Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Experimental Setup and Analysis Method
2.3. Experimental Details
3. Results and Discussion
3.1. The Synergistic Effect of nZVI-Heat Activated PS Degradation of CAP
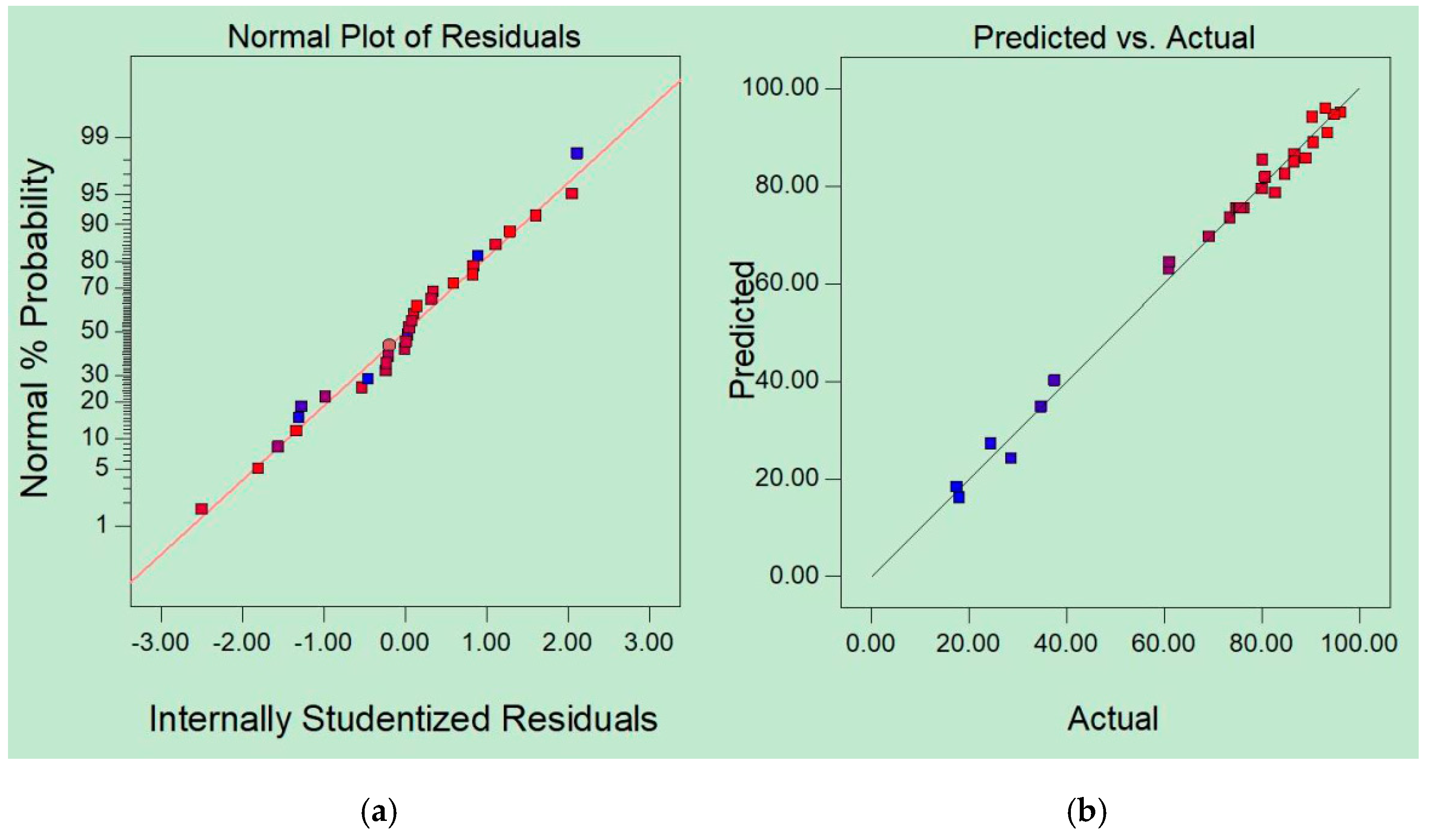
3.2. Model Analysis
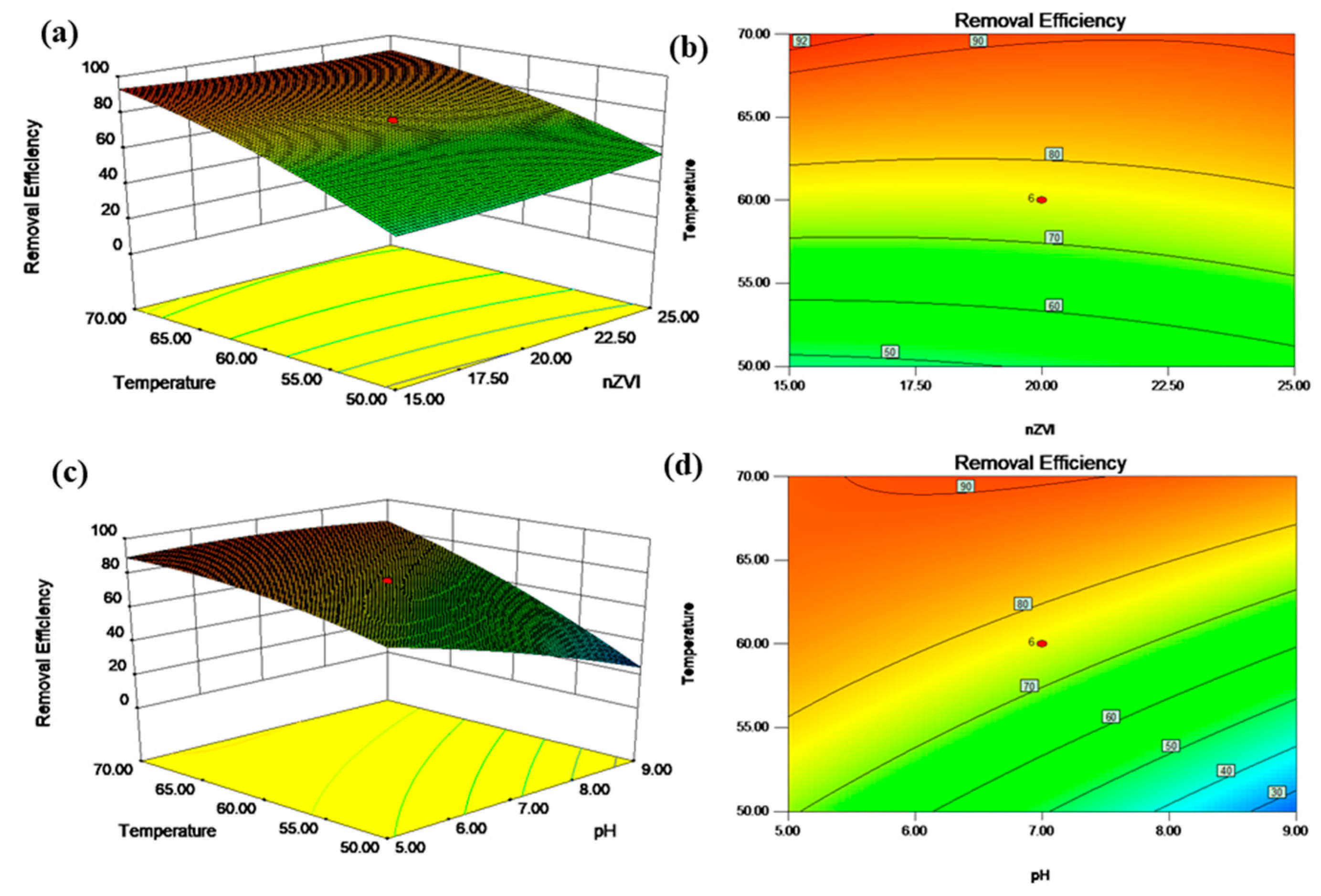
3.3. Interactive Effects of Operational Parameters
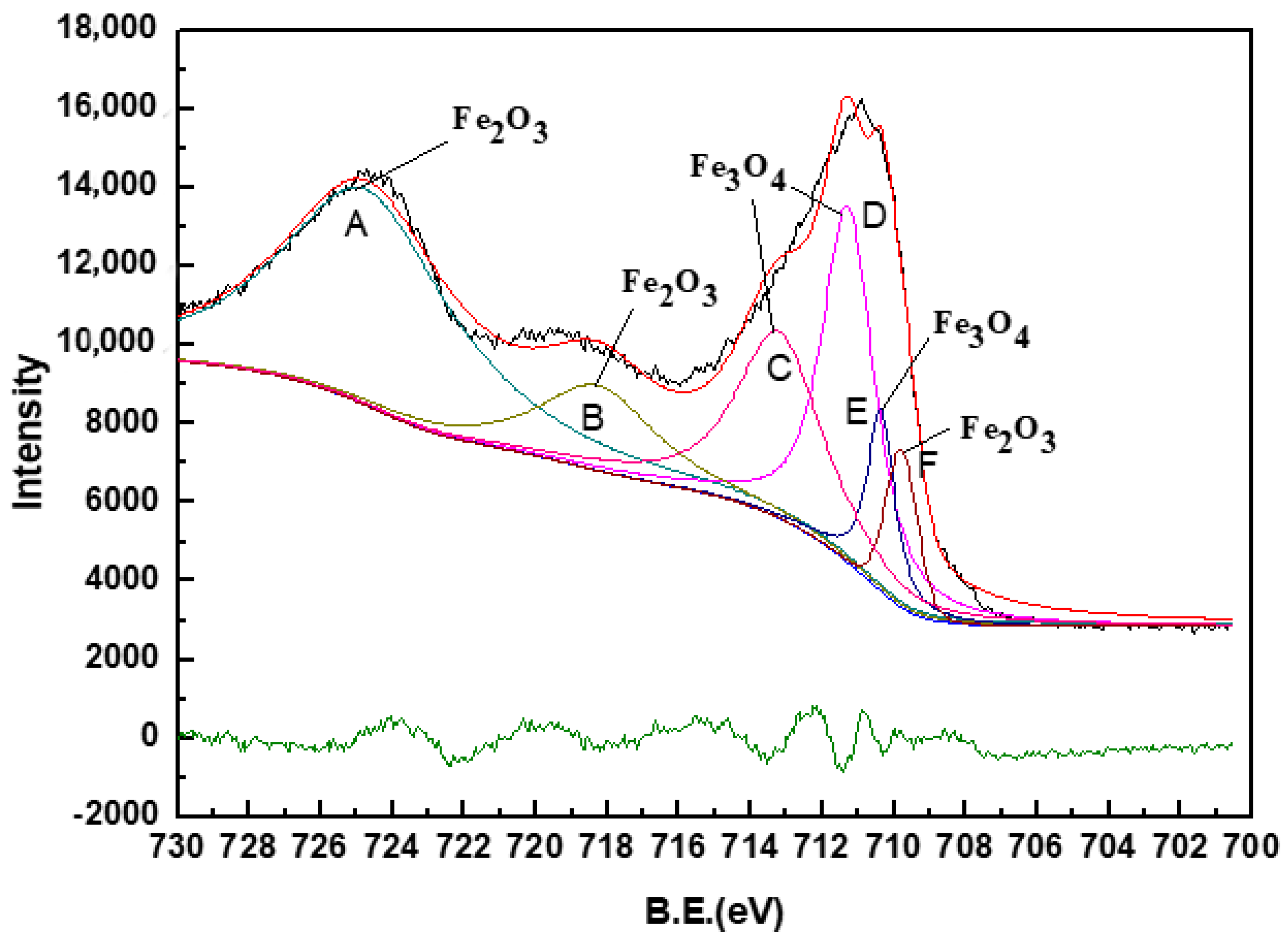
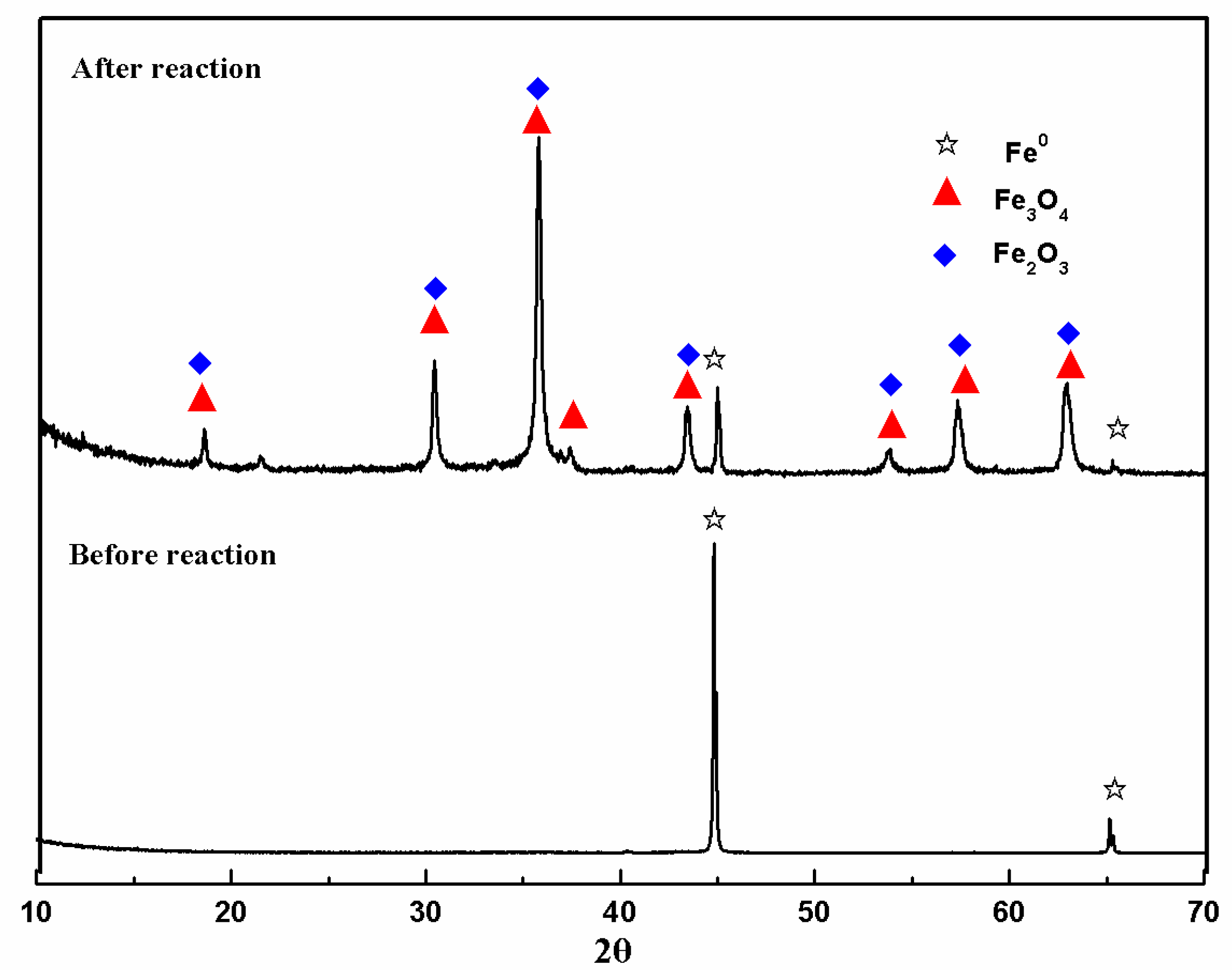
3.4. Fe-Speciation Analysis
3.5. Optimization of Removal Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Podzelinska, K.; Latimer, R.; Bhattacharya, A.; Vining, L.C.; Zechel, D.L.; Jia, Z. Chloramphenicol Biosynthesis: The Structure of CmlS, a Flavin-Dependent Halogenase Showing a Covalent Flavin–Aspartate Bond. J. Mol. Biol. 2010, 397, 316–331. [Google Scholar] [CrossRef]
- Samsonova, J.V.; Cannavan, A.; Elliott, C.T. A Critical Review of Screening Methods for the Detection of Chloramphenicol, Thiamphenicol, and Florfenicol Residues in Foodstuffs. Crit. Rev. Anal. Chem. 2012, 42, 50–78. [Google Scholar] [CrossRef]
- Ma, W.; Dai, J.; Dai, X.; Da, Z.; Yan, Y. Core–shell molecularly imprinted polymers based on magnetic chitosan microspheres for chloramphenicol selective adsorption. Mon. Chem. Chem. Mon. 2015, 146, 465–474. [Google Scholar] [CrossRef]
- Kramer, W.G.; Rensimer, E.R.; Ericsson, C.D.; Pickering, L.K. Comparative Bioavailability of Intravenous and Oral Chloramphenicol in Adults. J. Clin. Pharmacol. 1984, 24, 181–186. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.; Jung, J.; Kim, M.H.; Kim, C.S.; Kim, N.H.; Park, J. Occurrences and ecological risks of roxithromycin, trimethoprim, and chloramphenicol in the Han River, Korea. Environ. Toxicol. Chem. 2008, 27, 711–719. [Google Scholar] [CrossRef]
- Nie, M.H.; Yang, Y.; Zhang, Z.J.; Yan, C.X.; Wang, X.N.; Li, H.J.; Dong, W.B. Degradation of chloramphenicol by thermally activated persulfate in aqueous solution. Chem. Eng. J. 2014, 246, 373–382. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Yin, D.; Zhang, H.; Yu, Z. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 2011, 82, 822–828. [Google Scholar] [CrossRef]
- Badawy, M.I.; Wahaab, R.A.; El-Kalliny, A.S. Fenton-biological treatment processes for the removal of some pharmaceuticals from industrial wastewater. J. Hazard. Mater. 2009, 167, 567–574. [Google Scholar] [CrossRef]
- Chu, W.H.; Chu, T.F.; Du, E.D.; Yang, D.; Guo, Y.Q.; Gao, N.Y. Increased formation of halomethanes during chlorination of chloramphenicol in drinking water by UV irradiation, persulfate oxidation, and combined UV/persulfate pre-treatments. Ecotox. Environ. Safe 2016, 124, 147–154. [Google Scholar] [CrossRef]
- Shokri, M.; Jodat, A.; Modirshahla, N.; Behnajady, M.A. Photocatalytic degradation of chloramphenicol in an aqueous suspension of silver-doped TiO2 nanoparticles. Environ. Technol. 2013, 34, 1161–1166. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, B.; Yuan, S.; Wu, X.; Chen, J.; Wang, L. Adsorptive removal of chloramphenicol from wastewater by NaOH modified bamboo charcoal. Bioresour. Technol. 2010, 101, 7661–7664. [Google Scholar] [CrossRef]
- Dai, J.; He, J.; Xie, A.; Gao, L.; Pan, J.; Chen, X.; Zhou, Z.; Wei, X.; Yan, Y. Novel pitaya-inspired well-defined core–shell nanospheres with ultrathin surface imprinted nanofilm from magnetic mesoporous nanosilica for highly efficient chloramphenicol removal. Chem. Eng. J. 2016, 284, 812–822. [Google Scholar] [CrossRef]
- Qin, L.; Zhou, Z.; Dai, J.; Ma, P.; Zhao, H.; He, J.; Xie, A.; Li, C.; Yan, Y. Novel N-doped hierarchically porous carbons derived from sustainable shrimp shell for high-performance removal of sulfamethazine and chloramphenicol. J. Taiwan Inst. Chem. Eng. 2016, 62, 228–238. [Google Scholar] [CrossRef]
- Tran, V.S.; Ngo, H.H.; Guo, W.; Ton-That, C.; Li, J.; Li, J.; Liu, Y. Removal of antibiotics (sulfamethazine, tetracycline and chloramphenicol) from aqueous solution by raw and nitrogen plasma modified steel shavings. Sci. Total Environ. 2017, 601, 845–856. [Google Scholar] [CrossRef]
- Dai, J.; Tian, S.; Jiang, Y.; Chang, Z.; Xie, A.; Zhang, R.; Yan, Y. Facile synthesis of porous carbon sheets from potassium acetate via in-situ template and self-activation for highly efficient chloramphenicol removal. J. Alloys Compd. 2018, 732, 222–232. [Google Scholar] [CrossRef]
- Singh, K.P.; Singh, A.K.; Gupta, S.; Rai, P. Modeling and optimization of reductive degradation of chloramphenicol in aqueous solution by zero-valent bimetallic nanoparticles. Environ. Sci. Pollut. R 2012, 19, 2063–2078. [Google Scholar] [CrossRef]
- Xia, S.; Gu, Z.; Zhang, Z.; Zhang, J.; Hermanowicz, S.W. Removal of chloramphenicol from aqueous solution by nanoscale zero-valent iron particles. Chem. Eng. J. 2014, 257, 98–104. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Z.; Yuan, Z.L.; Zhang, J.; Guo, X.P.; Yang, Y.; He, F.; Zhao, Y.P.; Xu, J. Insight into the kinetics and mechanism of removal of aqueous chlorinated nitroaromatic antibiotic chloramphenicol by nanoscale zero-valent iron. Chem. Eng. J. 2018, 334, 508–518. [Google Scholar] [CrossRef]
- Wu, Y.W.; Yue, Q.Y.; Ren, Z.F.; Gao, B.Y. Immobilization of nanoscale zero-valent iron particles (nZVI) with synthesized activated carbon for the adsorption and degradation of Chloramphenicol (CAP). J. Mol. Liq. 2018, 262, 19–28. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Electrolytic and electro-irradiated technologies for the removal of chloramphenicol in synthetic urine with diamond anodes. Water Res. 2018, 128, 383–392. [Google Scholar] [CrossRef]
- Guo, N.; Wang, Y.K.; Yan, L.; Wang, X.H.; Wang, M.Y.; Xu, H.; Wang, S.G. Effect of bio-electrochemical system on the fate and proliferation of chloramphenicol resistance genes during the treatment of chloramphenicol wastewater. Water Res. 2017, 117, 95–101. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Kim, C.; Ahn, J.-Y.; Kim, T.Y.; Shin, W.S.; Hwang, I. Activation of Persulfate by Nanosized Zero-Valent Iron (NZVI): Mechanisms and Transformation Products of NZVI. Environ. Sci. Technol. 2018, 52, 3625–3633. [Google Scholar] [CrossRef]
- Fu, Y.; Peng, L.; Zeng, Q.; Yang, Y.; Song, H.; Shao, J.; Liu, S.; Gu, J. High efficient removal of tetracycline from solution by degradation and flocculation with nanoscale zerovalent iron. Chem. Eng. J. 2015, 270, 631–640. [Google Scholar] [CrossRef]
- Karim, S.; Bae, S.; Greenwood, D.; Hanna, K.; Singhal, N. Degradation of 17α-ethinylestradiol by nano zero valent iron under different pH and dissolved oxygen levels. Water Res. 2017, 125, 32–41. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Royer, S.; Zhang, H. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J. Hazard Mater. 2016, 302, 458–467. [Google Scholar] [CrossRef]
- Liu, F.Z.; Yi, P.; Wang, X.; Gao, H.; Zhang, H. Degradation of Acid Orange 7 by an ultrasound/ZnO-GAC/persulfate process. Sep. Purif. Technol. 2018, 194, 181–187. [Google Scholar] [CrossRef]
- Zou, X.L.; Zhou, T.; Mao, J.; Wu, X.H. Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe-0/persulfate Fenton-like system. Chem. Eng. J. 2014, 257, 36–44. [Google Scholar] [CrossRef]
- Safari, G.H.; Nasseri, S.; Mahvi, A.H.; Yaghmaeian, K.; Nabizadeh, R.; Alimohammadi, M. Optimization of sonochemical degradation of tetracycline in aqueous solution using sono-activated persulfate process. J. Environ. Health Sci. Eng. 2015, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Eslami, A.; Asadi, A.; Meserghani, M.; Bahrami, H. Optimization of sonochemical degradation of amoxicillin by sulfate radicals in aqueous solution using response surface methodology (RSM). J. Mol. Liq. 2016, 222, 739–744. [Google Scholar] [CrossRef]
- Li, R.B.; Cai, M.X.; Liu, H.J.; Liu, G.G.; Lv, W.Y. Thermo-activated peroxydisulfate oxidation of indomethacin: Kinetics study and influences of co-existing substances. Chemosphere 2018, 212, 1067–1075. [Google Scholar] [CrossRef]
- Tan, C.Q.; Fu, D.F.; Gao, N.Y.; Qin, Q.D.; Xu, Y.; Xiang, H.M. Kinetic degradation of chloramphenicol in water by UV/persulfate system. J. Photochem. Photobiol. A Chem. 2017, 332, 406–412. [Google Scholar] [CrossRef]
- Bahrami, H.; Eslami, A.; Nabizadeh, R.; Mohseni-Bandpi, A.; Asadi, A.; Sillanpaa, M. Degradation of trichloroethylene by sonophotolytic-activated persulfate processes: Optimization using response surface methodology. J. Clean Prod. 2018, 198, 1210–1218. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; Expósito, A.J.; Monsalve, V. Modeling the sonophoto-degradation/mineralization of carbamazepine in aqueous solution. Chem. Eng. J. 2016, 284, 503–512. [Google Scholar] [CrossRef]
- Kusic, H.; Peternel, I.; Koprivanac, N.; Bozic, A.L. Iron-Activated Persulfate Oxidation of an Azo Dye in Model Wastewater: Influence of Iron Activator Type on Process Optimization. J. Environ. Eng. 2011, 137, 454–463. [Google Scholar] [CrossRef]
- Turan, M.D.; Arslanoğlu, H.; Altundoğan, H.S. Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system. J. Taiwan Inst. Chem. Eng. 2015, 50, 49–55. [Google Scholar] [CrossRef]
- Yang, Q.; Zhong, Y.; Zhong, H.; Li, X.; Du, W.; Li, X.; Chen, R.; Zeng, G. A novel pretreatment process of mature landfill leachate with ultrasonic activated persulfate: Optimization using integrated Taguchi method and response surface methodology. Process Saf. Environ. Prot. 2015, 98, 268–275. [Google Scholar] [CrossRef]
- Tripathy, B.K.; Ramesh, G.; Debnath, A.; Kumar, M. Mature landfill leachate treatment using sonolytic-persulfate/hydrogen peroxide oxidation: Optimization of process parameters. Ultrason. Sonochem. 2019, 54, 210–219. [Google Scholar] [CrossRef]
- Abu Amr, S.S.; Aziz, H.A.; Adlan, M.N. Optimization of stabilized leachate treatment using ozone/persulfate in the advanced oxidation process. Waste Manag. 2013, 33, 1434–1441. [Google Scholar] [CrossRef]
- Chen, X.; Murugananthan, M.; Zhang, Y. Degradation of p-Nitrophenol by thermally activated persulfate in soil system. Chem. Eng. J. 2016, 283, 1357–1365. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, L.M.; Zhang, S.B.; Ou, L.H.; Liu, C.B.; Zheng, L.Y.; Yang, Y.F.; Ying, G.G.; Luo, S.L. Degradation of azole fungicide fluconazole in aqueous solution by thermally activated persulfate. Chem. Eng. J. 2017, 321, 113–122. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, M.; Yang, W.; Pan, Y.; Lu, X.; Serrano, K.G. Degradation and mechanism of 2,4-dichlorophenoxyacetic acid (2,4-D) by thermally activated persulfate oxidation. Chemosphere 2018, 212, 784–793. [Google Scholar] [CrossRef]
- Chen, J.; Qian, Y.; Liu, H.; Huang, T. Oxidative degradation of diclofenac by thermally activated persulfate: implication for ISCO. Environ. Sci. Pollut. R 2016, 23, 3824–3833. [Google Scholar] [CrossRef]
- Nasseri, S.; Mahvi, A.H.; Seyedsalehi, M.; Yaghmaeian, K.; Nabizadeh, R.; Alimohammadi, M.; Safari, G.H. Degradation kinetics of tetracycline in aqueous solutions using peroxydisulfate activated by ultrasound irradiation: Effect of radical scavenger and water matrix. J. Mol. Liq. 2017, 241, 704–714. [Google Scholar] [CrossRef]
- Zhu, J.; Li, B.Z. Degradation Kinetic and Remediation Effectiveness of 1,4-Dioxane-Contaminated Groundwater by a Sono-Activated Persulfate Process. J. Environ. Eng. 2018, 144, 04018098. [Google Scholar] [CrossRef]
- Rodriguez, S.; Vasquez, L.; Costa, D.; Romero, A.; Santos, A. Oxidation of Orange G by persulfate activated by Fe(II), Fe(III) and zero valent iron (ZVI). Chemosphere 2014, 101, 86–92. [Google Scholar] [CrossRef]
- Tan, C.Q.; Dong, Y.J.; Fu, D.F.; Gao, N.Y.; Ma, J.X.; Liu, X.Y. Chloramphenicol removal by zero valent iron activated peroxymonosulfate system: Kinetics and mechanism of radical generation. Chem. Eng. J. 2018, 334, 1006–1015. [Google Scholar] [CrossRef]
- Lu, L.L.; Zhai, P.P.; Chen, X.; Li, H.J.; Chovelon, J.M. Degradation of p-Aminobenzoic Acid by Zero-Valent Iron Activated Persulfate System. J. Environ. Eng. 2018, 144. [Google Scholar] [CrossRef]
- Vicente, F.; Santos, A.; Romero, A.; Rodriguez, S. Kinetic study of diuron oxidation and mineralization by persulphate: Effects of temperature, oxidant concentration and iron dosage method. Chem. Eng. J. 2011, 170, 127–135. [Google Scholar] [CrossRef]
- Nie, M.H.; Yan, C.X.; Li, M.; Wang, X.N.; Bi, W.L.; Dong, W.B. Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem. Eng. J. 2015, 279, 507–515. [Google Scholar] [CrossRef]
- Lin, H.; Wu, J.; Zhang, H. Degradation of clofibric acid in aqueous solution by an EC/Fe3+/PMS process. Chem. Eng. J. 2014, 244, 514–521. [Google Scholar] [CrossRef]
- Stefánsson, A. Iron(III) Hydrolysis and Solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [CrossRef]
- Xu, X.-R.; Li, X.-Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep. Purif. Technol. 2010, 72, 105–111. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xu, J.; Zhang, J.; Yang, Y.; Zhou, J.; Xu, X.; Lowry, G.V. Removal of Antibiotic Florfenicol by Sulfide-Modified Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2017, 51, 11269–11277. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Lin, T.-C.; Seshadri, G.; Kelber, J.A. A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Sun, C.; Wan, J.; He, H.; Wang, F.; Dai, Y.; Yang, S.; Lin, Y.; Zhan, X. Insights into removal of tetracycline by persulfate activation with peanut shell biochar coupled with amorphous Cu-doped FeOOH composite in aqueous solution. Environ. Sci. Pollut. R 2019, 26, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.-X.; Zhou, L.-X. Synthesis of iron oxyhydroxides of different crystal forms and their roles in adsorption and removal of Cr(Ⅵ)from aqueous solutions. Acta Petrol. ET Mineral. 2008, 27, 559–566. [Google Scholar]
- Yang, L.; He, L.; Xue, J.; Wu, L.; Ma, Y.; Li, H.; Peng, P.; Li, M.; Zhang, Z. Highly efficient nickel (II) removal by sewage sludge biochar supported α-Fe2O3 and α-FeOOH: Sorption characteristics and mechanisms. PLoS ONE 2019, 14, e0218114. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lei, M.; Zhu, L.; Anjum, M.N.; Zou, J.; Tang, H. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate. J. Hazard. Mater. 2011, 186, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.; Mohammadi, F.; Kakavandi, B.; Esrafili, A.; Rostamifasih, Z. Simultaneous catalytic degradation of 2,4-D and MCPA herbicides using sulfate radical-based Heterog oxidation over persulfate activated by natural hematite (α-Fe2O3/PS). J. Phys. Chem. Solids 2018, 117, 49–59. [Google Scholar] [CrossRef]

| Parameter | Character |
|---|---|
| Formula | C11H12Cl2N2O5 |
| Molecular weight | 323.13 |
| Solubility (mg/L), 25 °C | 2500 |
| Log Kow | 1.14 |
| pKa | 9.5 |
| CAS number | 56-75-7 |
| Molecular structure | 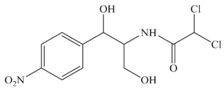 |
| Factors | Symbols | Level of Factors | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Nanoscale zero-valent iron (nZVI) (mg/L) | X1 | 10 | 15 | 20 | 25 | 30 |
| Peroxydisulfate (PS) concentration (mM) | X2 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 |
| Initial pH | X3 | 3 | 5 | 7 | 9 | 11 |
| Temperature (°C) | X4 | 40 | 50 | 60 | 70 | 80 |
| Run Number | Factors | Degradation Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Observed | Predicted | |
| 1 | 25 | 0.5 | 5 | 50 | 80.72 | 81.82 |
| 2 | 20 | 0.4 | 7 | 80 | 93.07 | 95.84 |
| 3 | 20 | 0.4 | 3 | 60 | 90.62 | 88.90 |
| 4 | 20 | 0.6 | 7 | 60 | 84.76 | 82.45 |
| 5 | 15 | 0.3 | 5 | 50 | 60.93 | 62.96 |
| 6 | 15 | 0.3 | 9 | 50 | 17.42 | 18.36 |
| 7 | 15 | 0.5 | 9 | 70 | 94.95 | 94.64 |
| 8 | 15 | 0.5 | 9 | 50 | 24.45 | 27.15 |
| 9 | 25 | 0.5 | 5 | 70 | 96.25 | 95.02 |
| 10 | 25 | 0.5 | 9 | 70 | 93.54 | 90.88 |
| 11 | 20 | 0.4 | 11 | 60 | 37.52 | 40.16 |
| 12 | 25 | 0.3 | 9 | 70 | 80.11 | 79.40 |
| 13 | 10 | 0.4 | 7 | 60 | 82.83 | 78.58 |
| 14 | 15 | 0.3 | 9 | 70 | 86.62 | 84.89 |
| 15 | 20 | 0.4 | 7 | 60 | 76.42 | 75.47 |
| 16 | 15 | 0.3 | 5 | 70 | 86.76 | 86.53 |
| 17 | 20 | 0.4 | 7 | 40 | 17.97 | 16.12 |
| 18 | 20 | 0.4 | 7 | 60 | 74.80 | 75.47 |
| 19 | 20 | 0.4 | 7 | 60 | 75.61 | 75.47 |
| 20 | 25 | 0.3 | 5 | 70 | 89.03 | 85.70 |
| 21 | 20 | 0.4 | 7 | 60 | 75.70 | 75.47 |
| 22 | 20 | 0.2 | 7 | 60 | 61.12 | 64.35 |
| 23 | 30 | 0.4 | 7 | 60 | 80.16 | 85.33 |
| 24 | 15 | 0.5 | 5 | 50 | 69.16 | 69.58 |
| 25 | 25 | 0.3 | 5 | 50 | 73.46 | 73.48 |
| 26 | 25 | 0.3 | 9 | 50 | 28.59 | 24.22 |
| 27 | 20 | 0.4 | 7 | 60 | 75.51 | 75.47 |
| 28 | 25 | 0.5 | 9 | 50 | 34.79 | 34.73 |
| 29 | 20 | 0.4 | 7 | 60 | 74.77 | 75.47 |
| 30 | 15 | 0.5 | 5 | 70 | 90.38 | 94.12 |
| Term | Coefficient Estimate | Standard Error | p-Value |
|---|---|---|---|
| Intercept | 75.47 | 1.31 | <0.0001 |
| X1 | 1.69 | 0.65 | 0.0208 |
| X2 | 4.53 | 0.65 | <0.0001 |
| X3 | −12.18 | 0.65 | <0.0001 |
| X4 | 19.93 | 0.65 | <0.0001 |
| X1X4 | −2.84 | 0.80 | 0.0029 |
| X3X4 | 10.74 | 0.80 | <0.0001 |
| X12 | 1.62 | 0.61 | 0.0181 |
| X32 | −2.73 | 0.61 | 0.0004 |
| X42 | −4.87 | 0.61 | <0.0001 |
| Term | Squares | df | Square | Value | Probability > F |
|---|---|---|---|---|---|
| Model | 16,615.92 | 14 | 1186.85 | 115.86 | <0.0001 |
| X1 | 68.28 | 1 | 68.28 | 6.66 | 0.0208 |
| X2 | 491.41 | 1 | 491.41 | 47.97 | <0.0001 |
| X3 | 3562.89 | 1 | 3562.89 | 347.80 | <0.0001 |
| X4 | 9532.92 | 1 | 9532.92 | 930.57 | <0.0001 |
| X1X2 | 2.98 | 1 | 2.98 | 0.29 | 0.5978 |
| X1X3 | 21.72 | 1 | 21.72 | 2.12 | 0.1660 |
| X1X4 | 128.71 | 1 | 128.71 | 12.56 | 0.0029 |
| X2X3 | 4.69 | 1 | 4.69 | 0.46 | 0.5091 |
| X2X4 | 0.94 | 1 | 0.94 | 0.092 | 0.7660 |
| X3X4 | 1845.13 | 1 | 1845.13 | 180.11 | <0.0001 |
| X12 | 72.15 | 1 | 72.15 | 7.04 | 0.0181 |
| X22 | 7.33 | 1 | 7.33 | 0.72 | 0.4110 |
| X32 | 205.08 | 1 | 205.08 | 20.02 | 0.0004 |
| X42 | 651.02 | 1 | 651.02 | 63.55 | <0.0001 |
| Residual | 153.66 | 15 | 10.24 | ||
| Lack of Fit | 151.75 | 10 | 15.17 | 39.61 | 0.0004 |
| Pure Error | 1.92 | 5 | 0.38 | ||
| Corrected Total | 16,769.58 | 29 | |||
| R2 | 0.9908 | ||||
| Adjusted R2 | 0.9823 | ||||
| Adequate Precision | 35.224 | ||||
| C.V.% | 4.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, H.; Xue, J.; He, L.; Ma, Y.; Wu, L.; Zhang, Z. Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization. Water 2020, 12, 131. https://doi.org/10.3390/w12010131
Yang L, Li H, Xue J, He L, Ma Y, Wu L, Zhang Z. Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization. Water. 2020; 12(1):131. https://doi.org/10.3390/w12010131
Chicago/Turabian StyleYang, Lie, Hong Li, Jianming Xue, Liuyang He, Yongfei Ma, Li Wu, and Zulin Zhang. 2020. "Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization" Water 12, no. 1: 131. https://doi.org/10.3390/w12010131
APA StyleYang, L., Li, H., Xue, J., He, L., Ma, Y., Wu, L., & Zhang, Z. (2020). Hydrothermal Enhanced Nanoscale Zero-Valent Iron Activated Peroxydisulfate Oxidation of Chloramphenicol in Aqueous Solutions: Fe-Speciation Analysis and Modeling Optimization. Water, 12(1), 131. https://doi.org/10.3390/w12010131








