Selection of Macroinvertebrate Indices and Metrics for Assessing Sediment Quality in the St. Lawrence River (QC, Canada)
Abstract
1. Introduction
2. Materials and Methods
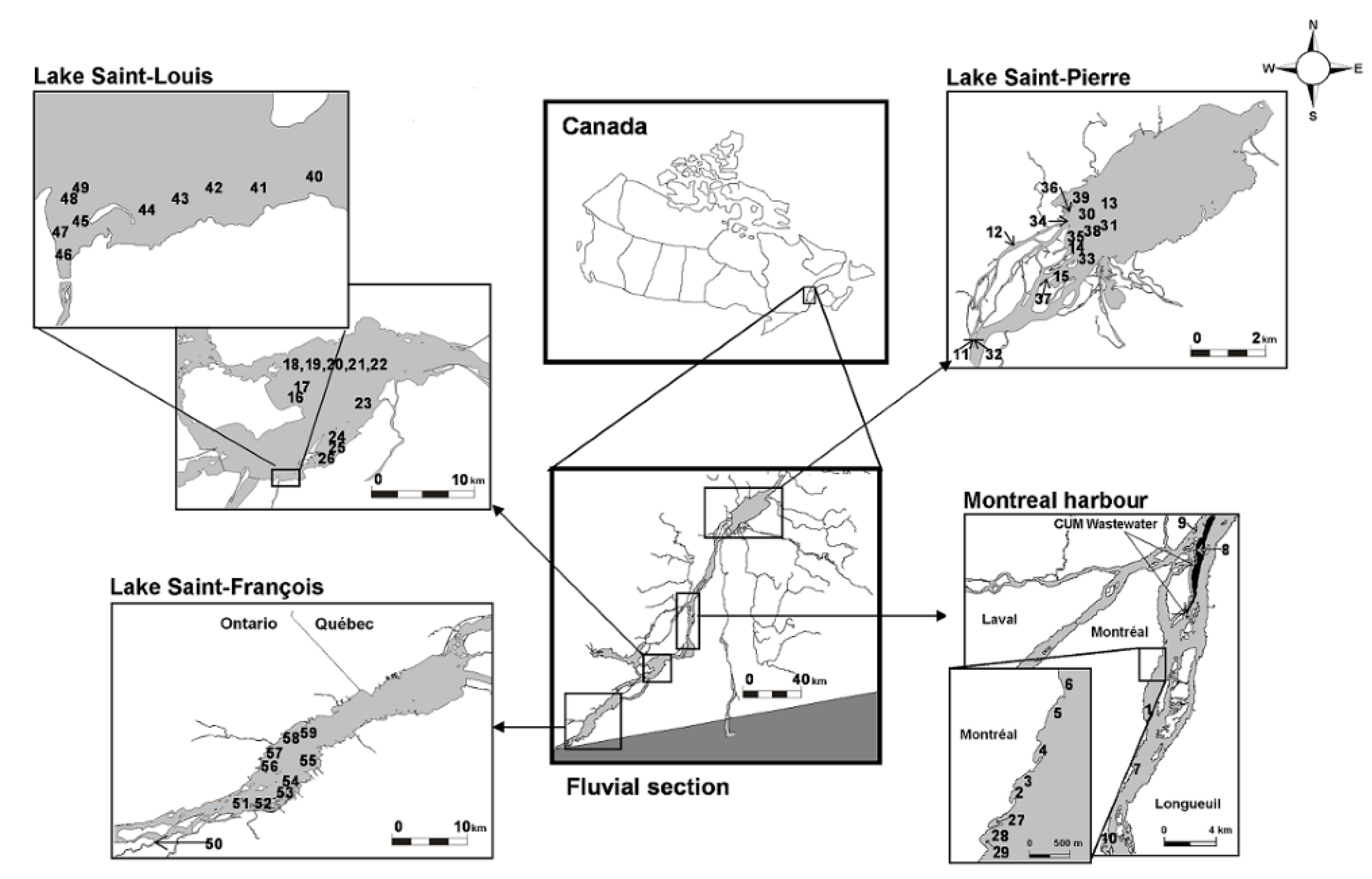
2.1. Sampling Sites at the St. Lawrence River
2.2. Sediment Sampling and Analysis
2.3. Macroinvertebrate Sampling and Analysis
2.4. Metrics Selection, Scoring, and Statistical Analysis
3. Results
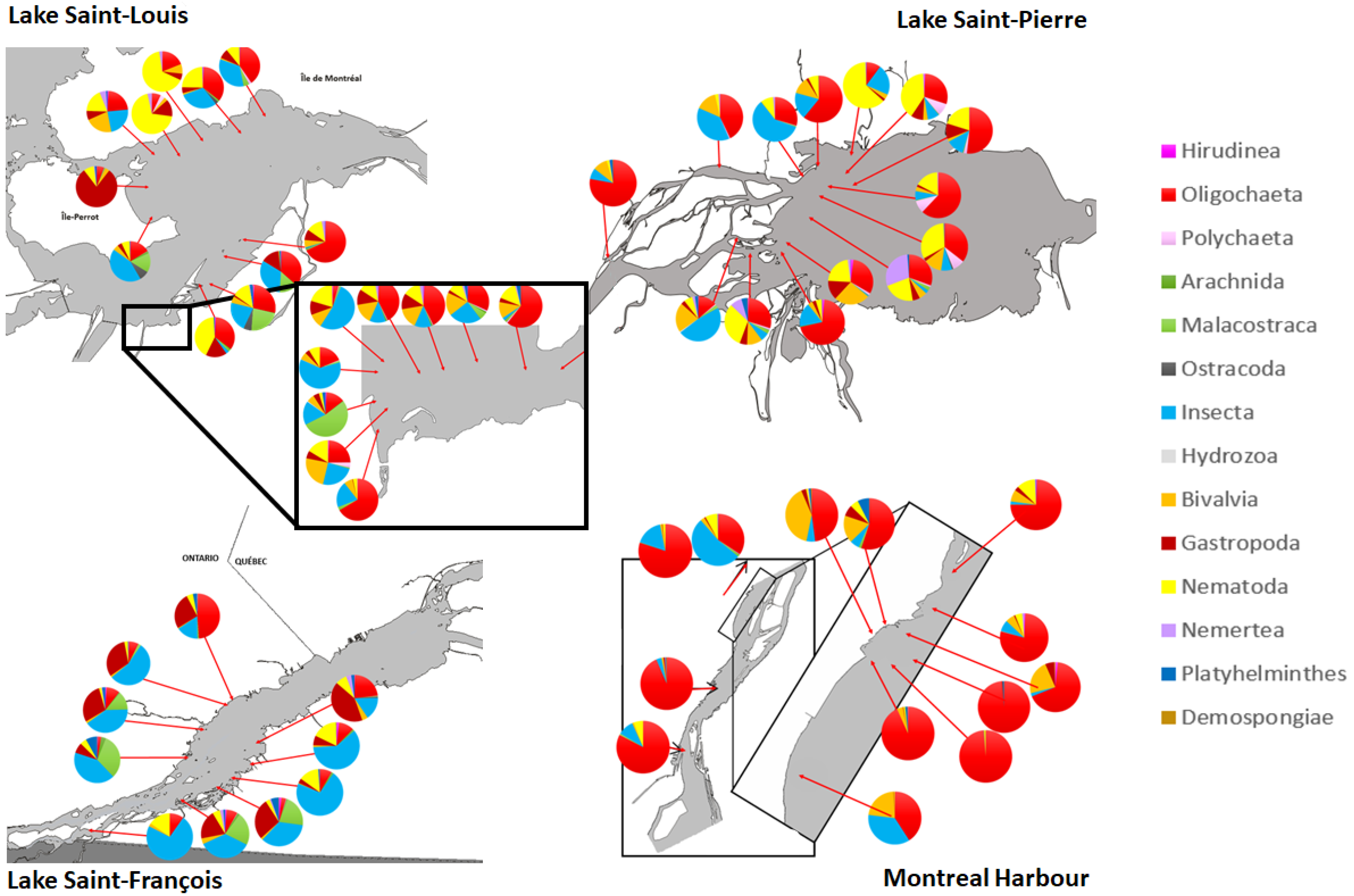
3.1. Typology of Macroinvertebrate Communities in the Fluvial Section
3.2. Selection of Macroinvertebrate Indices and Metrics
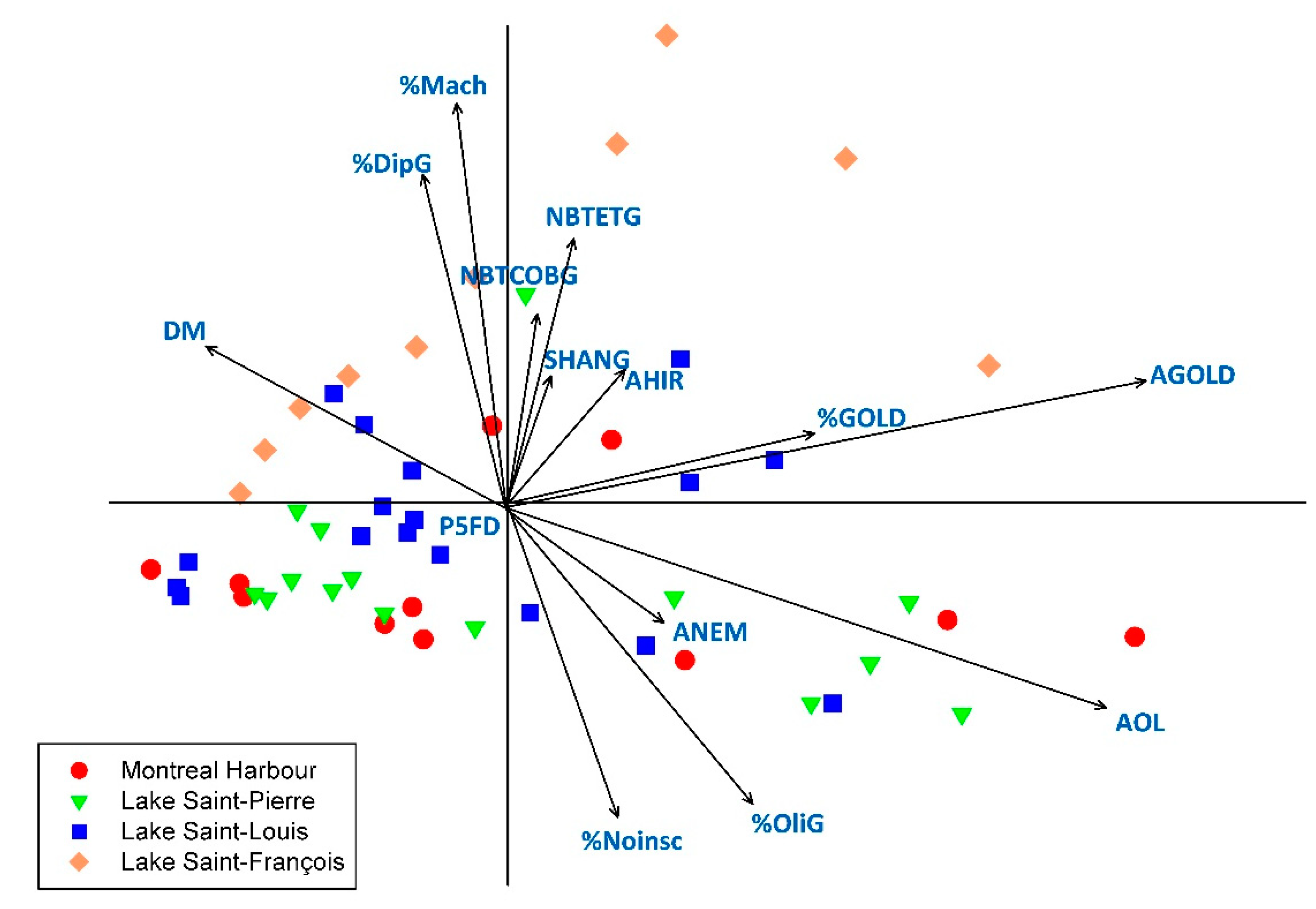
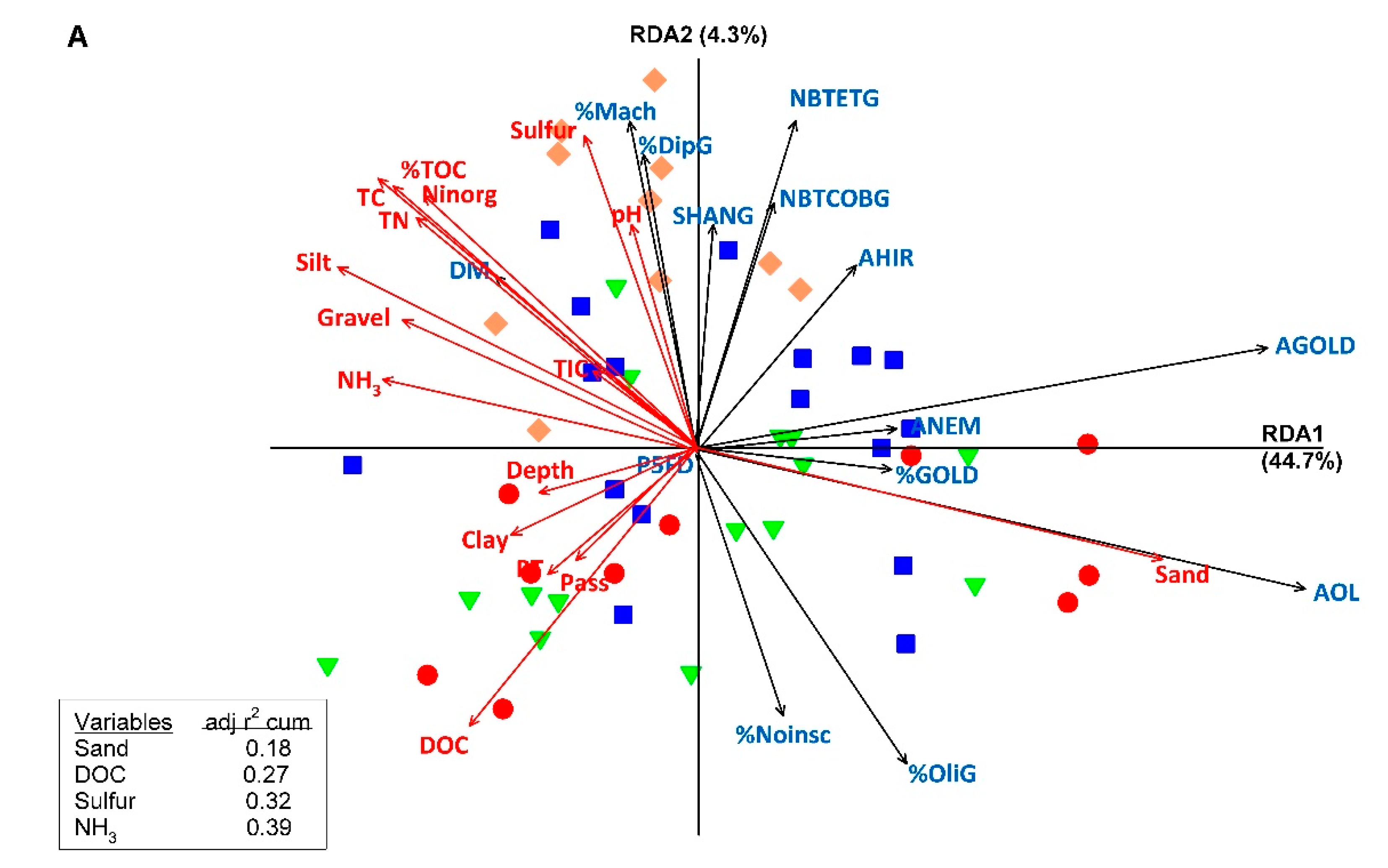
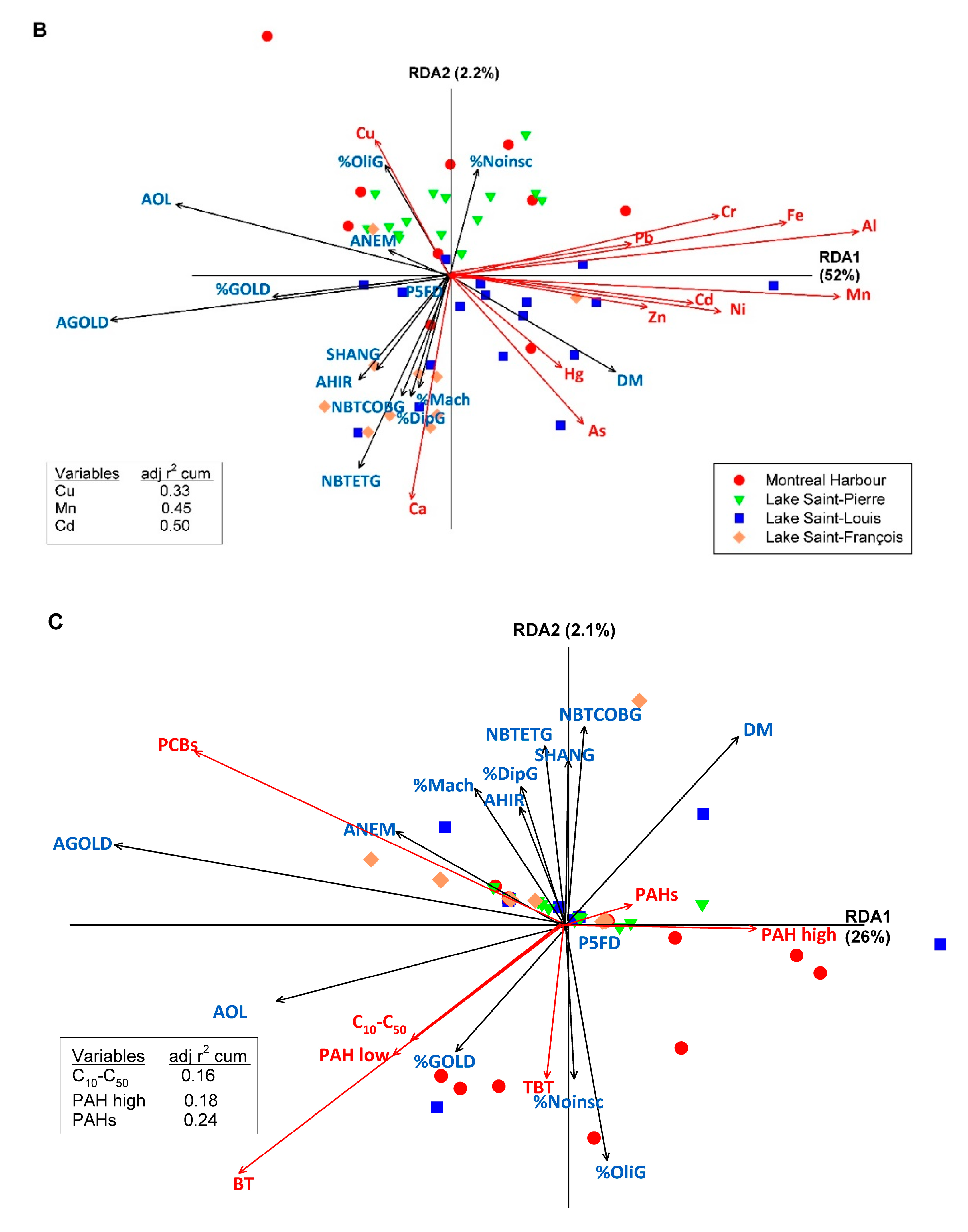
3.3. Relationships between Macroinvertebrates Metrics and Indices and Sediment Characteristics and Contamination
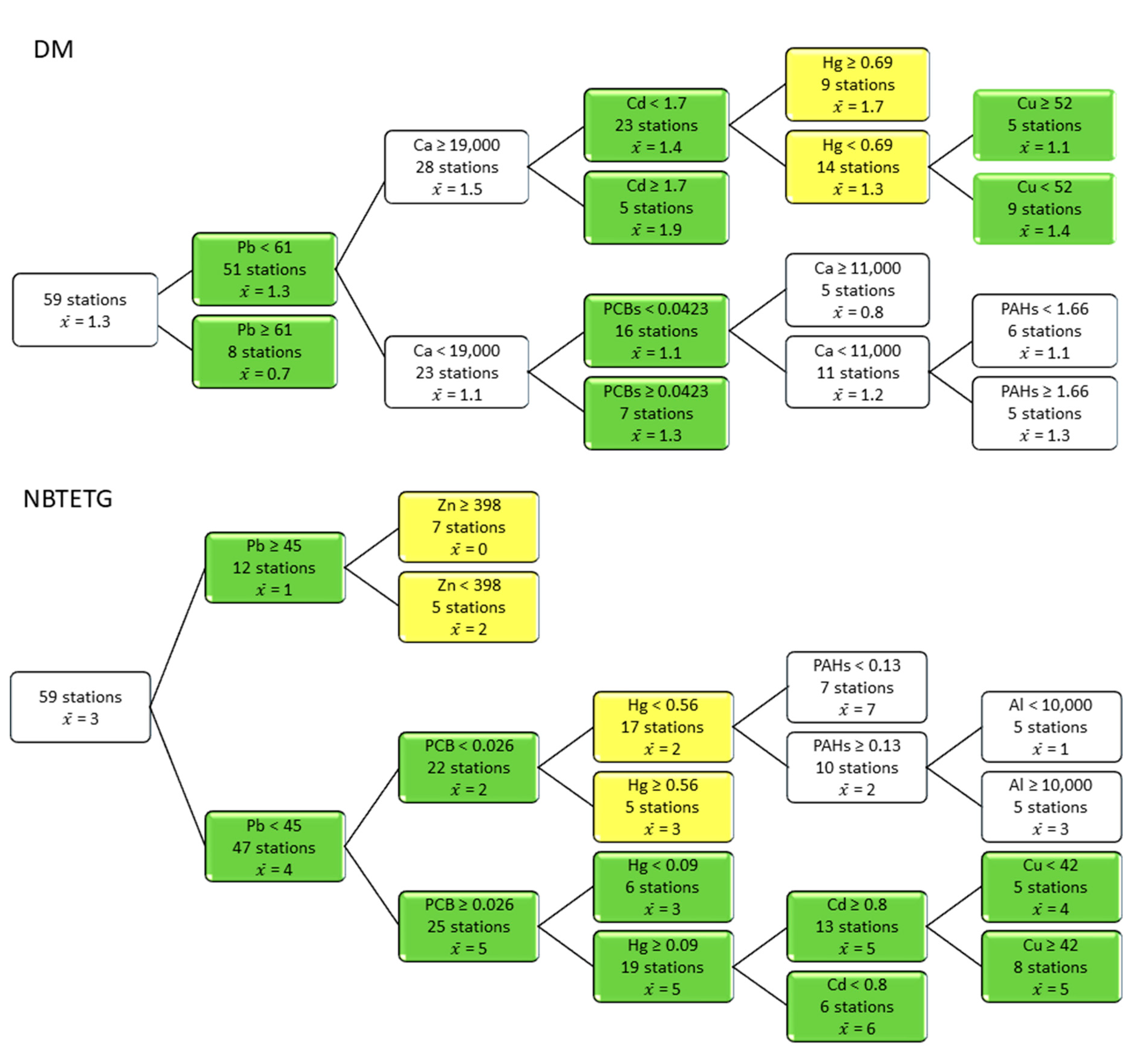
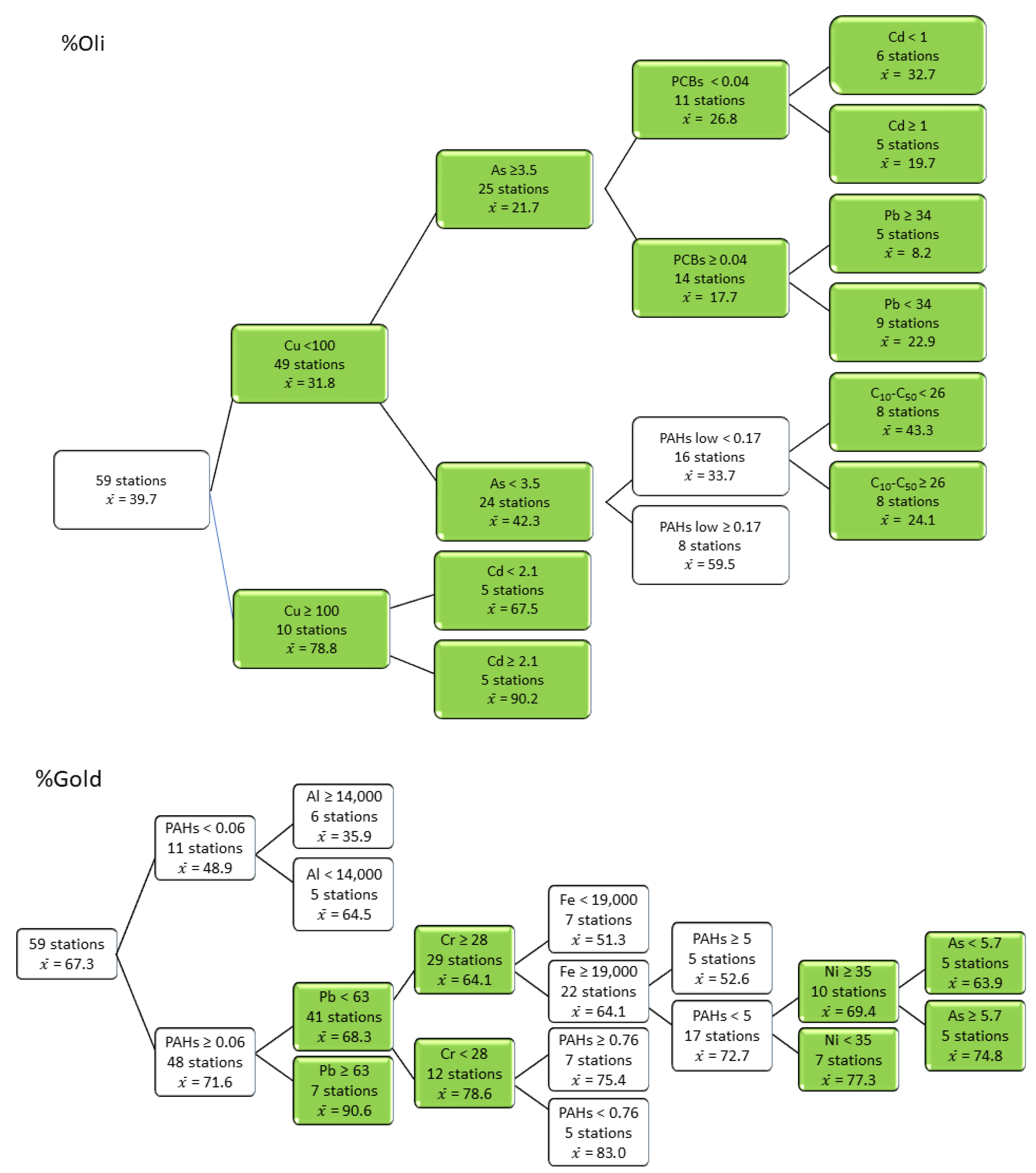
3.4. Responses of Metrics and Indices to Contaminant Thresholds
4. Discussion
4.1. The St. Lawrence River Case Study
4.2. Rational for the Selection and Inclusion of Indices and Metrics
4.3. Comparative Responses to Habitat Characteristics and Sediment Contamination
4.4. Bioassessment in a Large and Complex River: Limit and Pertinence
4.5. Conclusions and Recommendation for Bioassessment Programs
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hering, D.; Feld, C.K.; Moog, O.; Ofenböck, T. Cook book for the development of a Multimetric index for biological condition of aquatic ecosystems: Experiences from the European AQEM and STAR projects and related initiatives. Hydrobiologia 2006, 556, 311–324. [Google Scholar] [CrossRef]
- Reyjol, Y.; Argillier, C.; Bonne, W.; Borja, A.; Buijse, A.D.; Cardoso, A.C.; Daufresne, M.; Kernan, M.; Ferreira, M.T.; Poikane, S.; et al. Assessing the ecological status in the context of the European water framework directive (WFD): Where should we go now? Sci. Total Environ. 2014, 497/498, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Pinel-Alloul, B.; Méthot, G.; Borcard, D. Évaluation de L’état des Données sur les Macroinvertébrés Benthiques du Fleuve Saint-Laurent et de ses Tributaires Pour une Application d’un Modèle de Suivi Environnemental avec L’approche des Conditions de Référence (CABIN); Centre Saint-Laurent, E.C., Ed.; Université de Montréal: Montréal, QC, Canada, 2004; p. 68. [Google Scholar]
- U.S. Environmental Protection Agency. Methods for Evaluating Wetland Conditions: Developing an Invertebrate Index for Biological Integrity of Wetlands Office of Water; U.S.E.P.A.: Washington, DC, USA, 2002.
- Chessman, B.C. Rapid assessment of rivers using macroinvertebrates: A procedure based on habitat-specific sampling, family-level identification, and a biotic index. Aust. J. Ecol. 1995, 20, 122–129. [Google Scholar] [CrossRef]
- Chessman, B.C.; Royal, M.L. Bioassessment without reference sites: Use of environmental filters to predict natural assemblages of river macroinvertebrates. J. North Am. Benthol. Soc. 2004, 23, 599–615. [Google Scholar] [CrossRef]
- Metzeling, L.; Chessman, B.; Hardwick, R.; Wong, V. Rapid assessment of rivers using macroinvertebrates: The role of experience, and comparisons with quantitative methods. Hydrobiologia 2003, 510, 39–52. [Google Scholar] [CrossRef]
- Bonada, N.; Prat, N.; Resh, V.H.; Statzner, B. Developments in aquatic insect biomonitoring: A comparative analysis of recent approaches. Annu. Rev. Entomol. 2006, 51, 495–523. [Google Scholar] [CrossRef]
- Resh, V.H.; Norris, R.H.; Barbour, M.T. Design and implementation of rapid assessment approaches for water resource monitoring using benthic macroinvertebrates. Aust. J. Ecol. 1995, 20, 108–121. [Google Scholar] [CrossRef]
- Cayrou, J.; Compin, A.; Giani, N.; Céréghino, R. Associations spécifiques chez les macroinvertébrés benthiques et leur utilisation pour la typologie des cours d’eau. Cas du réseau hydrographique Adour-Garonne (France). Ann. Limnol. Int. J. Limnol. 2000, 36, 189–202. [Google Scholar] [CrossRef]
- Tripole, S.; Vallania, E.A.; Corigliano, M.C. Benthic macroinvertebrate tolerance to water acidification in the Grande River sub-basin (San Luis, Argentina). Limnetica 2008, 27, 29–38. [Google Scholar]
- Gabriels, W.; Lock, K.; De Pawn, N.; Goethals, P.L.M. Multimetrics macroinvertebrate Index Flanders (MMIF) for biological assessment of rivers and lakes in Flanders (Belgium). Limnologica 2010, 40, 199–207. [Google Scholar] [CrossRef]
- Lewin, I.; Czerniawska-Kusza, I.; Lawniczak, A.E.; Jusik, S. Biological indices applied to benthic macroinvertebrates at reference conditions of mountain streams in two ecoregions (Poland, the Slovak Republic). Hydrobiologia 2013, 709, 183–200. [Google Scholar] [CrossRef]
- Alemneh, T.; Ambelu, A.; Zaitchik, B.F.; Bahrndorff, S.; Mereta, S.T.; Pertoldi, C. Macroinvertebrate multi-metric index for Ethiopian highland streams. Hydrobiologia 2019, 843, 125–141. [Google Scholar] [CrossRef]
- Blocksom, K.A.; Johnson, B.R. Development of a regional macroinvertebrate index for large river bioassessment. Ecol. Indic. 2009, 9, 313–328. [Google Scholar] [CrossRef]
- Tessier, C.; Cattaneo, A.; Hudon, C.; Borcard, D. Invertebrate communities associated with metaphyton and emergent and submerged macrophytes in a large river. Can. J. Fish. Aquat. Sci. 2008, 70, 10–20. [Google Scholar] [CrossRef]
- Tall, L.; Méthot, G.; Armellin, A.; Pinel-Alloul, B. Bioassessment of benthic macroinvertebrates in wetland habitats of Lake Saint-Pierre (St. Lawrence River). J. Great Lakes Res. 2008, 34, 599–614. [Google Scholar] [CrossRef]
- Tall, L.; Armellin, A.; Pinel-Alloul, B.; Méthot, G.; Hudon, C. Effects of hydrological regime, landscape features, and environment on macroinvertebrates in St. Lawrence River wetlands. Hydrobiologia 2016, 778, 221–241. [Google Scholar] [CrossRef]
- Armitage, P.D.; Moss, D.; Wright, J.T.; Furse, M.T. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running water sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Armitage, P.D. The potential of RIPACS for predicting the effects of environmental change. Chapter 7. In Assessing the Biological Quality of Freshwaters: RIPACS and Other Techniques; Wright, J.F., Sutcliffe, D.W., Furse, M.T., Eds.; Freshwater Biological Association: Ambleside, UK, 2000; pp. 93–111. [Google Scholar]
- Burt, A.J.; McKee, P.M.; Hart, D.R.; Kauss, P.B. Effects of pollution on benthic invertebrate communities of the St. Marys River. Hydrobiologia 1991, 2019, 63–81. [Google Scholar] [CrossRef]
- Clements, W.H.; Cherry, D.S.; Van Hassel, J.H. Assessment of the impact of heavy metals on benthic communities at the Clinch River (Virginia): Evaluation of an index of community sensitivity. Can. J. Fish. Aquat. Sci. 1992, 49, 1686–1694. [Google Scholar] [CrossRef]
- Masson, S.; Desrosiers, M.; Pinel-Alloul, B.; Martel, L. Relating macroinvertebrate community structure to environmental characteristics and sediment contamination at the scale of the St. Lawrence River. Hydrobiologia 2010, 647, 35–50. [Google Scholar] [CrossRef]
- Angradi, T.R.; Pearson, M.S.; Bolgrein, D.W.; Jicha, T.M.; Taylor, D.L.; Hill, B.H. Multimetric macroinvertebrate indices for mid-continent US great rivers. J. North Am. Benthol. Soc. 2009, 28, 785–804. [Google Scholar] [CrossRef]
- Reynoldson, T.B.; Norris, R.H.; Resh, V.H.; Day, K.E.; Rosenberg, D.M. The reference condition: A comparison of multimetric and multivariate approaches to assess water-quality impairment using benthic macroinvertebrates. J. North Am. Benthol. Soc. 1997, 16, 833–852. [Google Scholar] [CrossRef]
- Bailey, R.C.; Kennedy, M.G.; Dervish, M.Z.; Taylor, R.M. Biological assessment of freshwater ecosystems using a reference condition approach: Comparing predicted and actual benthic invertebrate communities in Yukon streams. Freshw. Biol. 1998, 39, 765–774. [Google Scholar] [CrossRef]
- Czerniawska-Kusza, I. Comparing modified biological monitoring working partly score system and several biological indices based on macroinvertebrates for water quality assessment. Limnologica 2005, 35, 169–176. [Google Scholar] [CrossRef]
- Washington, H.G. Diversity, biotic and similarity indices: A review with special relevance to aquatic ecosystems. Water Res. 1984, 18, 653–694. [Google Scholar] [CrossRef]
- Koperski, P. Diversity of freshwater macrobenthos and its use in biological assessment: A critical review of current applications. Environ. Rev. 2011, 19, 16–31. [Google Scholar] [CrossRef]
- Buffagni, A.; Erba, S.; Cazzola, M.; Murray-Bligh, J.; Soszka, H.; Genoni, P. The STAR common metrics approch to the WFD intercalibration process: Full application for small, lowland rivers in three European countries. Hydrobiologia 2006, 566, 379–399. [Google Scholar] [CrossRef]
- Reynoldson, T.B.; Rosenberg, D.M.; Resh, V.H. Comparison of models predicting invertebrate assemblages for biomonitoring in the Fraser River catchment, British Columbia. Can. J. Fish. Aquat. Sci. 2001, 58, 1395–1410. [Google Scholar] [CrossRef]
- Odoutan, O.H.; Janssens de Bisthoven, L.; Abou, Y.; Eggermount, H. Biomonitoring of lakes using macroinvertebrates: Recommended indices and metrics for use in West Africa and developing countries. Hydrobiologia 2019, 826, 1–23. [Google Scholar] [CrossRef]
- Oertli, B.; Indermuehle, N.; Angélibert, S.; Hinden, H.; Stoll, A. Macroinvertebrate assemblages in 25 high alpine ponds of the Swiss National Parks (Cirque of Macun) and relation to environmental variables. Hydrobiologia 2008, 597, 29–41. [Google Scholar] [CrossRef]
- Walley, W.J.; Grbowitč, J.; Dzeroski, S. A reappraisal of saprobic values and indicator weights based on Slovenian river quality data. Water Res. 2001, 35, 4285–4292. [Google Scholar] [CrossRef]
- Hilsenhoff, W.L. Rapid field assessment of organic pollution with a family-level biotic index. J. North Am. Benthol. Soc. 1988, 7, 65–68. [Google Scholar] [CrossRef]
- Hilsenhoff, W.L. An improved biotic index for organic stream pollution. Great Lakes Entomol. 1987, 20, 31–39. [Google Scholar]
- Pinel-Alloul, B.; Méthot, G.; Lapierre, L.; Willsie, A. Macroinvertebrate community as a biological indicator of ecological and toxicological factors in Lake Saint-François (Québec). Environ. Pollut. 1996, 91, 65–87. [Google Scholar] [CrossRef]
- Ofenböck, T.; Moog, O.; Gerritsen, J.; Barbour, M. A stressor specific multimetric approach for monitoring running waters in Austria using benthic macro-invertebrates. Hydrobiologia 2004, 516, 251–268. [Google Scholar] [CrossRef]
- Touron-Poncet, H.; Bernadet, C.; Compin, A.N.B.; Céréghino, R. Implementing the Water Framework Directive in overseas Europe: A multimetric macroinvertebrate index for river bioassessment in Caribbean islands. Limnologica 2014, 47, 34–43. [Google Scholar] [CrossRef]
- Applegate, J.M.; Baumann, P.C.; Emery, E.B.; Wooten, M.S. First steps in developing a multimetric macroinvertebrate index for the Ohio River. River Res. Appl. 2007, 23, 683–697. [Google Scholar] [CrossRef]
- Zamora-Muñoz, C.; Alba-Tercedor, J. Bioassessment of organically polluted Spanish rivers, using a biotic index and multivariable methods. J. North Am. Benthol. Soc. 1996, 15, 332–352. [Google Scholar] [CrossRef]
- Barton, D.R.; Metcalfe-Smith, J.L. A comparison of sampling techniques and summary indices for assessment of water quality in the Yamaska River, Quebec, based on benthic macroinvertebrates. Environ. Monit. Assess. 1992, 21, 225–244. [Google Scholar] [CrossRef]
- Karr, J.R.; Fausch, K.D.; Angermeier, P.L.; Yant, P.R.; Scholosser, U. Assessing Biological Integrity in Running Waters: A Method and Its Rationale; Illinois Natural History Survey Special Publication No 5: Champaign, IL, USA, 1986. [Google Scholar]
- De Pauw, N.; Vanhooren, G. Method for biological quality assessment of watercourses in Belgium. Hydrobiologia 1983, 100, 153–168. [Google Scholar] [CrossRef]
- Armanini, D.G.; Horrigan, N.; Monk, W.A.; Peters, D.L.; Baird, D.J. Development of a benthic macroinvertebrate flow sensitivity index for Canadian rivers. River Res. Appl. 2011, 27, 723–737. [Google Scholar] [CrossRef]
- Schletterer, M.; Fureder, L.; Kusolev, V.V.; Beketov, M.A. Testing the coherence of several macroinvertebrates indices and environ mental factors in a large lowland river system (Volga River, Russia). Ecol. Indic. 2010, 10, 1083–1092. [Google Scholar] [CrossRef]
- Collier, K.J.; Hamer, M.P.; Moore, S.C. Littoral and benthic macroinvertebrate community responses to contrasting stressors in a large New Zealand river. N. Z. J. Mar. Freshw. Res. 2014, 48, 560–576. [Google Scholar] [CrossRef]
- Carignan, R.; Lorrain, S.; Lum, K. A 50-yr record of pollution by nutrients, trace metals, and organic chemicals in the St. Lawrence River. Can. J. Fish. Aquat. Sci. 1994, 51, 1088–1100. [Google Scholar] [CrossRef]
- Desrosiers, M.; Usseglio-Polatera, P.; Archaimbault, V.; Larras, F.; Méthot, G. Pinel-Alloul, B. Assessing anthropogenic pressure in the St. Lawrence River using traits of benthic macroinvertebrates. Sci. Total Environ. 2019, 649, 233–246. [Google Scholar] [CrossRef]
- Desrosiers, M.; Babut, M.P.; Pelletier, M.; Bélanger, C.; Thibodeau, S.; Martel, L. Efficiency of sediment quality guidelines for predicting toxicity: The case of the St. Lawrence River. Integr. Environ. Assess. Manag. 2010, 6, 225–239. [Google Scholar]
- Désy, J.C.; Archambault, J.F.; Pinel-Alloul, B.; Hubert, J.; Campbell, P.G.C. Relationships between total mercury in sediments and methyl mercury in the freshwater gastropod prosobranch Bithynia tentaculata in the St. Lawrence River, Quebec. Can. J. Fish. Aquat. Sci. 2000, 57, 164–173. [Google Scholar]
- Flessas, C.; Couillard, Y.; Pinel-alloul, B.; St-Cyr, L.; Campbell, P.G.C. Metal concentration in two freshwater gastropods (Mollusca) in the St. Lawrence River and relationships with environmental contamination. Can. J. Fish. Aquat. Sci. 2000, 57, 126–137. [Google Scholar] [CrossRef]
- Amyot, M.; Pinel-Alloul, B.; Campbell, P.G.C. Abiotic and seasonal factors influencing trace metal levels (Cd, Cu, Ni, Pb, and Zn) in the freshwater amphipod Gammarus fasciatus in two fluvial lakes of the St. Lawrence River. Can. J. Fish. Aquat. Sci. 1994, 51, 2003–2016. [Google Scholar] [CrossRef]
- Lévesque, D.; Cattaneo, A.; Hudon, C.; Gagnon, P. Predicting the risk of proliferation of the benthic cyanobacterium Lyngbia wollei in the St. Lawrence River. Can. J. Fish. Aquat. Sci. 2012, 69, 1585–1595. [Google Scholar] [CrossRef]
- Environnement Canada, Ministère du Développement durable, de l’Environnement et des Parcs du Québec. Criteria for the Assessment of Sediment Quality in Quebec and Application Frameworks: Prevention, Dredging and Remediation; Plan Saint-Laurent pour un développement durable: Québec, QC, Canada, 2007; p. 39.
- Klemm, D.J.; Lewis, P.A.; Fulk, F.; Lazorchak, J.M. Macroinvertebrate Field and Laboratory Methods for Evaluating the Biological Integrity of Surface Waters; U.S Environmental Protection Agency, Office of Research and Development of the Environmental Monitoring Systems Laboratory: Cincinnati, OH, USA, 1990; p. 206.
- Merritt, R.W.; Cummins, K.W. An Introduction to the Aquatic Insects of North America, 3rd ed.; Kendall/Hunt Publishing Company: Dubuque, Iowa, 1996; p. 442. [Google Scholar]
- Thorp, J.H.; Covich, A.P. Ecology and Classification of North American Freshwater Invertebrates, 2nd ed.; Academic Press: San Diego, CA, USA, 2001; p. 1038. [Google Scholar]
- Ter Braak, C.J.F. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1996, 67, 1167–1179. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4); Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology. In Development in Environmental Modelling, 3rd ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2012; Volume 24, p. 1006. [Google Scholar]
- De’ath, G. Multivariate regression trees: A new technique for modeling species-environment relationships. Ecology 2002, 83, 1105–1117. [Google Scholar]
- Ouelette, M.-H.; Legendre, P.; Borcard, D. Cascade multivariate regression tree: A novel approach for modelling nested explanatory sets. Methods Ecol. Evol. 2012, 3, 234–244. [Google Scholar] [CrossRef]
- Saulnier, I.; Gagnon, C. Background levels of metals in St. Lawrence River sediments: Implications for sediment quality criteria and environmental management. Integr. Environ. Assess. Manag. 2006, 2, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. Res. Appl. 2006, 22, 123–147. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The Riverine Ecosystem Synthesis: Towards Conceptual Cohesiveness in River Science; Academic Press: London, UK, 2008; p. 232. [Google Scholar]
- Stanford, J.A.; Ward, J.V. Revisiting the serial discontinuity model. River. River Res. Appl. 2001, 17, 303–310. [Google Scholar] [CrossRef]
- Wallace, J.B.; Grubaugh, J.W.; Whiles, M.R. Biotic indices and stream ecosystem processes: Results from an experimental study. Ecol. Appl. 1996, 6, 140–151. [Google Scholar] [CrossRef]
- Dorić, V.; Pozojević, I.; Vučković, N.; Ivković, M.; Mihaljević, Z. Lentic chironomid performance in species-based bioassessment proving: High-level taxonomy is not a dead end in monitoring. Ecol. Indic. 2020, 107041. in press. [Google Scholar]
- Beketov, M.A.; Liess, M. An indicator for effects of organic toxicants on lotic invertebrate communities: Independence of confounding environmental factors over an extensive river continuum. Environ. Pollut. 2008, 156, 980–987. [Google Scholar] [CrossRef]
- Hudon, C. Impact of water-level fluctuations on St. Lawrence River aquatic vegetation. Can. J. Fish. Aquat. Sci. 1997, 54, 2853–2865. [Google Scholar] [CrossRef]
- Hudon, C.; Carignan, R. Cumulative impacts of hydrology and human activities on water quality in the St. Lawrence River (Lake Saint-Pierre, Québec, Canada). Can. J. Fish. Aquat. Sci. 2008, 65, 1165–1180. [Google Scholar] [CrossRef]
- Hudon, C.; Gagnon, P.; Amyot, J.-P.; Létourneau, G.; Jean, M.; Plante, C.; Rioux, D.; Deschênes, M. Historical changes in herbaceous wetland distribution induced by hydrological conditionsin Lake Saint-Pierre (St. Lawrence River, Quebec, Canada). Hydrobiologia 2005, 539, 205–224. [Google Scholar] [CrossRef]
| Sediment Quality Class | Remediation of Contaminated Site Application Framework [55] | LSF | LSL | LSP | MH |
|---|---|---|---|---|---|
| 1 |
| n = 2 LSF51 LSL53 | n = 9 LSL16 LSL17 LSL18 LSL19 LSL20 LSL22 LSL23 LSL24 LSL26 | n = 12 LSP12 LSP13 LSP14 LSP15 LSP30 LSP31 LSP33 LSP34 LSP35 LSP37 LSP38 LSP39 | n = 2 PM8 PM9 |
| 2 |
| n = 5 LSF52 LSF56 LSF57 LSF58 LSF59 | n = 4 LSL21 LSL41 LSL43 LSL46 | n = 3 LSP11 LSP32 LSP36 | n = 2 PM2 PM5 |
| 3 |
| n = 3 LSF50 LSF54 LSF55 | n = 8 LSL25 LSL40 LSL42 LSL44 LSL45 LSL47 LSL48 LSL49 | n = 0 | n = 9 PM1 PM3 PM4 PM6 PM7 PM10 PM27 PM28 PM29 |
| Indices and Metrics | |
|---|---|
| Diversity indices | DSW, Hmax, ESW, DS, ES, M, BP, DH, DM, IG, PIE, TU, SIMF, SIMG, SHANF, SHANG, MARGF, MARGG, EVENF, EVENG |
| Metrics on taxa richness | NBFA, NBFOD, NBFEP, NBFTR, NBFDI, EPT, ETO, NBGE, NBGOD, NBGEP, NBGTR, NBGDI, NBGCH, NBTGaG, NBTGaF, NBTBiG, NBTBiF, NBTHiG, NBTHiF, NBTCrG, NBTCrF, NBTEG, NBTEF, NBTOdG, NBTOdF, NBTTrG, NBTTrF, NBTDiG, NBTDiF, NBTOtG, NBTOtF, NBTETG, NBTETF, NBTET/G, NBTET/F, NBTO/F, NBtEF, NBTOBF, NBTaxF, NBTODF, NBTODG, NBTEGA, NBTOLDG, NBTOD/G, NBTEG, NBTCOBG, NBFamG, NBGen, Ita |
| Metrics on taxa abundances | AOL, PFD, P2FD, P5FD, PCH, POL, PTR, PDI, PAM, PGA, PBI, PIN, %collector, %scraper, %predator, Mach%, NoIns%, %NemG, %GasG, %PolG, %OliG, %CruG, %TriG, %DipG, %HydG, %ETG, %ET/OLG, %AETG, %GOLDG, ANEM, APOL, AOLI, AHIR, ADIP, AHYD, AGOLD |
| Multi-metric biotic indices | IBGN, GFI, BMWP, ASPT, BBIF, BBIG, HAIF, HAIG, r/KF, r/KG, RETIF, RETIG, ICI |
| Metrics on functional traits [49] | Volt3, Life2, Sta3, Diss1, Diss3, Hab1, Hab2, Hab4, Att1, Att2, Att3, Att4, Form2, Form3, Arm1, Arm2, Arm3, Size2, Size4, Size5, Resp1, Resp2, Rep1, Rep2, Ovo2, Ovo3, Trop1, Trop4, Trop5, Foo1, Foo2, Foo4, Foo7, Vc1, Sub2, Sub3, Sub4, Sub5, Sub6 |
| Taxa | Indices and Metrics | Abbreviations | G/F | Equations |
|---|---|---|---|---|
| Macroinvertebrates | Diversity of Menhinick | DM | F | |
| Macroinvertebrates | Diversity Shannon-Wiener-Index | SHANG | G | |
| Oligochaeta | Taxonomic group (abundance) Oligochaeta | AOL | G | |
| Nematoda | Taxonomic group (abundance)—Nematoda | ANEM | G | |
| Hirudinea | Taxonomic group (abundance)—Hirudinea | AHIR | G | |
| Gastropoda, Oligochaeta, Diptera | Taxonomic group (abundance)—GOLD | AGOLD | G | |
| Oligochaeta | Taxonomic group [%]—Oligochaeta [%] | %Oli | G | |
| Diptera | Taxonomic group [%]—Diptera [%] | %Dip | G | |
| Gastropoda, Oligochaeta, Diptera | Taxonomic group [%]—GOLD | %GOLD | G | |
| Mollusca, Chironomidae, Achaeta | %abundance Mollusca, Achaeta, Chironomidae | %Mach | G | |
| Non-insect | %abundance Non-insect relative | NoIns% | G | |
| Macroinvertebrates | 5% first dominant families | P5FD | F | |
| Ephemeroptera, Trichoptera, Coleoptera, Odonata, Bivalvia | Taxonomic group (number of taxa)—ETCOB | NBTCOBG | G | |
| Ephemeroptera | Taxonomic group (number of taxa)— E-Taxa | NBTETG | G |
| Indices Metrics | Habitat Zones | Sediment Quality Classes | ||||
|---|---|---|---|---|---|---|
| LSF-LSL-LSP-MH | Prob. | 1-2-3 | Prob. | 1-2-3A-3B | Prob. | |
| Diversity | ||||||
| DM | A-A-A-B | 0.0004 | A-A-A | 0.3832 | B-AB-C-A | <0.0001 |
| SHANG | A-A-A-B | 0.0003 | A-A-A | 0.8649 | B-B-C-A | <0.0001 |
| Taxa richness | ||||||
| NBTETG | A-A-B-B | <0.0001 | A-A-A | 0.2897 | BC-AB-C-A | <0.0001 |
| NBTCOBG | A-A-A-B | <0.0001 | A-A-A | 0.4273 | B-AB-C-A | <0.0001 |
| Taxa abundance | ||||||
| AOL | B-AB-AB-A | 0.0085 | A-A-A | 0.4617 | B-B-A-B | 0.0075 |
| %OliG | C-BC-B-A | <0.0001 | A-A-A | 0.1796 | B-B-A-B | <0.0001 |
| %DipG | A-B-B-B | 0.0005 | A-A-A | 0.9226 | AB-AB-B-A | 0.0176 |
| P5FD | BC-C-B-A | <0.0001 | A-A-A | 0.8449 | B-BC-A-C | <0.0001 |
| ANEM | A-A-A-A | 0.0846 | A-B-AB | 0.0181 | A-B-B-AB | 0.0380 |
| AHIR | A-A-A-A | 0.2213 | A-A-A | 0.6814 | A-A-A-A | 0.5122 |
| AGOLD | A-A-A-A | 0.4945 | A-A-A | 0.5070 | A-A-A-A | 0.5290 |
| %Match | A-B-B-B | <0.0001 | A-A-A | 0.8731 | AB-AB-B-A | 0.0176 |
| %NoInsc | B-A-A-A | 0.0003 | A-A-A | 0.9340 | AB-AB-A-B | 0.0058 |
| %GOLDG | AB-B-B-A | 0.0006 | A-A-A | 0.1202 | B-AB-A-B | 0.0025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desrosiers, M.; Pinel-Alloul, B.; Spilmont, C. Selection of Macroinvertebrate Indices and Metrics for Assessing Sediment Quality in the St. Lawrence River (QC, Canada). Water 2020, 12, 3335. https://doi.org/10.3390/w12123335
Desrosiers M, Pinel-Alloul B, Spilmont C. Selection of Macroinvertebrate Indices and Metrics for Assessing Sediment Quality in the St. Lawrence River (QC, Canada). Water. 2020; 12(12):3335. https://doi.org/10.3390/w12123335
Chicago/Turabian StyleDesrosiers, Mélanie, Bernadette Pinel-Alloul, and Charlotte Spilmont. 2020. "Selection of Macroinvertebrate Indices and Metrics for Assessing Sediment Quality in the St. Lawrence River (QC, Canada)" Water 12, no. 12: 3335. https://doi.org/10.3390/w12123335
APA StyleDesrosiers, M., Pinel-Alloul, B., & Spilmont, C. (2020). Selection of Macroinvertebrate Indices and Metrics for Assessing Sediment Quality in the St. Lawrence River (QC, Canada). Water, 12(12), 3335. https://doi.org/10.3390/w12123335





