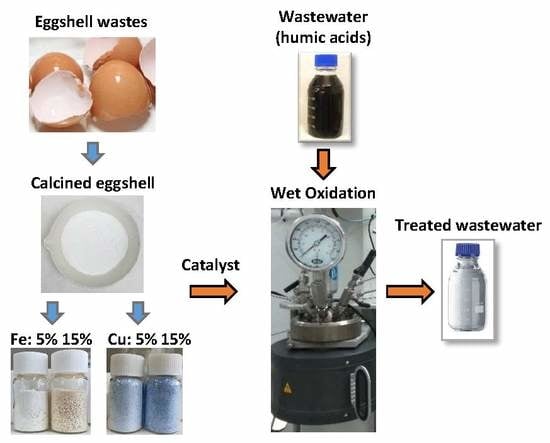
Eggshell-supported Catalysts for the Advanced Oxidation Treatment of Humic Acid Polluted Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the Catalysts
2.3. Techniques of Characterization
2.4. Catalytic Experiments (WO): Apparatus and Procedure
2.5. Catalytic Experiments: Sample Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Structural Characterization: XRD and FTIR Analyses
3.3. Textural Characterization
3.4. Microstructure: SEM and TEM microscopy
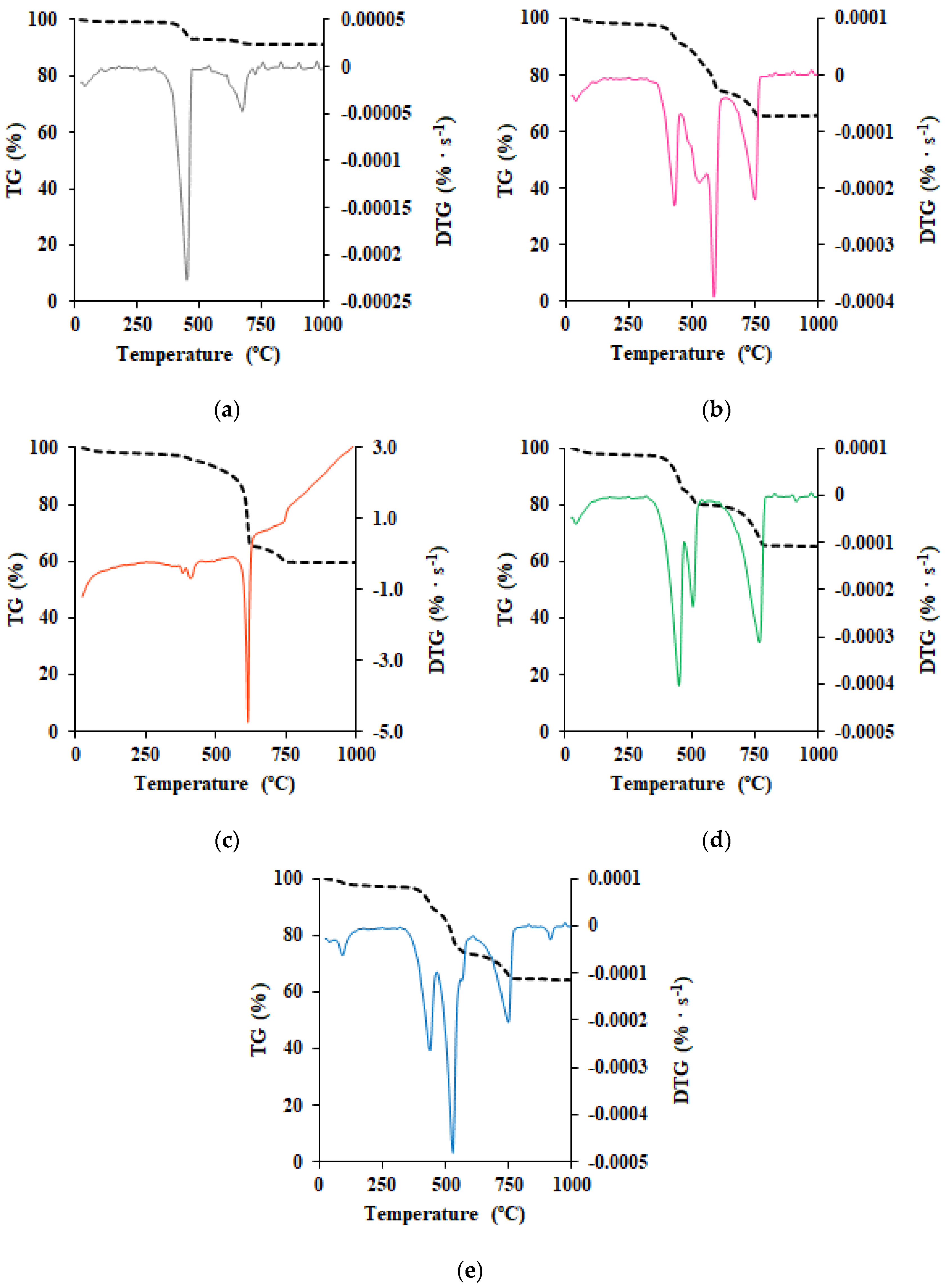
3.5. Thermal Stability
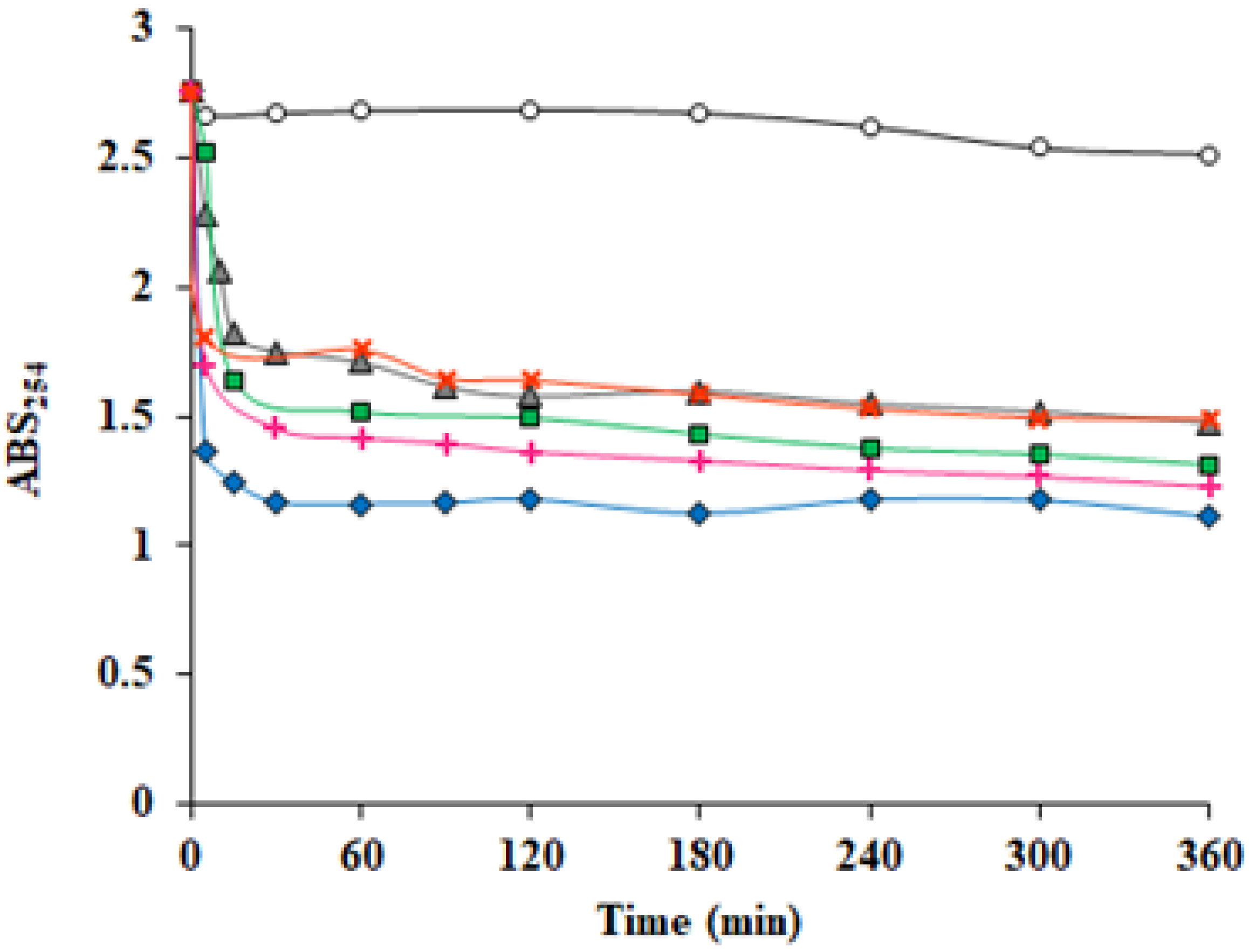
3.6. Catalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, J.-L.; Xia, X.; Haider, M.R.; Jiang, W.-L.; Tao, Y.; Liu, M.-J.; Wang, H.-c.; Ding, Y.-C.; Hou, Y.-N.; Cheng, H.-Y.; et al. Functional graphene oxide membrane preparation for organics/inorganic salts mixture separation aiming at advanced treatment of refractory wastewater. Sci. Total Environ. 2018, 628, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Mylapilli, S.V.P.; Reddy, S.N. Sub and supercritical water oxidation of pharmaceutical wastewater. J. Environ. Chem. Eng. 2019, 7, 103165. [Google Scholar] [CrossRef]
- Luan, M.; Jing, G.; Piao, Y.; Liu, D.; Jin, L. Treatment of refractory organic pollutants in industrial wastewater by wet air oxidation. Arab. J. Chem. 2017, 10, S769–S776. [Google Scholar] [CrossRef]
- Manjari, S.; Meenal, G.; Sushil, K.A.; Pawan, D. Removal of Refractory Organic Compounds from Wastewater by Various Advanced Oxidation Process—A Review. Curr. Environ. Eng. 2019, 6, 8–16. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; van Loosdrecht, M.C.M.; Yu, J.; Liu, R. Adaptation of semi-continuous anaerobic sludge digestion to humic acids. Water Res. 2019, 161, 329–334. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Li, Z.; Zhang, C.; Wan, C.; Zhang, Y.; Lee, D.-J. Inhibition of urease activity by humic acid extracted from sludge fermentation liquid. Bioresour. Technol. 2019, 290, 121767. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B.; Fan, C.M.; Sun, Y.P. Photoelectrocatalytic degradation of humic acid in aqueous solution using a Ti/TiO2 mesh photoelectrode. Water Res. 2002, 36, 2215–2224. [Google Scholar] [CrossRef]
- Oulego, P.; Collado, S.; Laca, A.; Díaz, M. Impact of leachate composition on the advanced oxidation treatment. Water Res. 2016, 88, 389–402. [Google Scholar] [CrossRef]
- Jiang, F.; Qiu, B.; Sun, D. Degradation of refractory organics from biologically treated incineration leachate by VUV/O3. Chem. Eng. J. 2019, 370, 346–353. [Google Scholar] [CrossRef]
- Delgado, J.J.; Pérez-Omil, J.A.; Rodríguez-Izquierdo, J.M.; Cauqui, M.A. The role of the carbonaceous deposits in the Catalytic Wet Oxidation (CWO) of phenol. Catal. Commun. 2006, 7, 639–643. [Google Scholar] [CrossRef]
- Mohite, R.G.; Garg, A. Performance of heterogeneous catalytic wet oxidation for the removal of phenolic compounds: Catalyst characterization and effect of pH, temperature, metal leaching and non-oxidative hydrothermal reaction. J. Environ. Chem. Eng. 2017, 5, 468–478. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Tardio, J.; Prasad, J.; Föger, K.; Akolekar, D.B.; Grocott, S.C. Wet Oxidation and Catalytic Wet Oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar] [CrossRef]
- Heponiemi, A.; Azalim, S.; Hu, T.; Lassi, U. Cerium Oxide Based Catalysts for Wet Air Oxidation of Bisphenol A. Top. Catal. 2015, 58, 1043–1052. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, I.W.; Huang, F. Toward large-scale water treatment using nanomaterials. Nano Today 2019, 27, 11–27. [Google Scholar] [CrossRef]
- Martins, R.; Domingues, E.; Bosio, M.; Quina, M.; Gmurek, M.; Quinta-Ferreira, R.; Gomes, J. Effect of Different Radiation Sources and Noble Metal Doped onto TiO2 for Contaminants of Emerging Concern Removal. Water 2019, 11, 894. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Hatamifard, A. Waste chicken eggshell as a natural valuable resource and environmentally benign support for biosynthesis of catalytically active Cu/eggshell, Fe3O4/eggshell and Cu/Fe3O4/eggshell nanocomposites. Appl. Catal. B Environ. 2016, 191, 209–227. [Google Scholar] [CrossRef]
- Chua, S.Y.; Periasamy, L.A.P.; Goh, C.M.H.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Walvekar, R.; Abdullah, E.C. Biodiesel synthesis using natural solid catalyst derived from biomass waste—A review. J. Ind. Eng. Chem. 2020, 81, 41–60. [Google Scholar] [CrossRef]
- Nath, B.; Kalita, P.; Das, B.; Basumatary, S. Highly efficient renewable heterogeneous base catalyst derived from waste Sesamum indicum plant for synthesis of biodiesel. Renew. Energy 2019. [Google Scholar] [CrossRef]
- Chenrayan, S.; Vediappan, K.; Murugan, N.; Kang, H.; Santhoshkumar, P.; Gnanamuthu, R.M.; Lee, C. Thermochemical Conversion of Eggshell as Biological Waste and its Application as a Functional Material for Lithium-ion Batteries. Chem. Eng. J. 2019, 372. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Eggshell waste as catalyst: A review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.S.; Roy, P.K. Development of waste derived nano-lakargiite bonded high alumina refractory castable for high temperature applications. Ceram. Int. 2019, 45, 16202–16213. [Google Scholar] [CrossRef]
- Karoshi, G.; Kolar, P.; Shah, S.B.; Gilleskie, G. Valorization of Eggshell Waste into Supported Copper Catalysts for Partial Oxidation of Methane. Int. J. Environ. Res. 2019. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Kolo, K.; Abdullah, S.M.; Hamad, S.M.; Khalid, H.S.; Yassein, A.T. Green synthesis of highly recyclable CuO/eggshell nanocomposite to efficient removal of aromatic containing compounds and reduction of 4-nitrophenol at room temperature. Surf. Interfaces 2018, 13, 205–215. [Google Scholar] [CrossRef]
- Sietsma, J.R.A.; Jos van Dillen, A.; de Jongh, P.E.; de Jong, K.P. Application of ordered mesoporous materials as model supports to study catalyst preparation by impregnation and drying. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., De Vos, D.E., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 162, pp. 95–102. [Google Scholar]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Oulego, P.; Collado, S.; Laca, A.; Díaz, M. Tertiary treatment of biologically pre-treated landfill leachates by non-catalytic wet oxidation. Chem. Eng. J. 2015, 273, 647–655. [Google Scholar] [CrossRef]
- Mahvi, A.; Maleki, A.; Rezaee, R.; Safari, M. Reduction of humic substances in water by application of ultrasound waves and ultraviolet irradiation. Iran. J. Environ. Health Sci. Eng. 2009, 6, 233–240. [Google Scholar]
- Fernandes, A.; Santos, D.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Electrochemical oxidation of humic acid and sanitary landfill leachate: Influence of anode material, chloride concentration and current density. Sci. Total Environ. 2016, 541, 282–291. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater, 20th ed.; APHA, AWWA, WEF, Eds.; APHA: Washington, DC, USA, 1999. [Google Scholar]
- Yaşar, F. Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: Its effects on some critical fuel properties and comparison with CaO. Fuel 2019, 255, 115828. [Google Scholar] [CrossRef]
- Goli, J.; Sahu, O. Development of heterogeneous alkali catalyst from waste chicken eggshell for biodiesel production. Renew. Energy 2018, 128, 142–154. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef]
- Alvarez-Torrellas, S.; Boutahala, M.; Boukhalfa, N.; Munoz, M. Effective Adsorption of Methylene Blue dye onto Magnetic Nanocomposites. Modeling and Reuse Studies. Appl. Sci. 2019, 9, 4563. [Google Scholar] [CrossRef]
- El-Trass, A.; ElShamy, H.; El-Mehasseb, I.; El-Kemary, M. CuO nanoparticles: Synthesis, characterization, optical properties and interaction with amino acids. Appl. Surf. Sci. 2012, 258, 2997–3001. [Google Scholar] [CrossRef]
- Ren, F.; Ding, Y.; Leng, Y. Infrared spectroscopic characterization of carbonated apatite: A combined experimental and computational study. J. Biomed. Mater. Res. Part A 2014, 102, 496–505. [Google Scholar] [CrossRef]
- Mosaddegh, E. Ultrasonic-assisted preparation of nano eggshell powder: A novel catalyst in green and high efficient synthesis of 2-aminochromenes. Ultrason. Sonochem. 2013, 20, 1436–1441. [Google Scholar] [CrossRef]
- Golmohammad, M.; Golestanifard, F.; Mirhabibi, A. Synthesis and Characterization of Maghemite as an Anode for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2016, 11, 6432–6442. [Google Scholar] [CrossRef]
- Witoon, T. Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. Ceram. Int. 2011, 37, 3291–3298. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Salehi, R.; Fathi, M.H.; Golniya, Z.; Daniels, A.U. Bioactive glass nanoparticles with negative zeta potential. Ceram. Int. 2011, 37, 2311–2316. [Google Scholar] [CrossRef]
- Nazari, M.; Ghasemi, N.; Maddah, H.; Motlagh, M.M. Synthesis and characterization of maghemite nanopowders by chemical precipitation method. J. Nanostruct. Chem. 2014, 4, 99. [Google Scholar] [CrossRef]
- Galván-Ruiz, M.; Hernández, J.; Baños, L.; Noriega-Montes, J.; Rodríguez-García, M.E. Characterization of Calcium Carbonate, Calcium Oxide, and Calcium Hydroxide as Starting Point to the Improvement of Lime for Their Use in Construction. J. Mater. Civ. Eng. 2009, 21, 694–698. [Google Scholar] [CrossRef]
- Ethiraj, A.S.; Kang, D.J. Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett. 2012, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Maurin, G.; Llewellyn, P. 1—Introduction. In Adsorption by Powders and Porous Solids, 2nd ed.; Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G., Eds.; Academic Press: Oxford, UK, 2014; pp. 1–24. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, C. Synthesis of dimethyl carbonate over waste eggshell catalyst. Catal. Today 2012, 190, 107–111. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Effects of Sample Preparation and Interpretation of Thermogravimetric Curves on Calcium Hydroxide in Hydrated Pastes and Mortars. Transp. Res. Rec. J. Transp. Res. Board 2012, 2290, 10–18. [Google Scholar] [CrossRef]
- Keely, W.M.; Maynor, H.W. Thermal Studies of Nickel, Cobalt, Iron and Copper Oxides and Nitrates. J. Chem. Eng. Data 1963, 8, 297–300. [Google Scholar] [CrossRef]
- Li, X.-G.; Lv, Y.; Ma, B.-G.; Wang, W.-Q.; Jian, S.-W. Decomposition kinetic characteristics of calcium carbonate containing organic acids by TGA. Arab. J. Chem. 2017, 10, S2534–S2538. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, G.; Peng, Y.; Liu, F.; Zhang, C.; Huang, B.; Zhong, Y.; He, Y.; Lai, M. 2,4,6-Trichlorophenol-promoted catalytic wet oxidation of humic substances and stabilized landfill leachate. Chem. Eng. J. 2014, 247, 216–222. [Google Scholar] [CrossRef]
- Hajimirzaee, S.; Soleimani Mehr, A.; Ghavipour, M.; Vatankhah, M.; Behbahani, R.M. Effect of metal loading on catalytic activity and selectivity of ZSM-5 zeolite catalyst in conversion of methanol to olefins and aromatics. Pet. Sci. Technol. 2017, 35, 279–286. [Google Scholar] [CrossRef]
- Urrea, J.L.; García, M.; Collado, S.; Oulego, P.; Díaz, M. Sludge hydrothermal treatments. Oxidising atmosphere effects on biopolymers and physical properties. J. Environ. Manag. 2018, 206, 284–290. [Google Scholar] [CrossRef]
- Fukushima, M.; Tatsumi, K.; Nagao, S. Degradation Characteristics of Humic Acid during Photo-Fenton Processes. Environ. Sci. Technol. 2001, 35, 3683–3690. [Google Scholar] [CrossRef]
- Da, X.; Ji, H.; Zhao, Z.; Lan, R.; Li, T.; Ma, J. Strongly prolonged hydroxyl radical production for Fenton-like reactions: The golden touch of Cu. Sep. Purif. Technol. 2019, 213, 500–506. [Google Scholar] [CrossRef]
- Miao, X.; Dai, H.; Chen, J.; Zhu, J. The enhanced method of hydroxyl radical generation in the heterogeneous UV-Fenton system with α-FeOOH as catalyst. Sep. Purif. Technol. 2018, 200, 36–43. [Google Scholar] [CrossRef]
- Cavalcante, A.d.M.; Lima, J.C.d.S.; Santos, L.d.M.; Oliveira, P.C.C.d.; Ribeiro Júnior, K.A.L.; Sant’ana, A.E.G. Comparative evaluation of the pH of calcium hydroxide powder in contact with carbon dioxide (CO2). Mater. Res. 2010, 13, 1–4. [Google Scholar] [CrossRef]
- Kolokassidou, C.; Pashalidis, I.; Costa, C.N.; Efstathiou, A.M.; Buckau, G. Thermal stability of solid and aqueous solutions of humic acid. Thermochim. Acta 2007, 454, 78–83. [Google Scholar] [CrossRef]







| Sample | % N | % C | % S | % H |
|---|---|---|---|---|
| Eggshell | 0.42 ± 0.05 | 1.12 ± 0.03 | 0.25 ± 0.06 | 1.63 ± 0.04 |
| Fe 5% | 2.90 ± 0.06 | 2.79 ± 0.04 | 0.27 ± 0.04 | 1.65 ± 0.07 |
| Fe 15% | 6.99 ± 0.07 | 2.23 ± 0.05 | 0.25 ± 0.05 | 1.21 ± 0.08 |
| Cu 5% | 1.66 ± 0.04 | 2.28 ± 0.03 | 0.25 ± 0.02 | 1.46 ± 0.08 |
| Cu 15% | 4.61 ± 0.07 | 2.48 ± 0.06 | 0.27 ± 0.03 | 1.65 ± 0.06 |
| Element | Eggshell | Fe 5% | Fe 15% | Cu 5% | Cu 15% |
|---|---|---|---|---|---|
| Ca | 98.48 ± 0.04 | 92.93 ± 0.04 | 83.81 ± 0.05 | 93.37 ± 0.05 | 83.80 ± 0.02 |
| Mg | 0.92 ± 0.07 | 0.85 ± 0.03 | 0.68 ± 0.03 | 0.88 ± 0.03 | 0.70 ± 0.04 |
| Na | 0.25 ± 0.03 | 0.33 ± 0.04 | 0.25 ± 0.02 | 0.29 ± 0.04 | 0.28 ± 0.03 |
| P | 0.29 ± 0.03 | 0.27 ± 0.02 | 0.23 ± 0.02 | 0.26 ± 0.03 | 0.27 ± 0.04 |
| K | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.02 |
| Fe | n.d. * | 5.42 ± 0.01 | 14.96 ± 0.06 | n.d. * | n.d. * |
| Cu | n.d. * | n.d. * | n.d. * | 5.14 ± 0.05 | 14.89 ± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oulego, P.; Laca, A.; Calvo, S.; Díaz, M. Eggshell-supported Catalysts for the Advanced Oxidation Treatment of Humic Acid Polluted Wastewaters. Water 2020, 12, 100. https://doi.org/10.3390/w12010100
Oulego P, Laca A, Calvo S, Díaz M. Eggshell-supported Catalysts for the Advanced Oxidation Treatment of Humic Acid Polluted Wastewaters. Water. 2020; 12(1):100. https://doi.org/10.3390/w12010100
Chicago/Turabian StyleOulego, Paula, Amanda Laca, Sonia Calvo, and Mario Díaz. 2020. "Eggshell-supported Catalysts for the Advanced Oxidation Treatment of Humic Acid Polluted Wastewaters" Water 12, no. 1: 100. https://doi.org/10.3390/w12010100
APA StyleOulego, P., Laca, A., Calvo, S., & Díaz, M. (2020). Eggshell-supported Catalysts for the Advanced Oxidation Treatment of Humic Acid Polluted Wastewaters. Water, 12(1), 100. https://doi.org/10.3390/w12010100