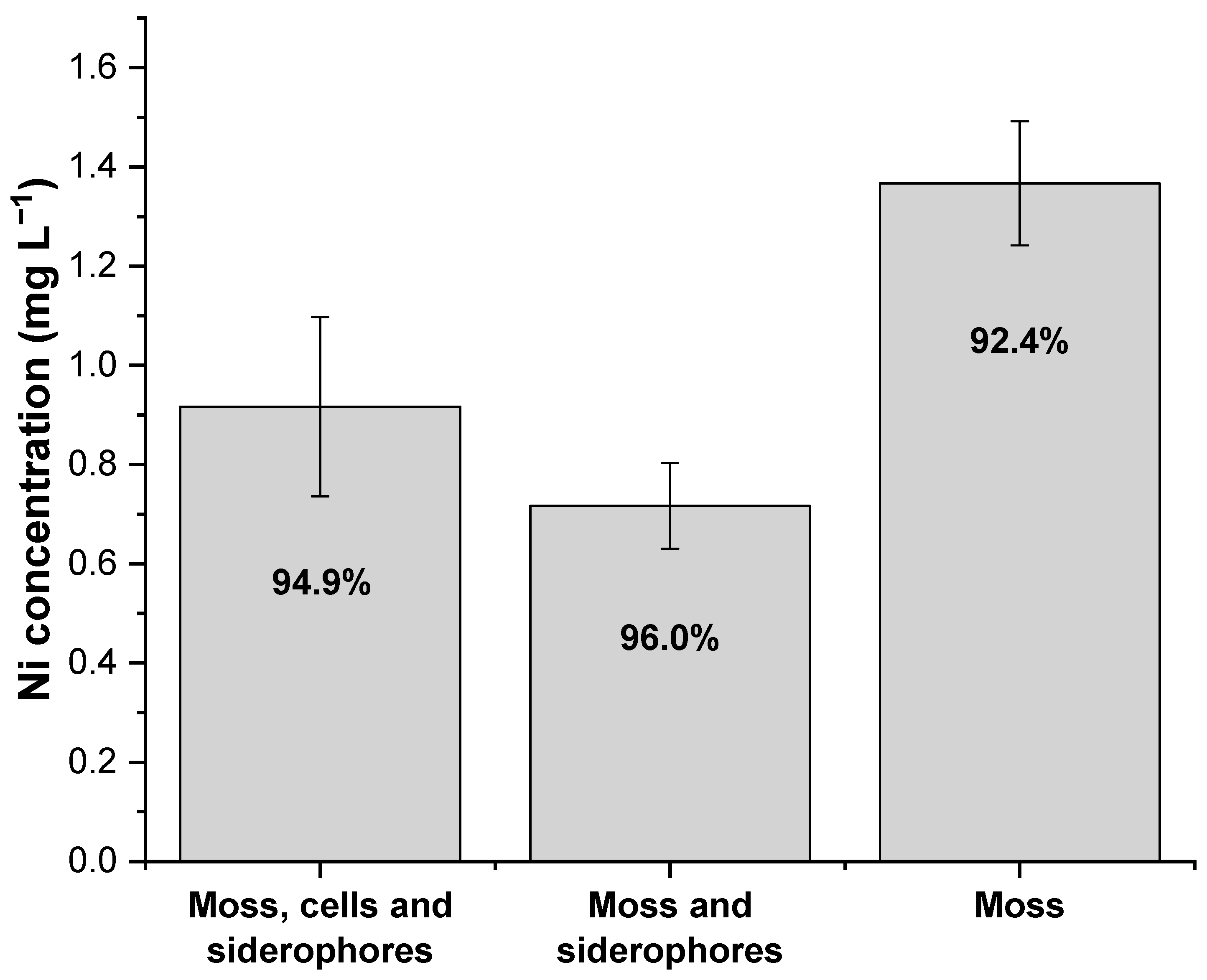
3.3. Evaluation of the Nickel Binding Efficiency of Hybrid Biosorbents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hider, R.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.B. Iron Transport in Microbes, Plants, and Animals. In Comparative Biochemistry of Microbial Iron Assimilation; Winkelmann, G., Van der Helm, D., Neilands, J.B., Eds.; VCH: Weinheim, Germany, 1987; pp. 3–33. [Google Scholar]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zloch, M.; Thiem, D.; Gadzala-Kopciuch, R.; Hrynkiewicz, K. Synthesis of siderophores by plant-associated metallotolerant bacteria under exposure to Cd 2+. Chemosphere 2016, 156, 312–325. [Google Scholar] [CrossRef] [PubMed]
- LePan, N. All the World’s Metals and Minerals in One Visualization. In Visual Capitalist; Visual Capitalist: Vancouver, WA, USA, 2020. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- International Labour Organization. International Chemical Safety Cards—Nickel; International Labour Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Yonebayashi, K. Studies on sustainable land use and soil ecosystems in tropical peat land. Tropics 2006, 15, 313–320. [Google Scholar] [CrossRef][Green Version]
- Fahmi, A.; Radjagukguk, B.; Purwanto, B.H.; Hanudin, E. The role of peat layers on iron dynamics in peatlands. J. Trop. Soils 2010, 15, 195–201. [Google Scholar] [CrossRef]
- Taskila, S.; Särkelä, R.; Tanskanen, J. Valuable applications for peat moss. Biomass Convers. Biorefin 2016, 6, 115–126. [Google Scholar] [CrossRef]
- Gunnarsson, U. Global patterns of Sphagnum productivity. J. Bryol. 2005, 27, 269–279. [Google Scholar] [CrossRef]
- Charman, D. Peatlands and Environmental Change; John Wiley & Sons Ltd.: Chichester, UK, 2002. [Google Scholar]
- Nakouti, I.; Sihanonth, P.; Hobbs, G. A New Approach to Isolating Siderophore-Producing Actinobacteria. Lett. Appl. Microbiol. 2012, 55, 68–72. [Google Scholar] [CrossRef]
- Wang, W.; Qiu, Z.; Tan, H.; Cao, L. Siderophore Production by Actinobacteria. Biometals 2014, 27, 623–631. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Tang, L.; Salvador, J.M. Copper adsorption by esterified and unesterified fractions of Sphagnum peat moss and its different humic substances. J. Hazard. Mater. 1996, 48, 191–206. [Google Scholar] [CrossRef]
- Taskila, S.; Leiviskä, T.; Haapalainen, O.; Tanskanen, J. Utilization of Industrial Microbe Side Streams for Biosorption of Heavy Metals from Wastewaters. J. Bioremed. Biodeg. 2015, 6, 10. [Google Scholar] [CrossRef]
- MACHEREY-NAGEL GmbH & Co. Genomic DNA from Microorganisms; MACHEREY-NAGEL GmbH & Co.: Duren, Germany, 2015. [Google Scholar]
- Villegas, M.E.D. Microbial siderophores. In Biotechnological Production of Siderophores; Varma, A., Chincholkar, S.B., Eds.; Springer: Berlin, Germany, 2007; Volume 12, pp. 219–231. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J. Microbiol. Methods 2001, 44, 89–95. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech. 2017, 7, 381. [Google Scholar] [CrossRef]
- Owen, J.G.; Ackerley, D.F. Characterization of pyoverdine and achromobactin in Pseudomonas syringae pv. phaseolicola 1448a. BMC Microbiol. 2011, 11, 218. [Google Scholar] [CrossRef]
- Payne, S.M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993, 1, 66–69. [Google Scholar] [CrossRef]
- Santos, D.T.; Sarrouh, B.F.; Rivaldi, J.D.; Converti, A.; Silva, S.S. Use of sugarcane bagasse as biomaterial for cell immobilization for xylitol production. J. Food Eng. 2008, 86, 542–548. [Google Scholar] [CrossRef]
- Finnish Environment Institute. SFS-EN ISO 11885:2009; Finnish Standards Association: Helsinki, Finland, 2009.
- Gogoi, H.; Leiviskä, T.; Heiderscheidt, E.; Postila, H.; Tanskanen, J. Removal of metals from industrial wastewater and urban runoff by mineral and bio-based sorbents. J. Environ. Manag. 2018, 209, 316–327. [Google Scholar] [CrossRef]
- Dimkpa, C. Microbial siderophores: Production, detection and application in agriculture and environment. Endocytobiosis Cell Res. 2016, 27, 7–16. [Google Scholar]
- Wilson, M.K.; Abergel, R.J.; Raymond, K.N.; Arceneaux, J.E.L.; Byers, B.R. Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Biochem. Biophys. Res. Commun. 2006, 348, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Boyer, G.L. Siderophore-mediated aluminum uptake by Bacillus megaterium ATCC 19213. Appl. Environ. Microbiol. 1996, 62, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 1–28. [Google Scholar] [CrossRef]
- Turick, C.E.; Knox, A.S.; Leverette, C.L.; Kritzas, Y.G. In situ uranium stabilization by microbial metabolites. J. Environ. Radioact. 2008, 99, 890–899. [Google Scholar] [CrossRef]
- Turick, C.E.; Knox, A.S.; Becnel, J.M.; Ekechukwu, A.A.; Milliken, C.E. Biopolymers. In Properties and Function of Pyomelanin; Elnashar, M., Ed.; IntechOpen: Rijeka, Croatia, 2010; pp. 450–472. [Google Scholar]
- Zheng, H.; Chatfield, C.H.; Liles, M.R.; Cianciotto, N.P. Secreted Pyomelanin of Legionella pneumophila Promotes Bacterial Iron Uptake and Growth under Iron-Limiting Conditions. Infect. Immun. 2013, 81, 4182–4191. [Google Scholar] [CrossRef]
- John, S.G.; Ruggiero, C.E.; Hersman, L.E.; Tung, C.; Neu, M.P. Siderophore Mediated Plutonium Accumulation by Microbacterium flavescens (JG-9). Environ. Sci. Technol. 2001, 35, 2942–2948. [Google Scholar] [CrossRef]
- Pasek, J.; Buechler, R.; Albrecht, R.; Boland, W.; Zeth, K. Structure and mechanism of iron translocation by a DPS protein from Microbacterium arborescens. J. Biol. Chem. 2011, 286, 34872–34882. [Google Scholar] [CrossRef]
- Cruz-Morales, P.; Ramos-Aboites, H.E.; Licona-Cassani, C.; Selem-Mójica, N.; Mejía-Ponce, P.M.; Souza-Saldívar, V.; Barona-Gómez, F. Actinobacteria phylogenomics, selective isolation from an iron oligotrophic environment and siderophore functional characterization, unveil new desferrioxamine traits. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Nguyen, N.; Kim, Y.; Hoang, V.; Min, J.; Hwang, K.; Yang, D. Microbacterium panaciterrae sp. nov., isolated from the rhizosphere of ginseng. Int. J. Syst. Evol. Microbiol. 2015, 65, 927–933. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.; Angle, J.S.; Delorme, T.A.; Chaney, R.L.; Van Berkum, P.; Moawad, H.; Ghanem, K.; Ghozlan, H.A. Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale. New Phytol. 2003, 158, 219–224. [Google Scholar] [CrossRef]
- Gallois, N. Proteogenomic insights into uranium tolerance of a Chernobyl’s Microbacterium bacterial isolate. J. Proteomics 2018, 177, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, M.; Montero-Álvarez, L.A.; Tobón-Avilés, A.; Fierros-Romero, G.; Rojas-Avelizapa, N.G. Microbacterium oxydans and Microbacterium liquefaciens: A biological alternative for the treatment of Ni-V-containing wastes. J. Environ. Sci. Health Part A 2015, 50, 602–610. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Prabhu, G.N.; Bindu, P. Optimization of Process Parameters for Siderophore Production Under Solid State Fermentation Using Polystyrene Beads as Inert Support. J. Sci. Ind. Res. 2016, 75, 621–625. [Google Scholar]
- Duffy, B.K.; Defago, G. Environmental Factors Modulating Antibiotic and Siderophore Biosynthesis by Pseudomonas fluorescens Biocontrol Strains. Appl. Environ. Microbiol. 1999, 65, 2429–2438. [Google Scholar] [CrossRef] [PubMed]
- Digat, B.; Mattar, J. Effects of temperature on growth and siderophore production of Pseudomonas fluorescens-putida strains. Symbiosis 1990, 9, 207–213. [Google Scholar]
- Saha, R.; Saha, N.; Donofrio, R.S.; Bestervelt, L.L. Microbial siderophores: A mini review. J. Basic Microbiol. 2013, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A. Removal of nickel from aqueous solution by the bacterium Bacillus thuringiensis. J. Hazard. Mater. 2007, 147, 518–523. [Google Scholar] [CrossRef]
- Selatnia, A.; Madani, A.; Bakhti, M.Z.; Kertous, L.; Mansouri, Y.; Yous, R. Biosorption of Ni2+ from aqueous solution by a NaOH-treated bacterial dead Streptomyces rimosus biomass. Miner. Eng. 2004, 17, 903–911. [Google Scholar] [CrossRef]
- Ho, Y.S.; John Wase, D.A.; Forster, C.F. Batch nickel removal from aqueous solution by sphagnum moss peat. Water Res. 1995, 29, 1327–1332. [Google Scholar] [CrossRef]
- Veglió, F.; Beolchini, F.; Gasbarro, A. Biosorption of toxic metals: An equilibrium study using free cells of Arthrobacter sp. Process Biochem. 1997, 32, 99–105. [Google Scholar] [CrossRef]
- Öztürk, A.; Artan, T.; Ayar, A. Biosorption of nickel(II) and copper(II) ions from aqueous solution by Streptomyces coelicolor A3(2). Colloids Surf. B 2004, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
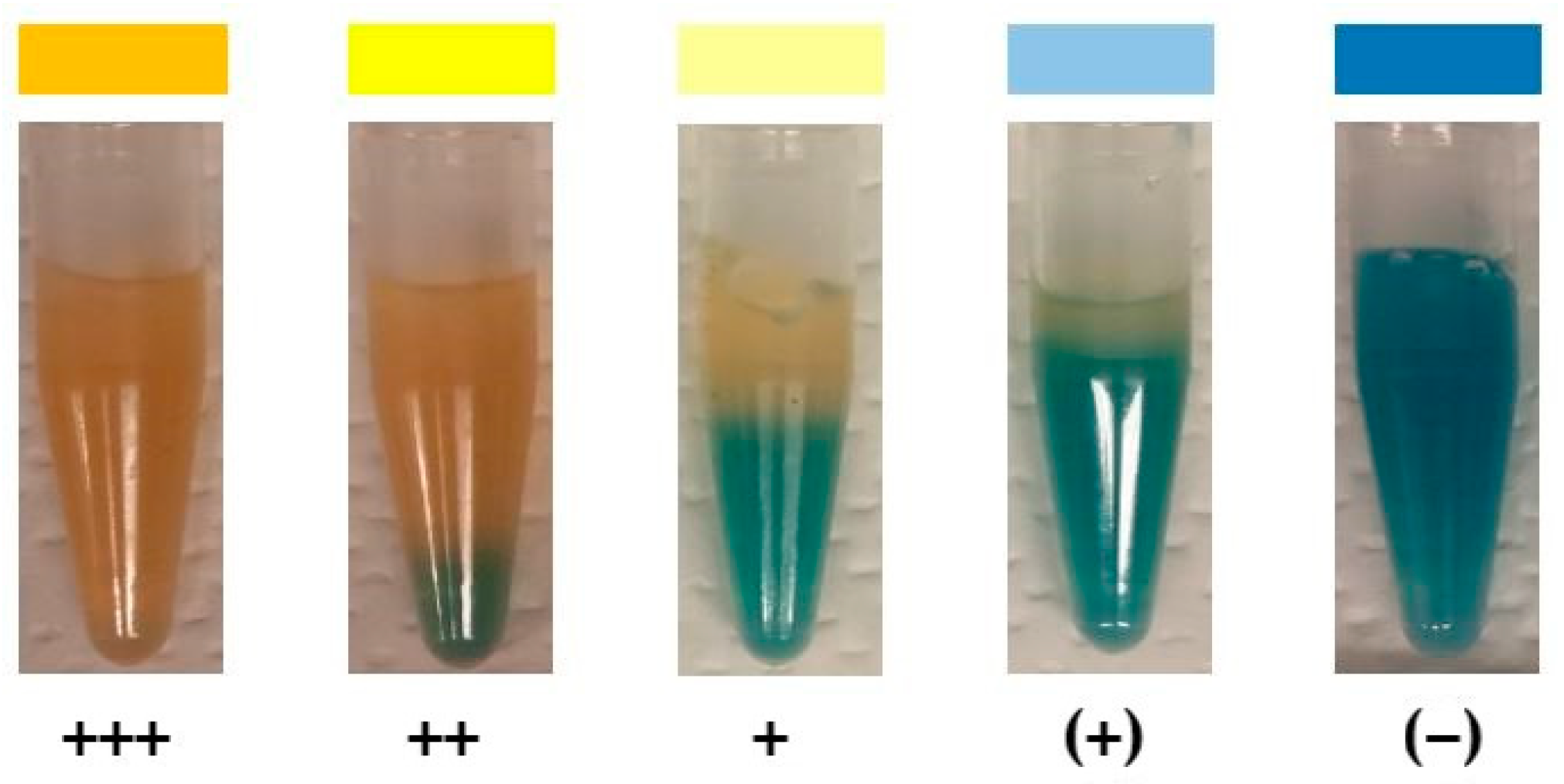
| Isolate No. | Soil Slice No. | Depth | Visible Iron Precipitate in Soil | Dilution of the Sample |
|---|---|---|---|---|
| 1 | 11 | C | no | 10−7 |
| 2 | 11 | C | no | 10−7 |
| (3) | 5 | A | no | 10−8 |
| 4 | 7 | A | no | 10−5 |
| 5 | 3 | A | no | 10−6 |
| 6 | 3 | A | no | 10−6 |
| 7 | 3 | A | no | 10−6 |
| 8 | 3 | C | no | 10−6 |
| 9 | 4 | B | yes | 10−5 |
| 10 | 6 | A | no | 10−6 |
| (11) | 8 | C | no | 10−7 |
| (12) | 4 | A | no | 10−8 |
| Ingredient [g L−1] | G-ASN | G-GLU | GLY-ASN | GLY-GLU | M-ASN | M-GLU | F-ASN | F-GLU | G-U | SA |
|---|---|---|---|---|---|---|---|---|---|---|
| L-Asn (mono-hydrate) | 2.0 | - | 2.0 | - | 2.0 | - | 2.0 | - | - | - |
| L-Glu | - | 2.0 | - | 2.0 | - | 2.0 | - | 2.0 | 1.0 | - |
| Urea | - | - | - | - | - | - | - | - | 0.85 | - |
| (NH4)2SO4 | - | - | - | - | - | - | - | - | - | 1.0 |
| Glycerol (100%) [mL] | - | - | 5.55 | 5.55 | - | - | - | - | - | - |
| Glucose | 7.0 | 7.0 | - | - | - | - | - | - | 10.0 | - |
| Skimmed milk | - | - | - | - | 7.0 | 7.0 | - | - | - | - |
| Fructose | - | - | - | - | - | - | 7.0 | 7.0 | - | - |
| Succinic acid | - | - | - | - | - | - | - | - | - | 4.0 |
| K2HPO4 | - | - | - | - | - | - | - | - | 0.56 | 6.0 |
| Na2HPO4 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | - | - |
| KH2PO4 | 0.44 | 0.44 | 0.44 | 0.44 | 0.44 | 0.44 | 0.44 | 0.44 | - | 3.0 |
| MgSO4·7H2O | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Ingredient | Solid | Liquid |
|---|---|---|
| Solution A | ||
| MOPS | 2.1 g | - |
| ddH2O | 70 mL | - |
| Solution B | ||
| HDTMA (CTAB) | 7.3 mg | 72.9 mg |
| 10 mM HCl solution | 10 mL | 40 mL |
| Solution C | ||
| FeCl3·6 H2O | 0.27 mg | 0.27 mg |
| 10 mM HCl solution (200 µL 0.5N HCl + 9.8 ml ddH2O) | 10 mL | 10 mL |
| Solution D | ||
| CAS | 5.18 mg | 60.5 mg |
| ddH2O | 10 mL | 50 mL |
| Agar | 3 g | - |
| Isolate No. | G-ASN | G-GLU | GLY-ASN | GLY-GLU | M-ASN | M-GLU | F-ASN | F-GLU | G-U | SA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1C | +++ | +++ | (+) | ++ | ++ | ++ | (+) | +++ | + | − |
| 2C | +++ | +++ | + | ++ | +++ | +++ | − | ++ | (+) | − |
| 3A | (+) | + | − | + | +++ | +++ | (+) | + | + | − |
| 3A B. | − | − | − | − | (+) | − | − | − | − | − |
| 3A M. | − | − | − | − | (+) | (+) | − | − | − | − |
| 4A | +++ | +++ | ++ | +++ | +++ | +++ | ++ | ++ | (+) | − |
| 5A | + | + | +++ | +++ | +++ | +++ | +++ | +++ | + | − |
| 6A | + | + | (+) | ++ | +++ | +++ | + | + | (+) | − |
| 7A | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | − |
| 8C | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | + | − |
| 9B | + | − | − | − | +++ | +++ | (+) | + | − | − |
| 10A | + | +++ | + | ++ | +++ | +++ | + | ++ | + | − |
| 11C | ++ | +++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | − |
| 11C B. | − | − | − | − | − | − | − | − | − | − |
| 11C M. | +++ | +++ | (+) | +++ | (+) | + | (+) | +++ | (+) | (+) |
| 12A | − | ++ | − | ++ | ++ | ++ | + | ++ | + | − |
| 12A B. | − | − | − | − | (+) | (+) | − | − | − | − |
| 12A M. | − | − | − | − | (+) | (+) | − | − | − | − |
| Biosorbent | Concentration of Biosorbent (g L−1) | C0 (mg L−1) | pH | T (°C) | q (mg g−1) | Ni Removal (%) | Reference |
|---|---|---|---|---|---|---|---|
| Sphagnum peat moss and Microbacterium sp. cells and siderophores | 5.56 | 18 | 6 | 22 | 3.08 ± 0.02 | 94.9 | This study |
| Sphagnum peat moss and Microbacterium sp. siderophores | 5.56 | 18 | 6 | 22 | 3.11 ± 0.01 | 96.0 | This study |
| Sphagnum peat moss | 5.56 | 18 | 6 | 22 | 2.99 ± 0.01 | 92.4 | This study |
| Streptomyces rimosus cells | 3 | 20 | 5.7 | 20 | 6 | 90.0 | [49] |
| Streptomyces rimosus cells | 3 | 100 | 5–5.7 | 20 | 16.3 | 48.9 | [49] |
| Bacillus thuringiensis cells | 1.0 | 119 | 6 | 35 | 21.5 | 18.0 | [48] |
| Arthrobacter sp. cells | 2.8 | 125 | 5–5.5 | 30 | 10.2 * | 22.8 | [51] |
| Arthrobacter sp. cells | 1.4 | 35 | 5–5.5 | 30 | 9 | 36.0 | [51] |
| Arthrobacter sp. cells | 1.4 | 150 | 5–5.5 | 30 | 12.7 * | 11.9 | [51] |
| Streptomyces coelicolor cells | 1 | 148 | 8 | 25 | 11.1 | 7.5 | [52] |
| Peat | 1 | 20 | 5.6-6 | 20 | 10 | 50.0 | [27] |
| Peat | 1 | 60 | 5.6–6 | 20 | 16 * | 26.7 | [27] |
| Peat | 4 | 200 | 7 | 25 | 9.18 * | 15.2 | [50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virpiranta, H.; Banasik, M.; Taskila, S.; Leiviskä, T.; Halttu, M.; Sotaniemi, V.-H.; Tanskanen, J. Isolation of Efficient Metal-Binding Bacteria from Boreal Peat Soils and Development of Microbial Biosorbents for Improved Nickel Scavenging. Water 2020, 12, 2000. https://doi.org/10.3390/w12072000
Virpiranta H, Banasik M, Taskila S, Leiviskä T, Halttu M, Sotaniemi V-H, Tanskanen J. Isolation of Efficient Metal-Binding Bacteria from Boreal Peat Soils and Development of Microbial Biosorbents for Improved Nickel Scavenging. Water. 2020; 12(7):2000. https://doi.org/10.3390/w12072000
Chicago/Turabian StyleVirpiranta, Hanna, Michal Banasik, Sanna Taskila, Tiina Leiviskä, Maiju Halttu, Ville-Hermanni Sotaniemi, and Juha Tanskanen. 2020. "Isolation of Efficient Metal-Binding Bacteria from Boreal Peat Soils and Development of Microbial Biosorbents for Improved Nickel Scavenging" Water 12, no. 7: 2000. https://doi.org/10.3390/w12072000
APA StyleVirpiranta, H., Banasik, M., Taskila, S., Leiviskä, T., Halttu, M., Sotaniemi, V.-H., & Tanskanen, J. (2020). Isolation of Efficient Metal-Binding Bacteria from Boreal Peat Soils and Development of Microbial Biosorbents for Improved Nickel Scavenging. Water, 12(7), 2000. https://doi.org/10.3390/w12072000






