Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Experimental Setup and Analysis Method
2.3. Experimental Details
3. Results and Discussion
3.1. The Synergistic Effect of nZVI-Heat Activated PS Degradation of CAP
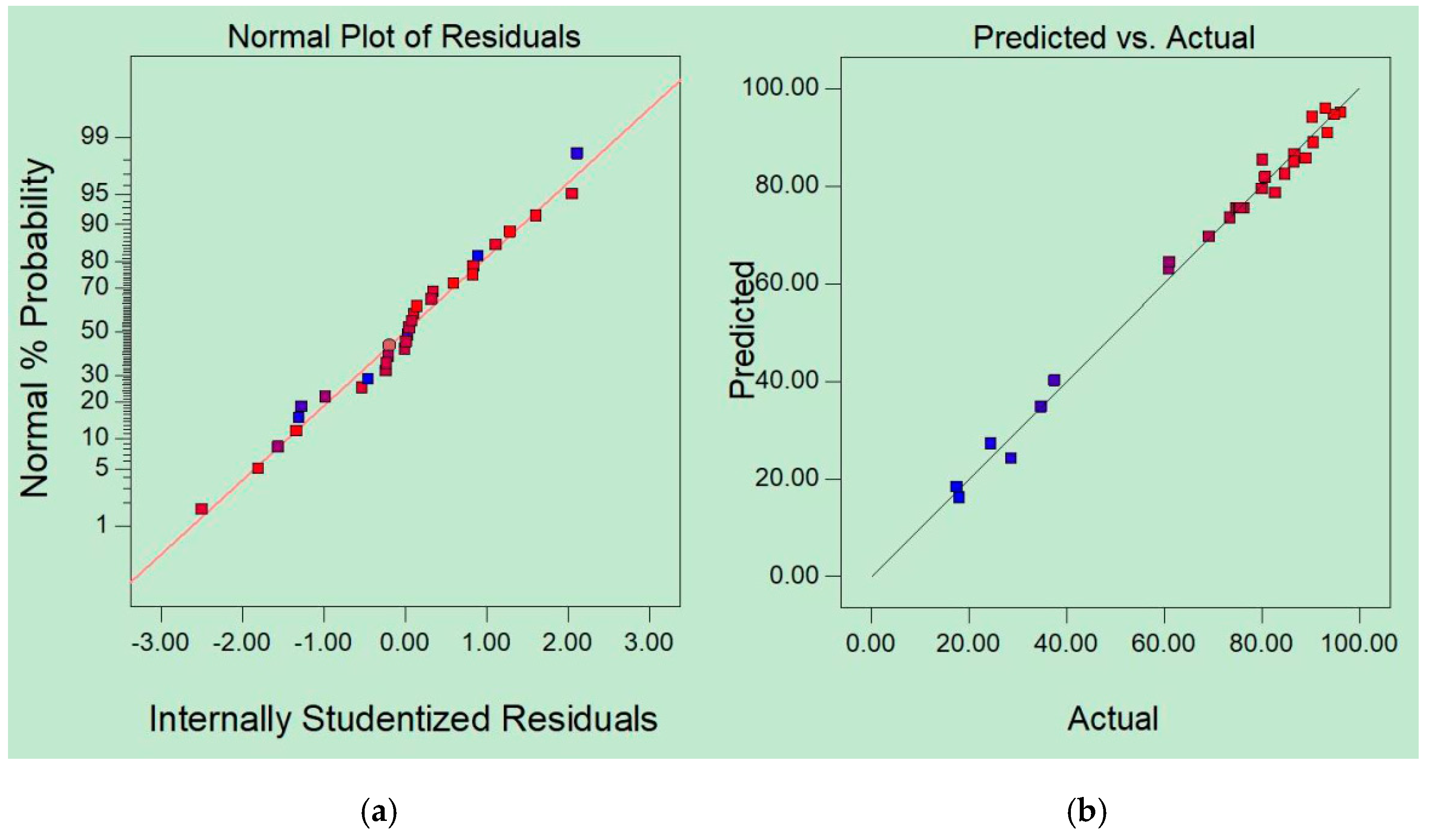
3.2. Model Analysis
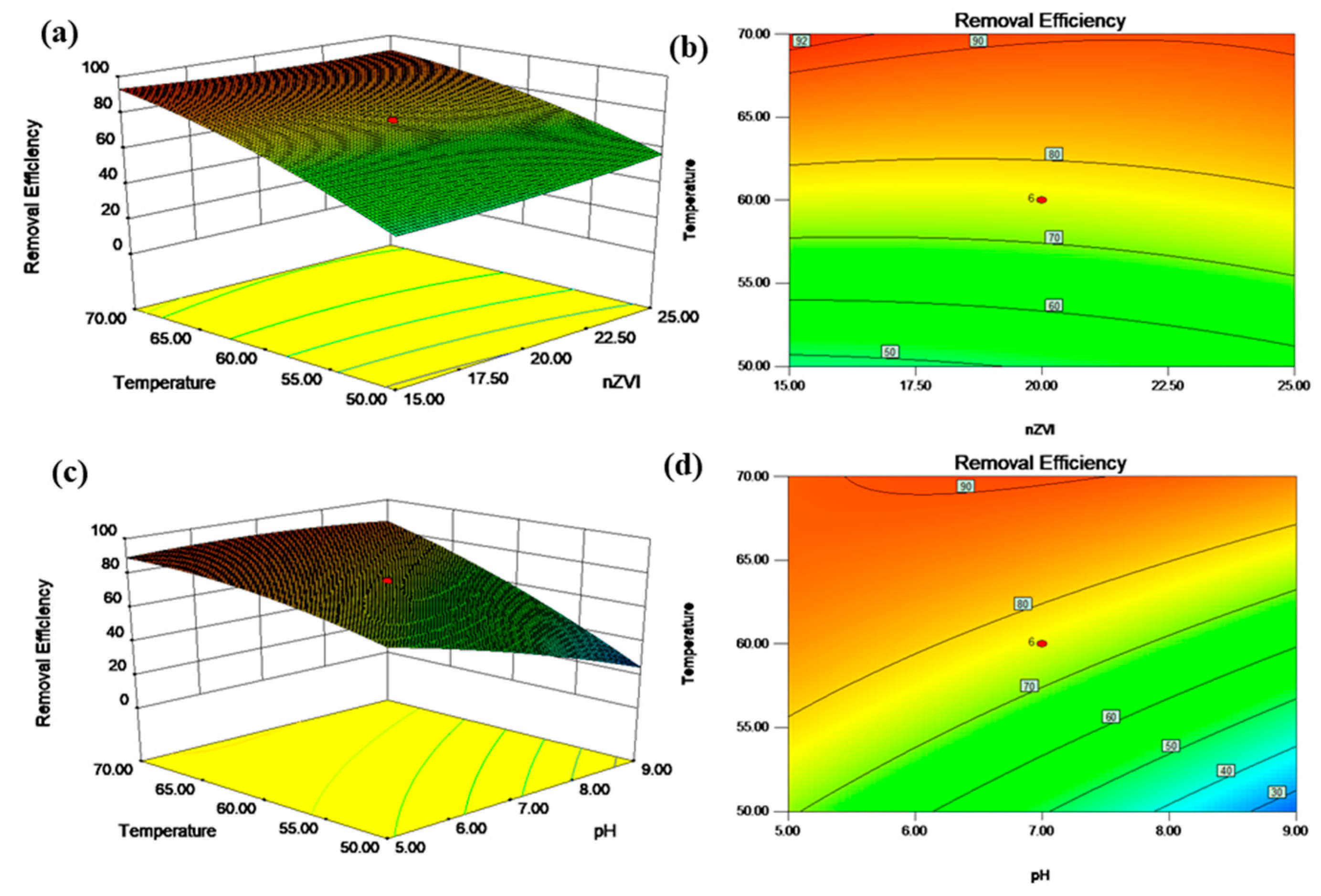
3.3. Interactive Effects of Operational Parameters
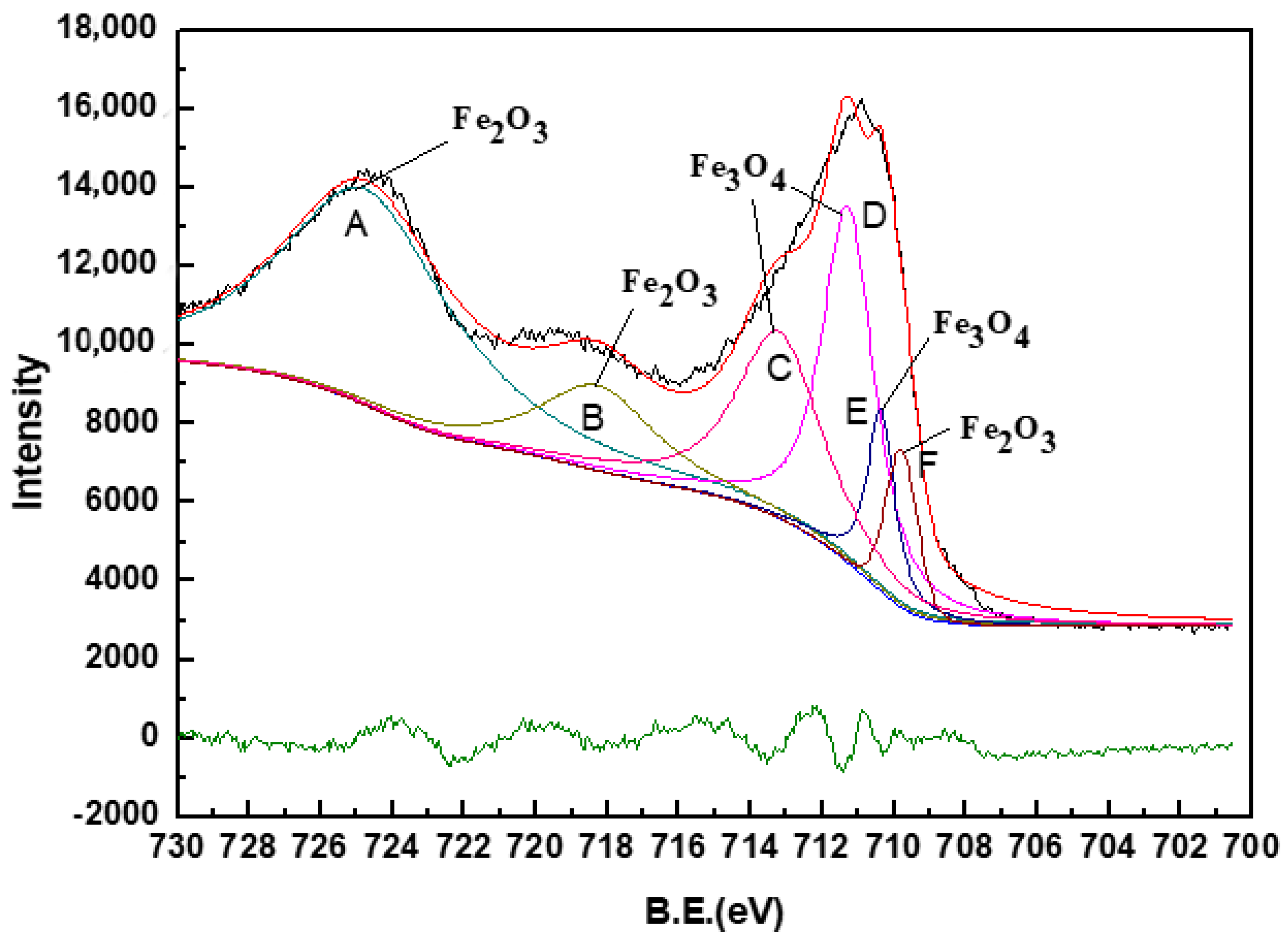
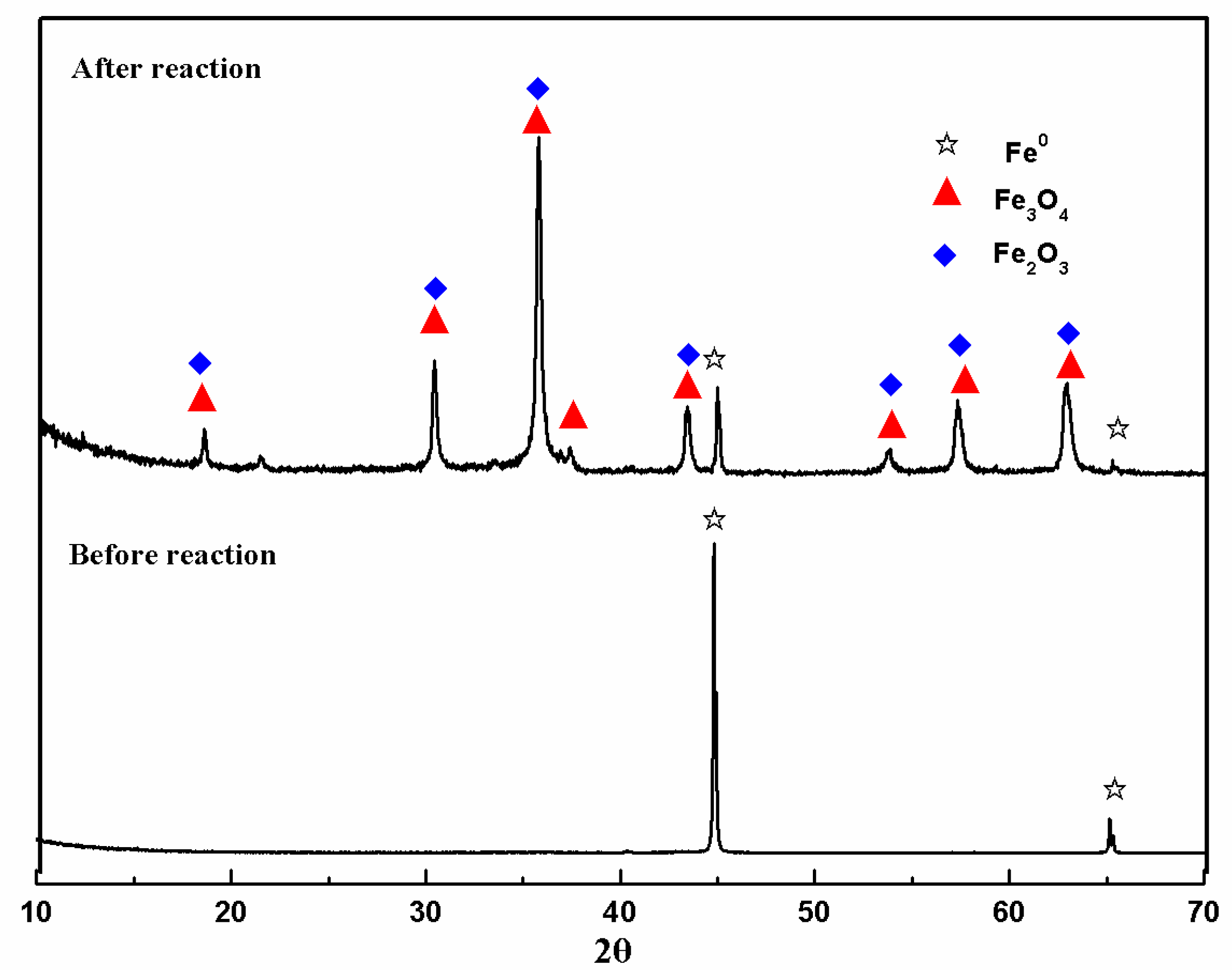
3.4. Fe-Speciation Analysis
3.5. Optimization of Removal Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Podzelinska, K.; Latimer, R.; Bhattacharya, A.; Vining, L.C.; Zechel, D.L.; Jia, Z. Chloramphenicol Biosynthesis: The Structure of CmlS, a Flavin-Dependent Halogenase Showing a Covalent Flavin–Aspartate Bond. J. Mol. Biol. 2010, 397, 316–331. [Google Scholar] [CrossRef]
- Samsonova, J.V.; Cannavan, A.; Elliott, C.T. A Critical Review of Screening Methods for the Detection of Chloramphenicol, Thiamphenicol, and Florfenicol Residues in Foodstuffs. Crit. Rev. Anal. Chem. 2012, 42, 50–78. [Google Scholar] [CrossRef]
- Ma, W.; Dai, J.; Dai, X.; Da, Z.; Yan, Y. Core–shell molecularly imprinted polymers based on magnetic chitosan microspheres for chloramphenicol selective adsorption. Mon. Chem. Chem. Mon. 2015, 146, 465–474. [Google Scholar] [CrossRef]
- Kramer, W.G.; Rensimer, E.R.; Ericsson, C.D.; Pickering, L.K. Comparative Bioavailability of Intravenous and Oral Chloramphenicol in Adults. J. Clin. Pharmacol. 1984, 24, 181–186. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.; Jung, J.; Kim, M.H.; Kim, C.S.; Kim, N.H.; Park, J. Occurrences and ecological risks of roxithromycin, trimethoprim, and chloramphenicol in the Han River, Korea. Environ. Toxicol. Chem. 2008, 27, 711–719. [Google Scholar] [CrossRef]
- Nie, M.H.; Yang, Y.; Zhang, Z.J.; Yan, C.X.; Wang, X.N.; Li, H.J.; Dong, W.B. Degradation of chloramphenicol by thermally activated persulfate in aqueous solution. Chem. Eng. J. 2014, 246, 373–382. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Yin, D.; Zhang, H.; Yu, Z. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 2011, 82, 822–828. [Google Scholar] [CrossRef]
- Badawy, M.I.; Wahaab, R.A.; El-Kalliny, A.S. Fenton-biological treatment processes for the removal of some pharmaceuticals from industrial wastewater. J. Hazard. Mater. 2009, 167, 567–574. [Google Scholar] [CrossRef]
- Chu, W.H.; Chu, T.F.; Du, E.D.; Yang, D.; Guo, Y.Q.; Gao, N.Y. Increased formation of halomethanes during chlorination of chloramphenicol in drinking water by UV irradiation, persulfate oxidation, and combined UV/persulfate pre-treatments. Ecotox. Environ. Safe 2016, 124, 147–154. [Google Scholar] [CrossRef]
- Shokri, M.; Jodat, A.; Modirshahla, N.; Behnajady, M.A. Photocatalytic degradation of chloramphenicol in an aqueous suspension of silver-doped TiO2 nanoparticles. Environ. Technol. 2013, 34, 1161–1166. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, B.; Yuan, S.; Wu, X.; Chen, J.; Wang, L. Adsorptive removal of chloramphenicol from wastewater by NaOH modified bamboo charcoal. Bioresour. Technol. 2010, 101, 7661–7664. [Google Scholar] [CrossRef]
- Dai, J.; He, J.; Xie, A.; Gao, L.; Pan, J.; Chen, X.; Zhou, Z.; Wei, X.; Yan, Y. Novel pitaya-inspired well-defined core–shell nanospheres with ultrathin surface imprinted nanofilm from magnetic mesoporous nanosilica for highly efficient chloramphenicol removal. Chem. Eng. J. 2016, 284, 812–822. [Google Scholar] [CrossRef]
- Qin, L.; Zhou, Z.; Dai, J.; Ma, P.; Zhao, H.; He, J.; Xie, A.; Li, C.; Yan, Y. Novel N-doped hierarchically porous carbons derived from sustainable shrimp shell for high-performance removal of sulfamethazine and chloramphenicol. J. Taiwan Inst. Chem. Eng. 2016, 62, 228–238. [Google Scholar] [CrossRef]
- Tran, V.S.; Ngo, H.H.; Guo, W.; Ton-That, C.; Li, J.; Li, J.; Liu, Y. Removal of antibiotics (sulfamethazine, tetracycline and chloramphenicol) from aqueous solution by raw and nitrogen plasma modified steel shavings. Sci. Total Environ. 2017, 601, 845–856. [Google Scholar] [CrossRef]
- Dai, J.; Tian, S.; Jiang, Y.; Chang, Z.; Xie, A.; Zhang, R.; Yan, Y. Facile synthesis of porous carbon sheets from potassium acetate via in-situ template and self-activation for highly efficient chloramphenicol removal. J. Alloys Compd. 2018, 732, 222–232. [Google Scholar] [CrossRef]
- Singh, K.P.; Singh, A.K.; Gupta, S.; Rai, P. Modeling and optimization of reductive degradation of chloramphenicol in aqueous solution by zero-valent bimetallic nanoparticles. Environ. Sci. Pollut. R 2012, 19, 2063–2078. [Google Scholar] [CrossRef]
- Xia, S.; Gu, Z.; Zhang, Z.; Zhang, J.; Hermanowicz, S.W. Removal of chloramphenicol from aqueous solution by nanoscale zero-valent iron particles. Chem. Eng. J. 2014, 257, 98–104. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Z.; Yuan, Z.L.; Zhang, J.; Guo, X.P.; Yang, Y.; He, F.; Zhao, Y.P.; Xu, J. Insight into the kinetics and mechanism of removal of aqueous chlorinated nitroaromatic antibiotic chloramphenicol by nanoscale zero-valent iron. Chem. Eng. J. 2018, 334, 508–518. [Google Scholar] [CrossRef]
- Wu, Y.W.; Yue, Q.Y.; Ren, Z.F.; Gao, B.Y. Immobilization of nanoscale zero-valent iron particles (nZVI) with synthesized activated carbon for the adsorption and degradation of Chloramphenicol (CAP). J. Mol. Liq. 2018, 262, 19–28. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Electrolytic and electro-irradiated technologies for the removal of chloramphenicol in synthetic urine with diamond anodes. Water Res. 2018, 128, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Wang, Y.K.; Yan, L.; Wang, X.H.; Wang, M.Y.; Xu, H.; Wang, S.G. Effect of bio-electrochemical system on the fate and proliferation of chloramphenicol resistance genes during the treatment of chloramphenicol wastewater. Water Res. 2017, 117, 95–101. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Kim, C.; Ahn, J.-Y.; Kim, T.Y.; Shin, W.S.; Hwang, I. Activation of Persulfate by Nanosized Zero-Valent Iron (NZVI): Mechanisms and Transformation Products of NZVI. Environ. Sci. Technol. 2018, 52, 3625–3633. [Google Scholar] [CrossRef]
- Fu, Y.; Peng, L.; Zeng, Q.; Yang, Y.; Song, H.; Shao, J.; Liu, S.; Gu, J. High efficient removal of tetracycline from solution by degradation and flocculation with nanoscale zerovalent iron. Chem. Eng. J. 2015, 270, 631–640. [Google Scholar] [CrossRef]
- Karim, S.; Bae, S.; Greenwood, D.; Hanna, K.; Singhal, N. Degradation of 17α-ethinylestradiol by nano zero valent iron under different pH and dissolved oxygen levels. Water Res. 2017, 125, 32–41. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Royer, S.; Zhang, H. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J. Hazard Mater. 2016, 302, 458–467. [Google Scholar] [CrossRef]
- Liu, F.Z.; Yi, P.; Wang, X.; Gao, H.; Zhang, H. Degradation of Acid Orange 7 by an ultrasound/ZnO-GAC/persulfate process. Sep. Purif. Technol. 2018, 194, 181–187. [Google Scholar] [CrossRef]
- Zou, X.L.; Zhou, T.; Mao, J.; Wu, X.H. Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe-0/persulfate Fenton-like system. Chem. Eng. J. 2014, 257, 36–44. [Google Scholar] [CrossRef]
- Safari, G.H.; Nasseri, S.; Mahvi, A.H.; Yaghmaeian, K.; Nabizadeh, R.; Alimohammadi, M. Optimization of sonochemical degradation of tetracycline in aqueous solution using sono-activated persulfate process. J. Environ. Health Sci. Eng. 2015, 13, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslami, A.; Asadi, A.; Meserghani, M.; Bahrami, H. Optimization of sonochemical degradation of amoxicillin by sulfate radicals in aqueous solution using response surface methodology (RSM). J. Mol. Liq. 2016, 222, 739–744. [Google Scholar] [CrossRef]
- Li, R.B.; Cai, M.X.; Liu, H.J.; Liu, G.G.; Lv, W.Y. Thermo-activated peroxydisulfate oxidation of indomethacin: Kinetics study and influences of co-existing substances. Chemosphere 2018, 212, 1067–1075. [Google Scholar] [CrossRef]
- Tan, C.Q.; Fu, D.F.; Gao, N.Y.; Qin, Q.D.; Xu, Y.; Xiang, H.M. Kinetic degradation of chloramphenicol in water by UV/persulfate system. J. Photochem. Photobiol. A Chem. 2017, 332, 406–412. [Google Scholar] [CrossRef]
- Bahrami, H.; Eslami, A.; Nabizadeh, R.; Mohseni-Bandpi, A.; Asadi, A.; Sillanpaa, M. Degradation of trichloroethylene by sonophotolytic-activated persulfate processes: Optimization using response surface methodology. J. Clean Prod. 2018, 198, 1210–1218. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; Expósito, A.J.; Monsalve, V. Modeling the sonophoto-degradation/mineralization of carbamazepine in aqueous solution. Chem. Eng. J. 2016, 284, 503–512. [Google Scholar] [CrossRef]
- Kusic, H.; Peternel, I.; Koprivanac, N.; Bozic, A.L. Iron-Activated Persulfate Oxidation of an Azo Dye in Model Wastewater: Influence of Iron Activator Type on Process Optimization. J. Environ. Eng. 2011, 137, 454–463. [Google Scholar] [CrossRef]
- Turan, M.D.; Arslanoğlu, H.; Altundoğan, H.S. Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system. J. Taiwan Inst. Chem. Eng. 2015, 50, 49–55. [Google Scholar] [CrossRef]
- Yang, Q.; Zhong, Y.; Zhong, H.; Li, X.; Du, W.; Li, X.; Chen, R.; Zeng, G. A novel pretreatment process of mature landfill leachate with ultrasonic activated persulfate: Optimization using integrated Taguchi method and response surface methodology. Process Saf. Environ. Prot. 2015, 98, 268–275. [Google Scholar] [CrossRef]
- Tripathy, B.K.; Ramesh, G.; Debnath, A.; Kumar, M. Mature landfill leachate treatment using sonolytic-persulfate/hydrogen peroxide oxidation: Optimization of process parameters. Ultrason. Sonochem. 2019, 54, 210–219. [Google Scholar] [CrossRef]
- Abu Amr, S.S.; Aziz, H.A.; Adlan, M.N. Optimization of stabilized leachate treatment using ozone/persulfate in the advanced oxidation process. Waste Manag. 2013, 33, 1434–1441. [Google Scholar] [CrossRef]
- Chen, X.; Murugananthan, M.; Zhang, Y. Degradation of p-Nitrophenol by thermally activated persulfate in soil system. Chem. Eng. J. 2016, 283, 1357–1365. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, L.M.; Zhang, S.B.; Ou, L.H.; Liu, C.B.; Zheng, L.Y.; Yang, Y.F.; Ying, G.G.; Luo, S.L. Degradation of azole fungicide fluconazole in aqueous solution by thermally activated persulfate. Chem. Eng. J. 2017, 321, 113–122. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, M.; Yang, W.; Pan, Y.; Lu, X.; Serrano, K.G. Degradation and mechanism of 2,4-dichlorophenoxyacetic acid (2,4-D) by thermally activated persulfate oxidation. Chemosphere 2018, 212, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Qian, Y.; Liu, H.; Huang, T. Oxidative degradation of diclofenac by thermally activated persulfate: implication for ISCO. Environ. Sci. Pollut. R 2016, 23, 3824–3833. [Google Scholar] [CrossRef]
- Nasseri, S.; Mahvi, A.H.; Seyedsalehi, M.; Yaghmaeian, K.; Nabizadeh, R.; Alimohammadi, M.; Safari, G.H. Degradation kinetics of tetracycline in aqueous solutions using peroxydisulfate activated by ultrasound irradiation: Effect of radical scavenger and water matrix. J. Mol. Liq. 2017, 241, 704–714. [Google Scholar] [CrossRef]
- Zhu, J.; Li, B.Z. Degradation Kinetic and Remediation Effectiveness of 1,4-Dioxane-Contaminated Groundwater by a Sono-Activated Persulfate Process. J. Environ. Eng. 2018, 144, 04018098. [Google Scholar] [CrossRef]
- Rodriguez, S.; Vasquez, L.; Costa, D.; Romero, A.; Santos, A. Oxidation of Orange G by persulfate activated by Fe(II), Fe(III) and zero valent iron (ZVI). Chemosphere 2014, 101, 86–92. [Google Scholar] [CrossRef]
- Tan, C.Q.; Dong, Y.J.; Fu, D.F.; Gao, N.Y.; Ma, J.X.; Liu, X.Y. Chloramphenicol removal by zero valent iron activated peroxymonosulfate system: Kinetics and mechanism of radical generation. Chem. Eng. J. 2018, 334, 1006–1015. [Google Scholar] [CrossRef]
- Lu, L.L.; Zhai, P.P.; Chen, X.; Li, H.J.; Chovelon, J.M. Degradation of p-Aminobenzoic Acid by Zero-Valent Iron Activated Persulfate System. J. Environ. Eng. 2018, 144. [Google Scholar] [CrossRef]
- Vicente, F.; Santos, A.; Romero, A.; Rodriguez, S. Kinetic study of diuron oxidation and mineralization by persulphate: Effects of temperature, oxidant concentration and iron dosage method. Chem. Eng. J. 2011, 170, 127–135. [Google Scholar] [CrossRef]
- Nie, M.H.; Yan, C.X.; Li, M.; Wang, X.N.; Bi, W.L.; Dong, W.B. Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem. Eng. J. 2015, 279, 507–515. [Google Scholar] [CrossRef]
- Lin, H.; Wu, J.; Zhang, H. Degradation of clofibric acid in aqueous solution by an EC/Fe3+/PMS process. Chem. Eng. J. 2014, 244, 514–521. [Google Scholar] [CrossRef]
- Stefánsson, A. Iron(III) Hydrolysis and Solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [CrossRef]
- Xu, X.-R.; Li, X.-Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep. Purif. Technol. 2010, 72, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xu, J.; Zhang, J.; Yang, Y.; Zhou, J.; Xu, X.; Lowry, G.V. Removal of Antibiotic Florfenicol by Sulfide-Modified Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2017, 51, 11269–11277. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Lin, T.-C.; Seshadri, G.; Kelber, J.A. A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Sun, C.; Wan, J.; He, H.; Wang, F.; Dai, Y.; Yang, S.; Lin, Y.; Zhan, X. Insights into removal of tetracycline by persulfate activation with peanut shell biochar coupled with amorphous Cu-doped FeOOH composite in aqueous solution. Environ. Sci. Pollut. R 2019, 26, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.-X.; Zhou, L.-X. Synthesis of iron oxyhydroxides of different crystal forms and their roles in adsorption and removal of Cr(Ⅵ)from aqueous solutions. Acta Petrol. ET Mineral. 2008, 27, 559–566. [Google Scholar]
- Yang, L.; He, L.; Xue, J.; Wu, L.; Ma, Y.; Li, H.; Peng, P.; Li, M.; Zhang, Z. Highly efficient nickel (II) removal by sewage sludge biochar supported α-Fe2O3 and α-FeOOH: Sorption characteristics and mechanisms. PLoS ONE 2019, 14, e0218114. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lei, M.; Zhu, L.; Anjum, M.N.; Zou, J.; Tang, H. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate. J. Hazard. Mater. 2011, 186, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.; Mohammadi, F.; Kakavandi, B.; Esrafili, A.; Rostamifasih, Z. Simultaneous catalytic degradation of 2,4-D and MCPA herbicides using sulfate radical-based Heterog oxidation over persulfate activated by natural hematite (α-Fe2O3/PS). J. Phys. Chem. Solids 2018, 117, 49–59. [Google Scholar] [CrossRef]

| Parameter | Character |
|---|---|
| Formula | C11H12Cl2N2O5 |
| Molecular weight | 323.13 |
| Solubility (mg/L), 25 °C | 2500 |
| Log Kow | 1.14 |
| pKa | 9.5 |
| CAS number | 56-75-7 |
| Molecular structure | 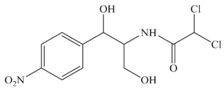 |
| Factors | Symbols | Level of Factors | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Nanoscale zero-valent iron (nZVI) (mg/L) | X1 | 10 | 15 | 20 | 25 | 30 |
| Peroxydisulfate (PS) concentration (mM) | X2 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 |
| Initial pH | X3 | 3 | 5 | 7 | 9 | 11 |
| Temperature (°C) | X4 | 40 | 50 | 60 | 70 | 80 |
| Run Number | Factors | Degradation Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Observed | Predicted | |
| 1 | 25 | 0.5 | 5 | 50 | 80.72 | 81.82 |
| 2 | 20 | 0.4 | 7 | 80 | 93.07 | 95.84 |
| 3 | 20 | 0.4 | 3 | 60 | 90.62 | 88.90 |
| 4 | 20 | 0.6 | 7 | 60 | 84.76 | 82.45 |
| 5 | 15 | 0.3 | 5 | 50 | 60.93 | 62.96 |
| 6 | 15 | 0.3 | 9 | 50 | 17.42 | 18.36 |
| 7 | 15 | 0.5 | 9 | 70 | 94.95 | 94.64 |
| 8 | 15 | 0.5 | 9 | 50 | 24.45 | 27.15 |
| 9 | 25 | 0.5 | 5 | 70 | 96.25 | 95.02 |
| 10 | 25 | 0.5 | 9 | 70 | 93.54 | 90.88 |
| 11 | 20 | 0.4 | 11 | 60 | 37.52 | 40.16 |
| 12 | 25 | 0.3 | 9 | 70 | 80.11 | 79.40 |
| 13 | 10 | 0.4 | 7 | 60 | 82.83 | 78.58 |
| 14 | 15 | 0.3 | 9 | 70 | 86.62 | 84.89 |
| 15 | 20 | 0.4 | 7 | 60 | 76.42 | 75.47 |
| 16 | 15 | 0.3 | 5 | 70 | 86.76 | 86.53 |
| 17 | 20 | 0.4 | 7 | 40 | 17.97 | 16.12 |
| 18 | 20 | 0.4 | 7 | 60 | 74.80 | 75.47 |
| 19 | 20 | 0.4 | 7 | 60 | 75.61 | 75.47 |
| 20 | 25 | 0.3 | 5 | 70 | 89.03 | 85.70 |
| 21 | 20 | 0.4 | 7 | 60 | 75.70 | 75.47 |
| 22 | 20 | 0.2 | 7 | 60 | 61.12 | 64.35 |
| 23 | 30 | 0.4 | 7 | 60 | 80.16 | 85.33 |
| 24 | 15 | 0.5 | 5 | 50 | 69.16 | 69.58 |
| 25 | 25 | 0.3 | 5 | 50 | 73.46 | 73.48 |
| 26 | 25 | 0.3 | 9 | 50 | 28.59 | 24.22 |
| 27 | 20 | 0.4 | 7 | 60 | 75.51 | 75.47 |
| 28 | 25 | 0.5 | 9 | 50 | 34.79 | 34.73 |
| 29 | 20 | 0.4 | 7 | 60 | 74.77 | 75.47 |
| 30 | 15 | 0.5 | 5 | 70 | 90.38 | 94.12 |
| Term | Coefficient Estimate | Standard Error | p-Value |
|---|---|---|---|
| Intercept | 75.47 | 1.31 | <0.0001 |
| X1 | 1.69 | 0.65 | 0.0208 |
| X2 | 4.53 | 0.65 | <0.0001 |
| X3 | −12.18 | 0.65 | <0.0001 |
| X4 | 19.93 | 0.65 | <0.0001 |
| X1X4 | −2.84 | 0.80 | 0.0029 |
| X3X4 | 10.74 | 0.80 | <0.0001 |
| X12 | 1.62 | 0.61 | 0.0181 |
| X32 | −2.73 | 0.61 | 0.0004 |
| X42 | −4.87 | 0.61 | <0.0001 |
| Term | Squares | df | Square | Value | Probability > F |
|---|---|---|---|---|---|
| Model | 16,615.92 | 14 | 1186.85 | 115.86 | <0.0001 |
| X1 | 68.28 | 1 | 68.28 | 6.66 | 0.0208 |
| X2 | 491.41 | 1 | 491.41 | 47.97 | <0.0001 |
| X3 | 3562.89 | 1 | 3562.89 | 347.80 | <0.0001 |
| X4 | 9532.92 | 1 | 9532.92 | 930.57 | <0.0001 |
| X1X2 | 2.98 | 1 | 2.98 | 0.29 | 0.5978 |
| X1X3 | 21.72 | 1 | 21.72 | 2.12 | 0.1660 |
| X1X4 | 128.71 | 1 | 128.71 | 12.56 | 0.0029 |
| X2X3 | 4.69 | 1 | 4.69 | 0.46 | 0.5091 |
| X2X4 | 0.94 | 1 | 0.94 | 0.092 | 0.7660 |
| X3X4 | 1845.13 | 1 | 1845.13 | 180.11 | <0.0001 |
| X12 | 72.15 | 1 | 72.15 | 7.04 | 0.0181 |
| X22 | 7.33 | 1 | 7.33 | 0.72 | 0.4110 |
| X32 | 205.08 | 1 | 205.08 | 20.02 | 0.0004 |
| X42 | 651.02 | 1 | 651.02 | 63.55 | <0.0001 |
| Residual | 153.66 | 15 | 10.24 | ||
| Lack of Fit | 151.75 | 10 | 15.17 | 39.61 | 0.0004 |
| Pure Error | 1.92 | 5 | 0.38 | ||
| Corrected Total | 16,769.58 | 29 | |||
| R2 | 0.9908 | ||||
| Adjusted R2 | 0.9823 | ||||
| Adequate Precision | 35.224 | ||||
| C.V.% | 4.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, H.; Xue, J.; He, L.; Ma, Y.; Wu, L.; Zhang, Z. Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization. Water 2020, 12, 131. https://doi.org/10.3390/w12010131
Yang L, Li H, Xue J, He L, Ma Y, Wu L, Zhang Z. Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization. Water. 2020; 12(1):131. https://doi.org/10.3390/w12010131
Chicago/Turabian StyleYang, Lie, Hong Li, Jianming Xue, Liuyang He, Yongfei Ma, Li Wu, and Zulin Zhang. 2020. "Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization" Water 12, no. 1: 131. https://doi.org/10.3390/w12010131
APA StyleYang, L., Li, H., Xue, J., He, L., Ma, Y., Wu, L., & Zhang, Z. (2020). Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization. Water, 12(1), 131. https://doi.org/10.3390/w12010131








