Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Method
2.2.1. Synthesis of Zeolite Y
2.2.2. Characterization of Zeolite Y
2.2.3. Groundwater Sampling
2.2.4. Continuous Retrieval and Re-Use of Zeolite Y
2.2.5. Chemical Analysis and Batch Adsorption Experiment
2.2.6. Kinetic Studies
2.3. Theoretical Model Equations
3. Results
3.1. Characterization of Zeolite Y
3.2. Characterization of Water Samples
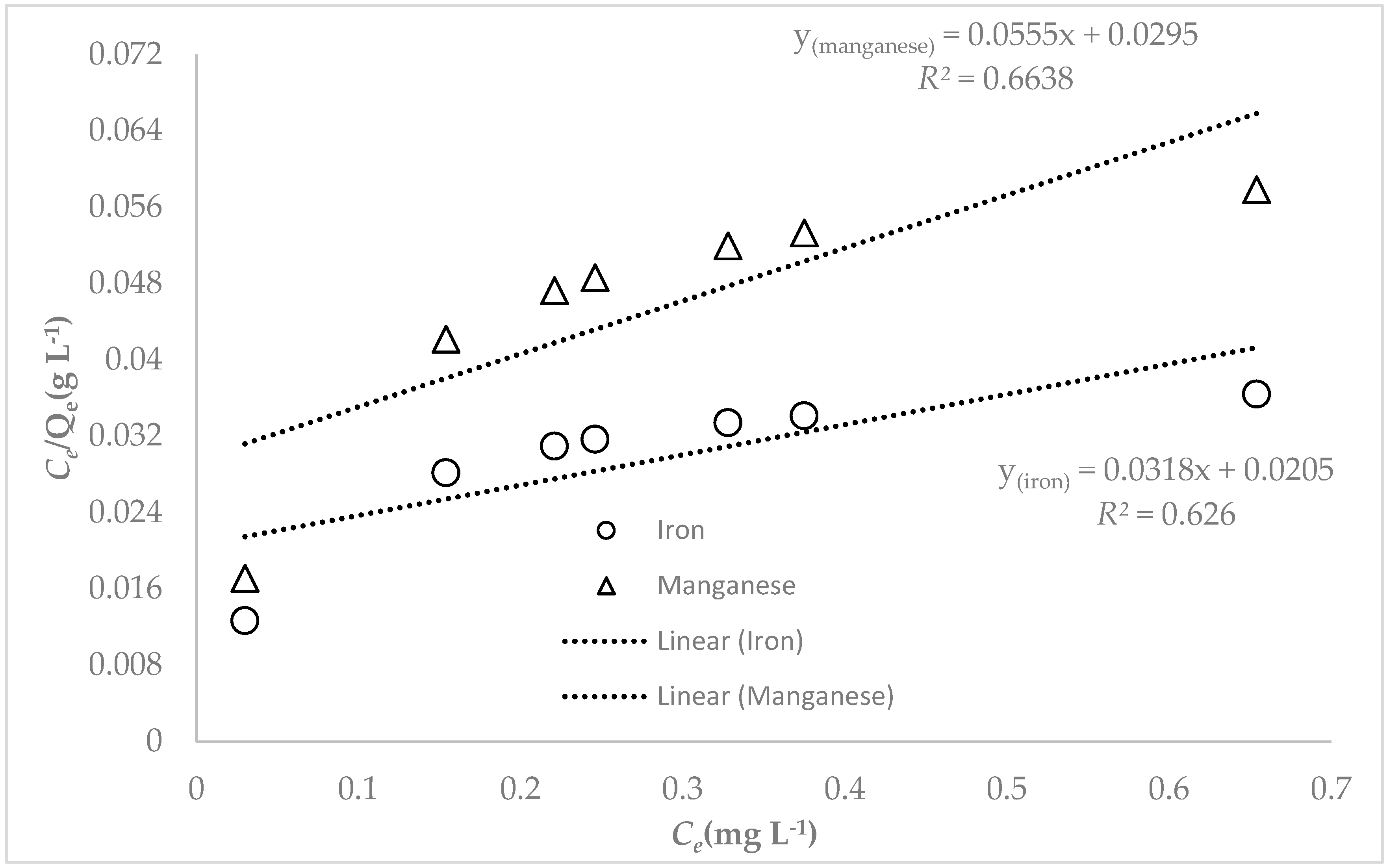
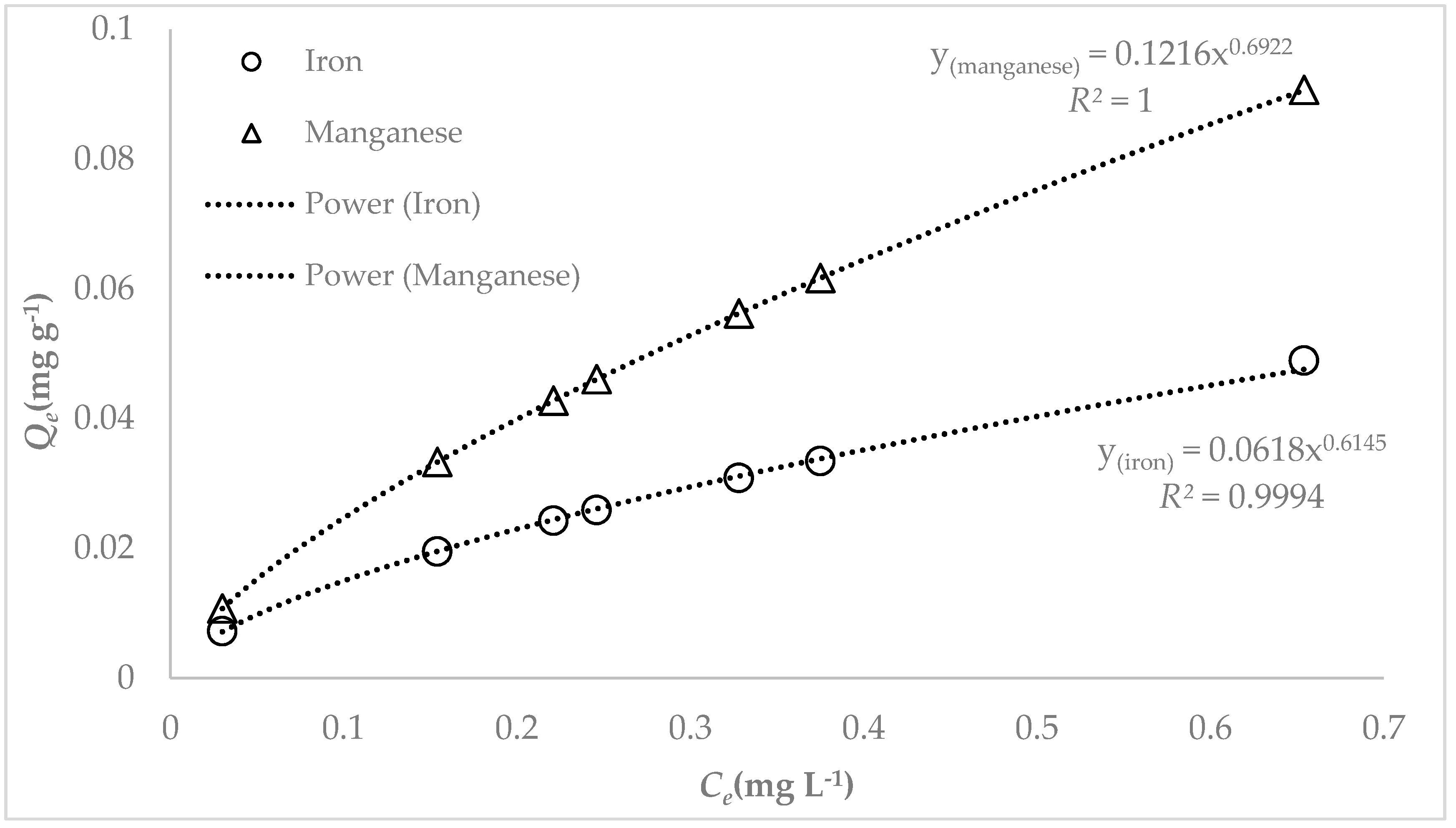
3.3. Adsorption Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| m = | mass of zeolite Y |
| Ce = | Equilibrium concentration |
| Qe = | Adsorption at equilibrium |
| Qt = | Adsorption at time t |
| Q0.5 = | adsorption of iron at concentration of 0.5 mg L−1 |
| Q0.02 = | adsorption of manganese at concentration of 0.02 mg L−1 |
| KL = | Langmuir constant parameter |
| KF = | Freundlich constant parameter |
| K1 = | Pseudo-first order rate constant |
| K2 = | Pseudo-second order rate constant |
| Ki = | Intra-particle diffusion constant |
| Kd = | diffusion constant |
References
- Barloková, D.; Ilavský, J. Removal of Iron and Manganese from Water Using Filtration by Natural Materials. Pol. J. Environ. Stud. 2010, 19, 1117–1122. [Google Scholar]
- bin Jusoh, A.; Cheng, W.H.; Low, W.M.; Nora’aini, A.; Megat Mohd Noor, M.J. Study on the removal of iron and manganese in groundwater by granular activated carbon. Desalination 2005, 182, 347–353. [Google Scholar] [CrossRef]
- Aziz, H.A.; Smith, P.G. Removal of manganese from water using crushed dolomite filtration technique. Water Res. 1996, 30, 489–492. [Google Scholar] [CrossRef]
- Dorthel, J.; Jensk, B.S.; Thomash, C. Speciation of Dissolved Iron(II) and Manganese(II) in a Groundwater Pollution Plume. Environ. Sci. Technol. 1998, 32, 2657–2664. [Google Scholar]
- AWWA; ASCE. Water Treatment Plant Design, 2nd ed.; McGraw-Hill Inc.: New York, NY, USA, 1990. [Google Scholar]
- García-Mendieta, A.; Solache-Ríos, M.; Olguín, M.T. Evaluation of the sorption properties of a Mexican clinoptilolite-rich tuff for iron, manganese and iron–manganese systems. Microporous Mesoporous Mater. 2009, 118, 489–495. [Google Scholar] [CrossRef]
- Inglezakisa, V.J.; Doulab, M.K.; Aggelatou, V.; Zorpas, A.A. Removal of iron and manganese from underground water by use of natural minerals in batch mode treatment. Desalin. Water Treat. 2010, 18, 341–346. [Google Scholar] [CrossRef]
- WHO. World Health Organization Guidelines, 3rd ed.; WHO Press: Geneva, Switzerland, 2004; pp. 166–196. [Google Scholar]
- Sarin, P.; Snoeyink, V.L.; Bebee, J.; Jim, K.K.; Beckett, M.A.; Clement, J.A. Iron release from corroded iron pipes in drinking water distribution systems: Effect of dissolved oxygen. Water Res. 2004, 38, 1259–1269. [Google Scholar] [CrossRef]
- Aschner, A.; Erikson, K.M.; Hernández, E.H.; Tjalkens, R. Manganese and its Role in Parkinson’s Disease: From Transport to Neuropathology. Neuromol. Med. 2009, 11, 252–266. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 2nd ed.; WHO Press: Geneva, Switzerland, 2011; pp. 1–21. [Google Scholar]
- Ahmad, M. Iron and Manganese Removal from Groundwater: Geochemical Modeling of the Vyredox Method. Master’s Thesis, University of Oslo, Oslo, Norway, 2012. [Google Scholar]
- Awuah, J.B.; Dzade, N.Y.; Tia, R.; Adei, E.; Kwakye-Awuah, B.; Richard, C.; de Leeuw, N.H. A density functional theory study of arsenic immobilization by the Al(III)-modified zeolite clinoptilolite. Phys. Chem. Chem. Phys. 2016, 18, 11297–11305. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Coll. Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Singer, P.A.; Salamanca-Buentell, F.; Daar, A. Harnessing Nanotechnology to Improve Global Equity. Issues Sci. Technol. 2005, 21, 57–64. [Google Scholar]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef]
- Tsitsishvili, G.V.; Andronikashvili, T.G.; Kirov, G.N.; Filizova, L.D. Natural Zeolite; Ellis Horwood: New York, NY, USA, 1992. [Google Scholar]
- Yusof, A.M.; Malek, N.A.N.N. Removal of Cr (VI) and as (V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Beachner, R.; McManama, Z.; Hanlie, H. Sorption of arsenic by surfactant-modified zeolite and kaolinite. Micropor Mesopor Mater. 2007, 105, 291–297. [Google Scholar] [CrossRef]
- Payne, K.; Abdel-Fattah, T. Adsorption of Arsenate and Arsenite by Iron-Treated Activated Carbon and Zeolites: Effects of pH, Temperature, and Ionic Strength. J. Envrion. Sci. Health Part A 2005, 40, 723–749. [Google Scholar] [CrossRef]
- Ahmed, S.; Chughtai, S.; Keane, M.A. The removal of cadmium and lead from aqueous solution by ion exchange with Na–Y zeolite. Sep. Purif. Technol. 1998, 13, 57–64. [Google Scholar] [CrossRef]
- Keane, M.A. Microporous Materials. Role of the alkali metal co-cation in the ion exchange of Y zeolites III. Equilibrium properties of the Ni/Cu/Na-Y and Ni/Cu/K-Y zeolite systems. Microporous Mater. 1995, 4, 359–368. [Google Scholar] [CrossRef]
- Shevade, S.; Ford, R.G. Use of synthetic zeolites for arsenate removal from pollutant water. Water Res. 2004, 38, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Kwakye-Awuah, B.; Williams, C.; Kenward, M.A.; Radecka, I. Antimicrobial action and efficiency of silver-loaded zeolite X. J. Appl. Microbiol. 2008, 104, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Kwakye-Awuah, B.; Labik, L.K.; Nkrumah, I.; Williams, C. Removal of ammonium ion by laboratory-synthesized zeolite LTA adsorption from waters samples affected by mining activities in Ghana. J. Water Health 2014, 12, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Zhang, L.; Keane, M.A. Removal of iron from aqueous solutions by ion exchange with NA-Y zeolite. Sep. Sci. Technol. 2013, 36, 1509–1525. [Google Scholar] [CrossRef]
- Mozgawa, W. The influence of some heavy metals cations on the FTIR spectra of zeolites. J. Mol. Struct. 2000, 555, 299–304. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Von-Kiti, E.; Nkrumah, I.; Ikyereve, R.E.; Williams, C. Parametric, Equilibrium, and Kinetic Study of the Removal of Salt Ions from Ghanaian Seawater by Adsorption onto Zeolite X. Desalin. Water Treat. 2016, 57, 21654–21663. [Google Scholar] [CrossRef]
- Coutinho, D.; Balkus, J.K., Jr. Preparation and characterization of zeolite X membranes via pulsed-laser deposition. Microporous Mesoporous Mater. 2002, 52, 79–91. [Google Scholar] [CrossRef]
- Saha, U.K.; Taniguuchi, S.; Sakurai, K. Simultaneous sorption of cadmium, zinc, and lead on hydroxyaluminum- and hydroxyaluminosilicate-montmorillonite complexes. Soil Sci. Soc. Am. J. 2002, 66, 117–128. [Google Scholar] [CrossRef]
- Fu, G.; Allen, H.E.; Cowan, C.E. Adsorption of cadmium and copper by manganese oxide. Soil Sci. 1991, 152, 72–81. [Google Scholar] [CrossRef]
- Gouzinis, A.; Kosmidis, N.; Vayenas, D.V.; Lyberatos, G. Removal of Mn and Simultaneous Removal of NH3, Fe, and Mn from Potable Water Using a Trickling Filter. Water Res. 1998, 32, 2442–2450. [Google Scholar] [CrossRef]
- Farrag, A.H.A.; Moghny, T.A.; Gad Mohamed, A.M.G.; Saleem, S.S.; Fathy, M. Abu Zenima synthetic zeolite for removing iron and manganese from Assiut governorate groundwater, Egypt. Appl. Water Sci. 2017, 7, 3087–3094. [Google Scholar] [CrossRef]
- Yavuz, O.; Altunkaynak, Y.; Guzel, F. Equilibrium and Kinetics Study for Adsorption of 2,4-Dinitrophenol from Aqueous Solutions by Using Cucumis Sativus Peels and Kidney Bean Shells as New Low-cost Adsorbents. Water Res. 2003, 37, 948. [Google Scholar] [CrossRef]
- Wei-Wei, B.; Hai-Feng, Z.; Shu-Cai, G.; Xue-Chun, X.; Gui-Juan, J.; Ke-Yan, Z. Adsorption of Heavy Metal Ions from Aqueous Solutions by Zeolite Based on Oil Shale Ash: Kinetic and Equilibrium Studies. Chem. Res. Chin. Univ. 2013, 29, 126–131. [Google Scholar]
- Araby, R.E.; Hawash, S.; Diwani, G.E. Treatment of iron and manganese in simulated groundwater via ozone technology. Desalination 2009, 249, 1345–1349. [Google Scholar] [CrossRef]
- Novembre, D.; Sabatino, B.D.; Gimeno, D.; Garcia-Valles, M.; Martınez-Manent, S. Synthesis of Na–X zeolites from tripolaceous deposits (Crotone, Italy) and volcanic zeolitised rocks (Vico volcano, Italy). Microporous Mesoporous Mater. 2004, 75, 1–11. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Zhang, Z.; Shi, J.; Yang, J. Removal of copper(II) and lead(II) from aqueous solution by manganese oxide coated sand I. Characterization and kinetic study. J. Hazard. Mater. B 2006, 137, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Wark, M.; Lutz, W.; Schulz-Ekloff, G.; Dyer, A. Quantitative monitoring of side products during high loading of zeolites by heavy metals via pH measurements. Zeolites 1993, 13, 658. [Google Scholar] [CrossRef]
- Silvio, R.T.; Rubio, T.J. Removal of Mn2+ from aqueous solution by manganese oxide coated zeolite. Miner. Eng. 2010, 23, 1131–1138. [Google Scholar]
- Sefa-Ntiri, B.; Kwakye-Awuah, B.; Mensah-Amoah, P.; Williams, C. Removal of Pb2+ and Fe2+ from Water Samples in Ghana by Synthetic Zeolites as Measured by Atomic Absorption Spectroscopy and Light Transmission Experiments. Int. J. Sci. Res. 2015, 4, 220–227. [Google Scholar]
- Qin, S.; Ma, F.; Huang, P. Membrane Processes: Development, Monitoring and Modelling from the Nano to the Macro Scale. J. Desalin. 2009, 245, 183–193. [Google Scholar] [CrossRef]
- Vasanth, K.K.; Ramamurthi, V.S.S. Modeling the mechanism involved during the sorption of methylene blue onto fly ash. J. Colloid Interface Sci. 2005, 284, 14–21. [Google Scholar]
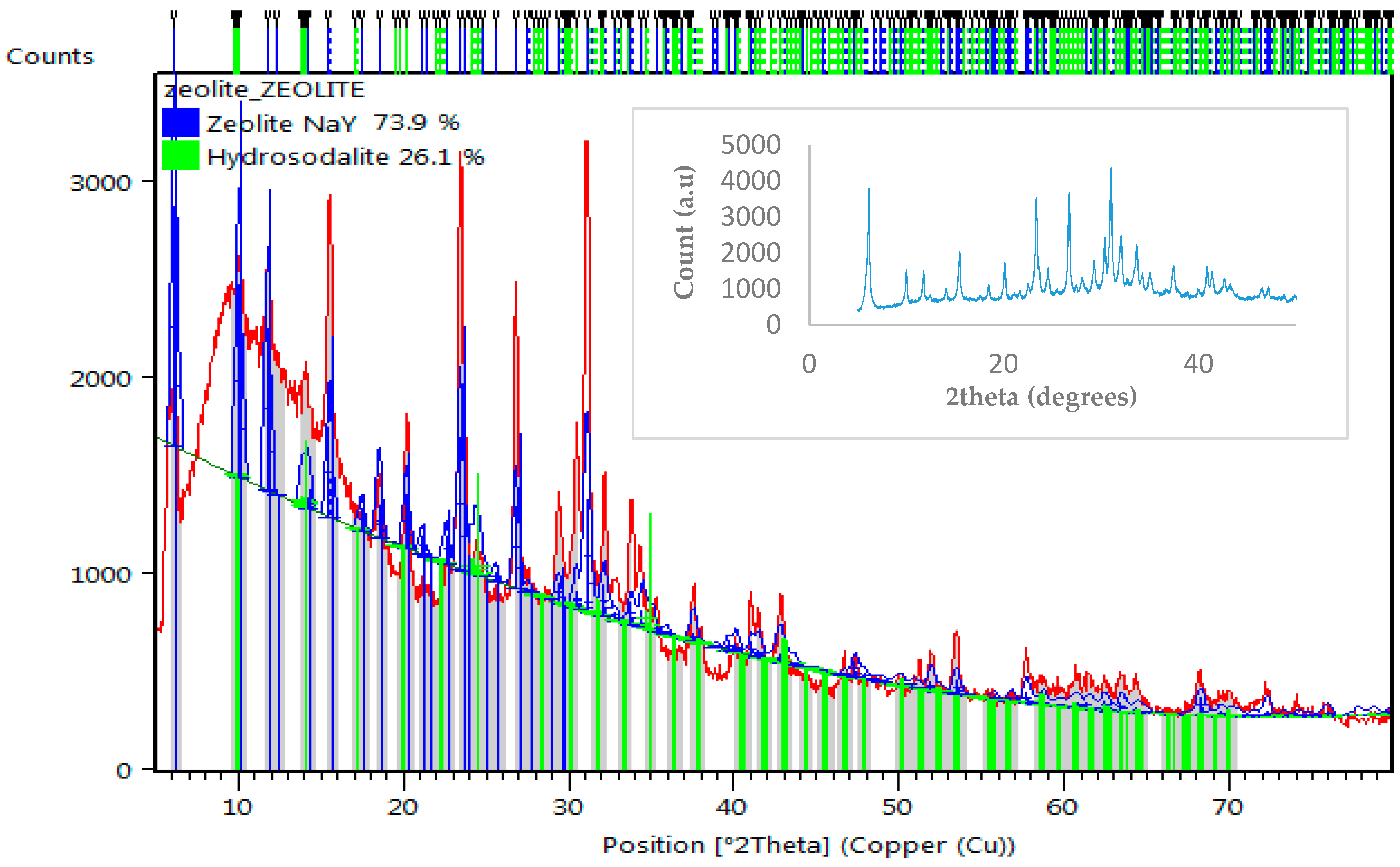
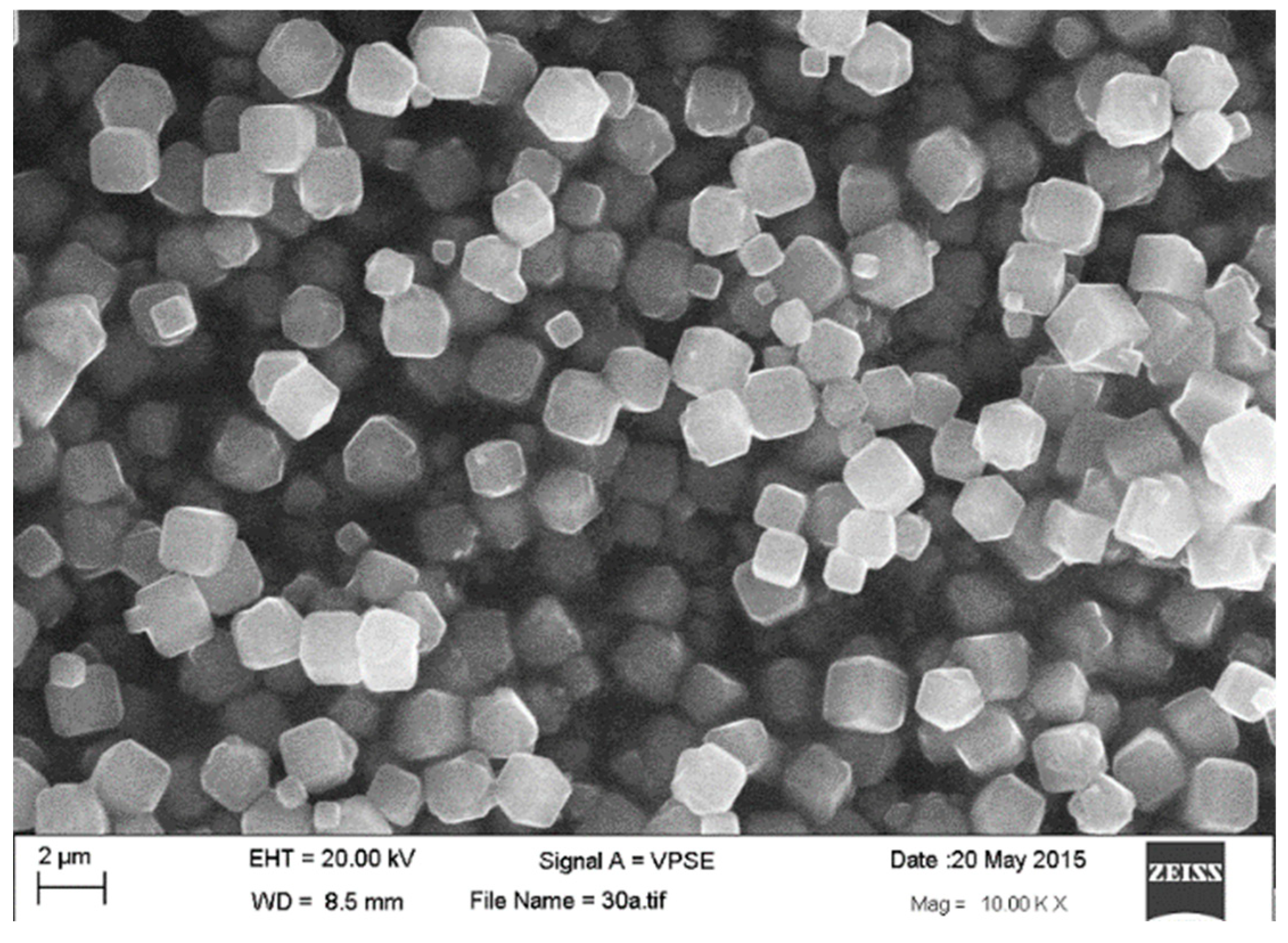
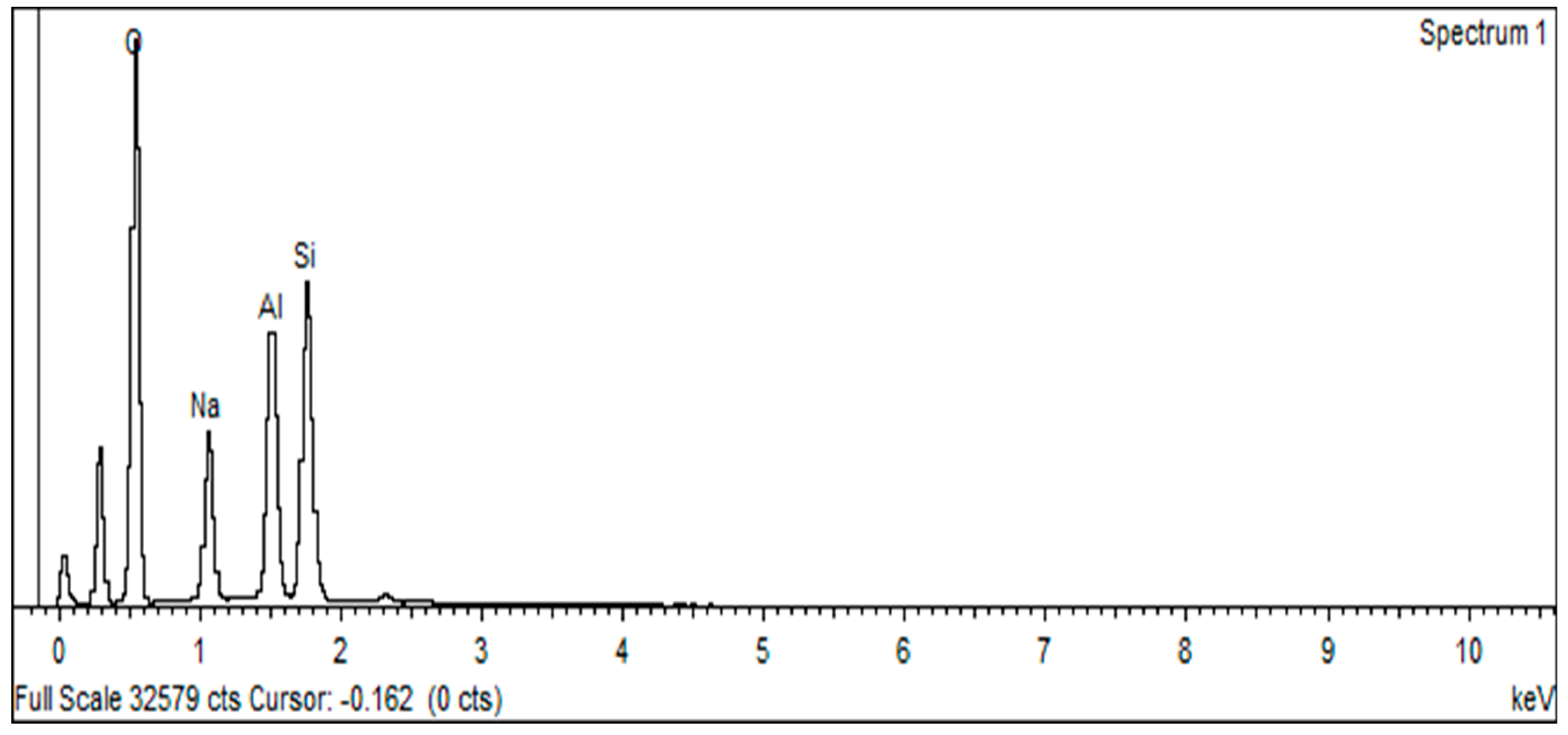
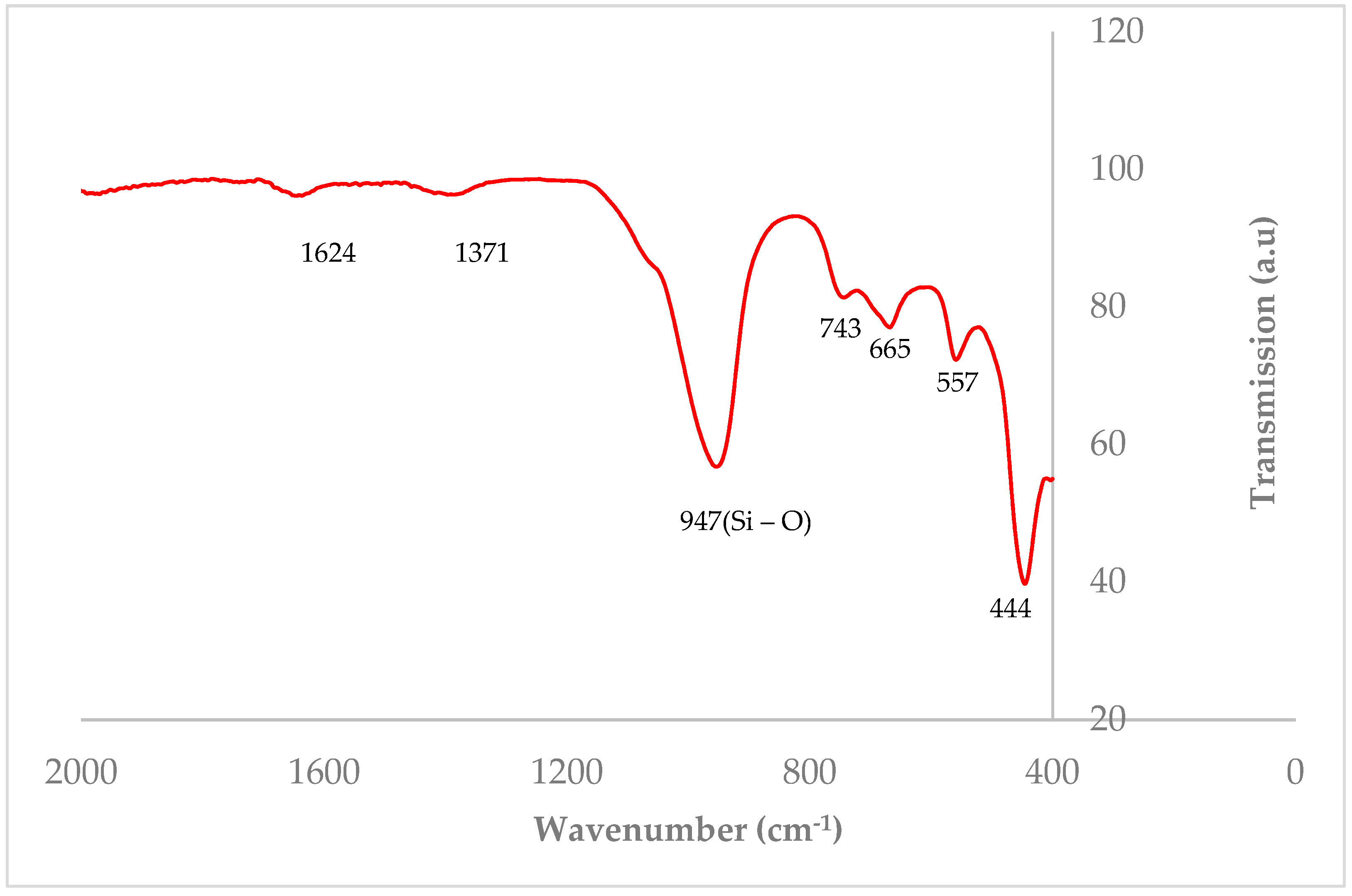
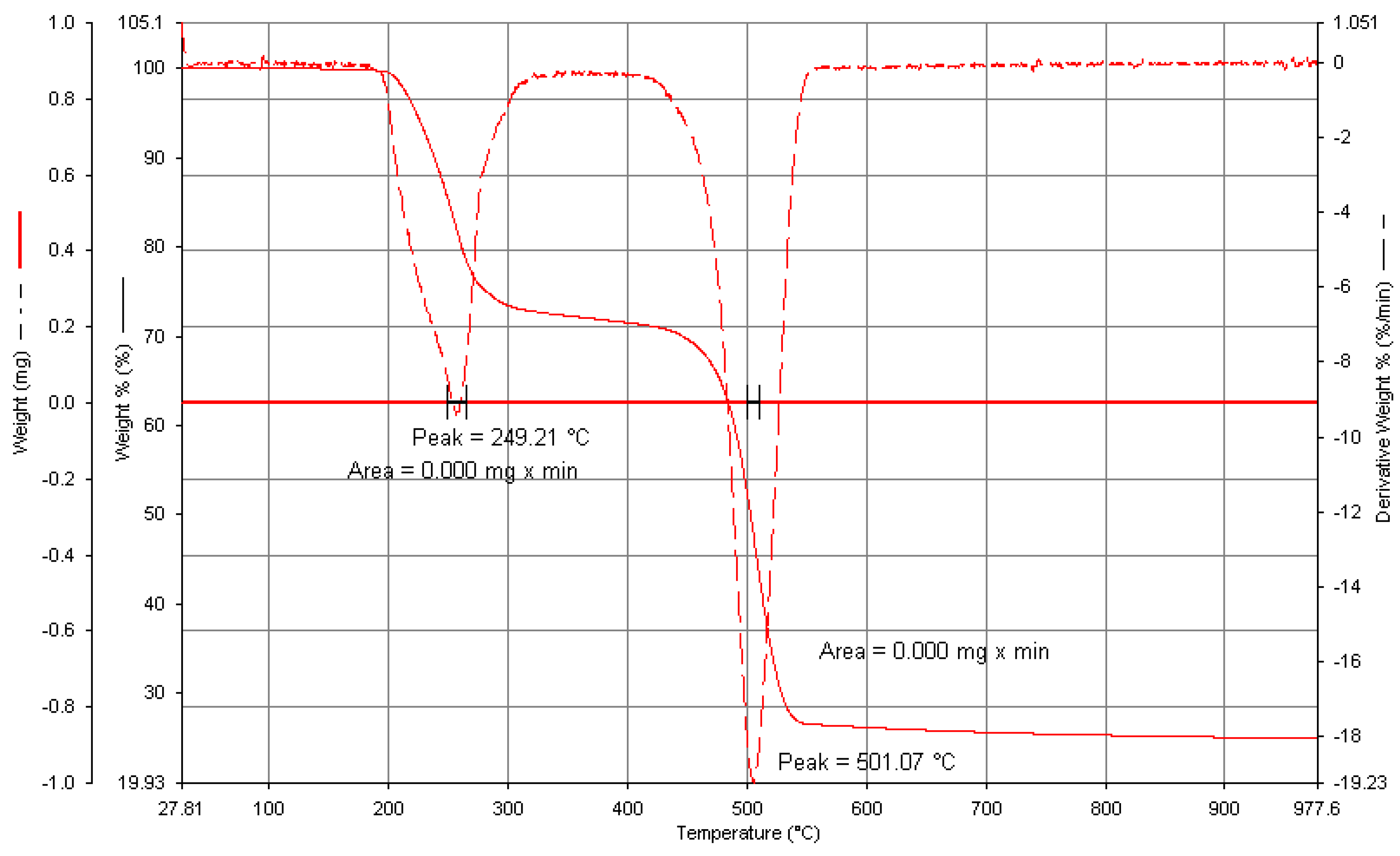
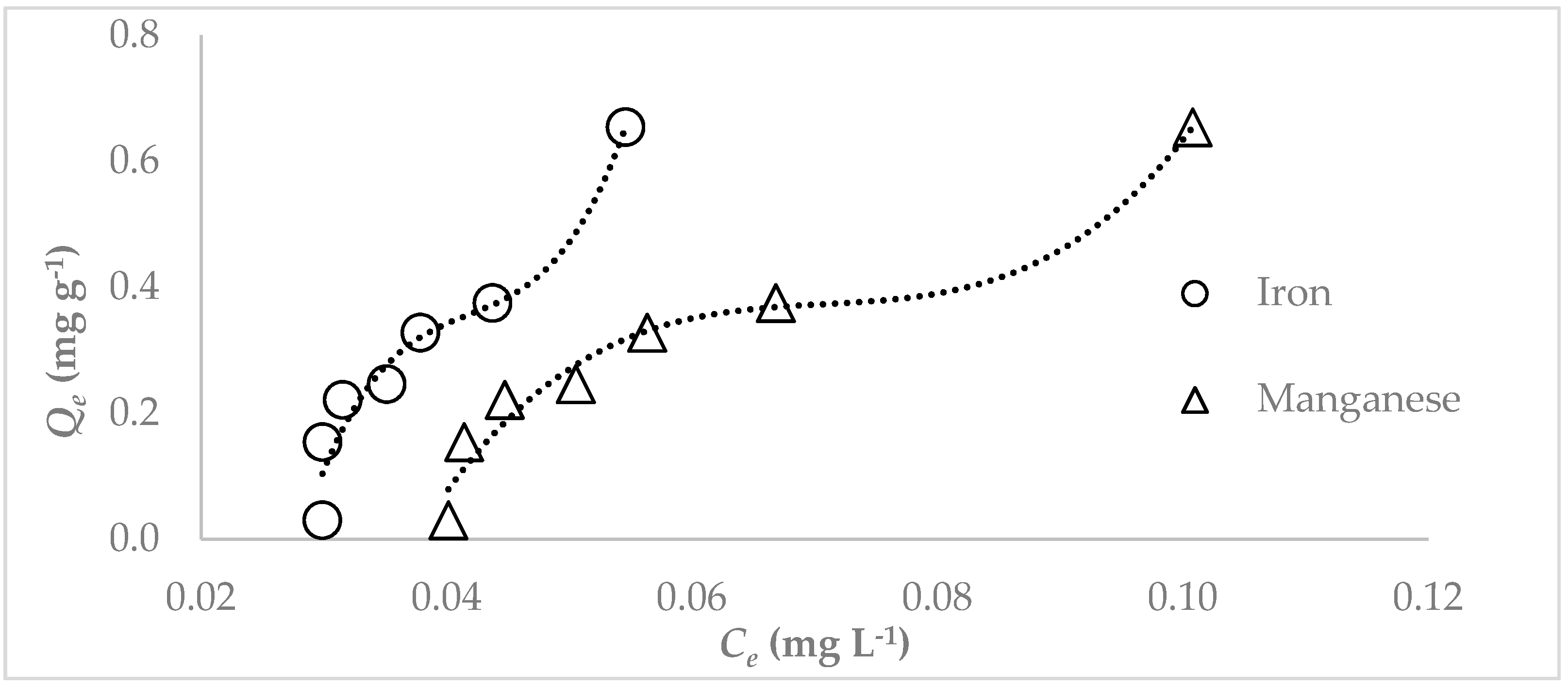

| Element | % Atomic |
|---|---|
| Na | 5.13 |
| Al | 35.04 |
| Si | 58.09 |
| K | 1.03 |
| Ca | 0.67 |
| LoI | 0.04 |
| Total | 100 |
| Parameter | Units | Cape Coast | Ashesi | Kumasi | GSA * Standard | WHO Standard | |||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||||
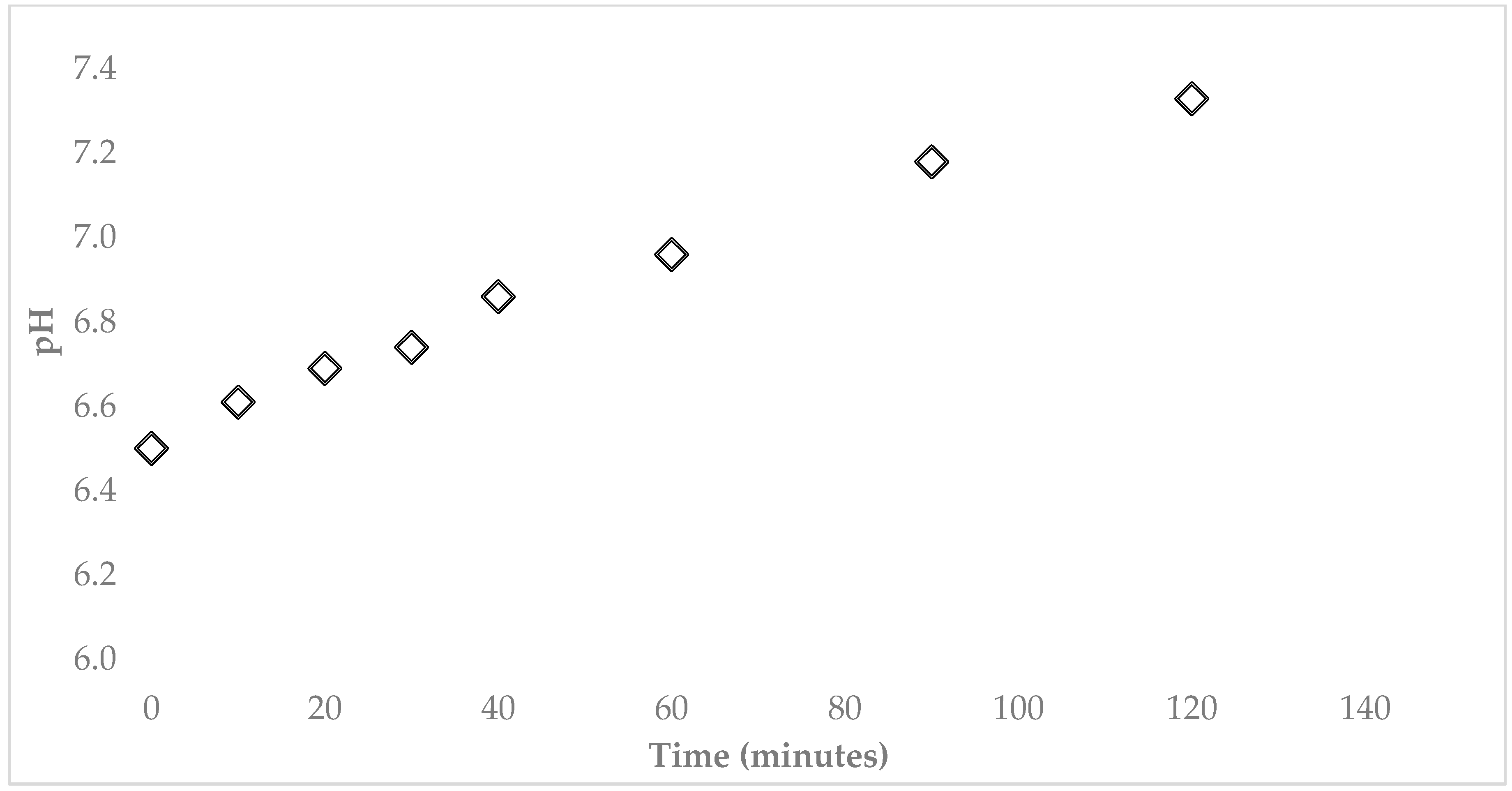
| pH @ 25 °C | 6.60 | 7.17 | 6.50 | 7.33 | 6.88 | 7.35 | 6.5–8.5 | 6.5–8.5 | |
| Turbidity | NTU | 2.6 | 1.2 | 2.2 | 1.3 | 2.4 | 1.3 | 5 or less | 50 or less |
| Total dissolved solids | NTU | 288 | 280 | 286 | 278 | 290 | 282 | 1000 | 300 or less |
| Apparent Color | - | 19 | 15 | 20 | 16 | 19 | 15 | - | 1000 |
| Conductivity | µS cm−1 | 500 | 370 | 570 | 375 | 590 | 375 | 2500 | 2500 |
| Total hardness | mg L−1 | 118 | 40 | 50 | 39 | 50 | 39 | 500 | Not included |
| Ca hardness | mg L−1 | 6.49 | 6 | 6.5 | 6 | 6.8 | 6 | 50 | 500 |
| Mg hardness | mg L−1 | 25 | b.d | 26 | b.d | 25 | b.d | 50 | 50 |
| mg L−1 | 23 | 21 | 20 | 21 | 22 | 20 | 400 | 200 | |
| Chlorides as Cl- | mg L−1 | 87 | 89 | 86 | 250 | 250 | |||
| Sulphate as | mg L−1 | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 200 |
| Nitrate | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | |
| Nitrite | mg L−1 | b.d | b.d | b.d | b.d | b.d | b.d | 0.3 | 0.5 |
| Cyanide | mg L−1 | b.d | b.d | b.d | b.d | b.d | b.d | 0.07 | 0.05 |
| Mercury | mg L−1 | b.d | b.d | b.d | b.d | b.d | b.d | 0.001 | 0.001 |
| Lead as Pb2+ | mg L−1 | b.d | b.d | b.d | b.d | b.d | b.d | 0.01 | 0.01 |
| Arsenic as As5+ | mg L−1 | b.d | b.d | b.d | b.d | b.d | b.d | 0.01 | 0.01 |
| Cadmium as Cd2+ | mg L−1 | b.d | b.d | b.d | b.d | b.d | b.d | 0.03 | 0.01 |
| Manganese | mg L−1 | 1.61 | 0.08 | 1.54 | 0.05 | 1.35 | 0.04 | 0.05 | 0.2 |
| Iron | mg L−1 | 1.15 | 0.06 | 1.12 | 0.05 | 1.01 | 0.04 | 0.3 | 0.3 |
| Activity | Sample Source | Initial Concentration (mg L−1) | Final Concentration (mg L−1) | Removal Efficiency (%) | |||
|---|---|---|---|---|---|---|---|
| Iron | Manganese | Iron | Manganese | Iron | Manganese | ||
| First exposure | Cape Coast | 1.15 | 1.61 | 0.02 | 0.04 | 98.26 | 97.52 |
| Ashesi | 1.12 | 1.54 | 0.02 | 0.04 | 98.21 | 97.37 | |
| Kumasi | 1.01 | 1.35 | 0.03 | 0.03 | 97.03 | 97.78 | |
| First Retrieval | Cape Coast | 1.16 | 1.6 | 0.02 | 0.04 | 98.28 | 97.50 |
| Ashesi | 1.14 | 1.52 | 0.03 | 0.04 | 97.37 | 98.03 | |
| Kumasi | 1.10 | 1.37 | 0.02 | 0.03 | 98.18 | 97.81 | |
| Second retrieval | Cape coast | 1.2 | 1.61 | 0.02 | 0.04 | 98.33 | 97.52 |
| Ashesi | 1.14 | 1.54 | 0.03 | 0.03 | 97.37 | 98.05 | |
| Kumasi | 1.01 | 1.35 | 0.02 | 0.03 | 98.02 | 97.78 | |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Fe | 31.45 | 1.551 | 0.626 | 0.0618 | 0.6145 | 0.979 |
| Mn | 18.02 | 1.881 | 0.664 | 0.1216 | 0.6922 | 0.981 |
| Pseudo First Order | Pseudo Second Order | Intra Particle Diffusion | Liquid Film | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ) | ) | |||||||||
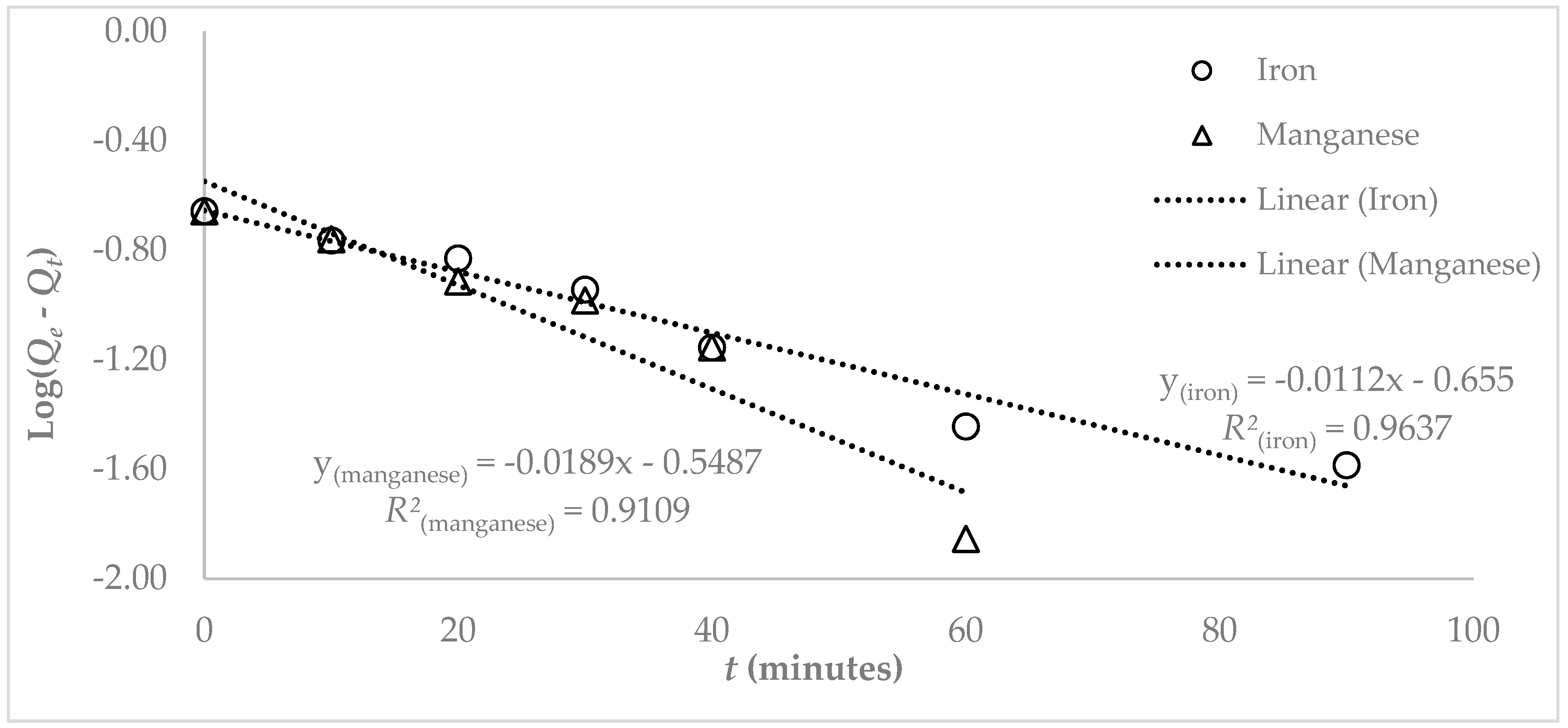
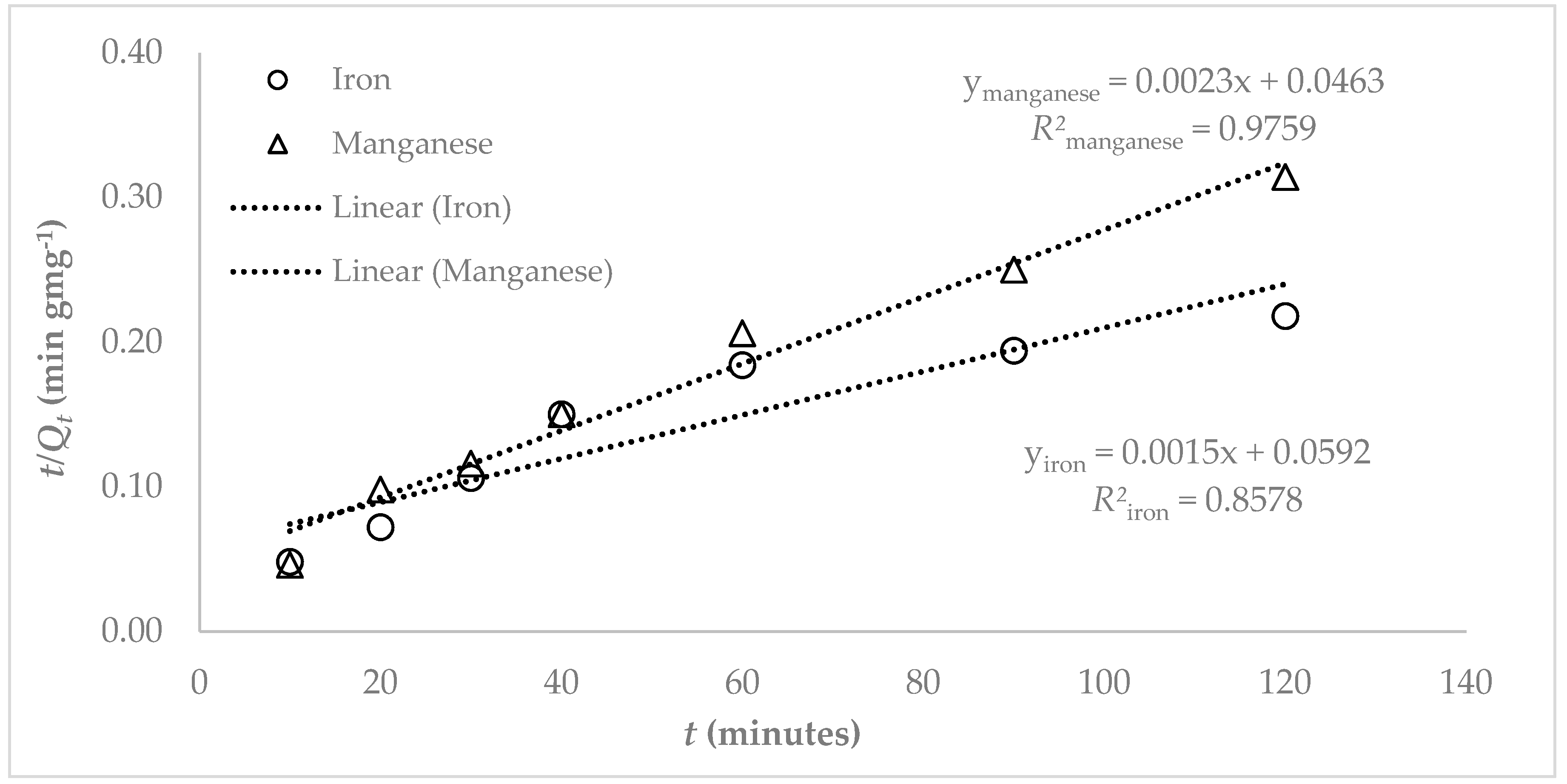
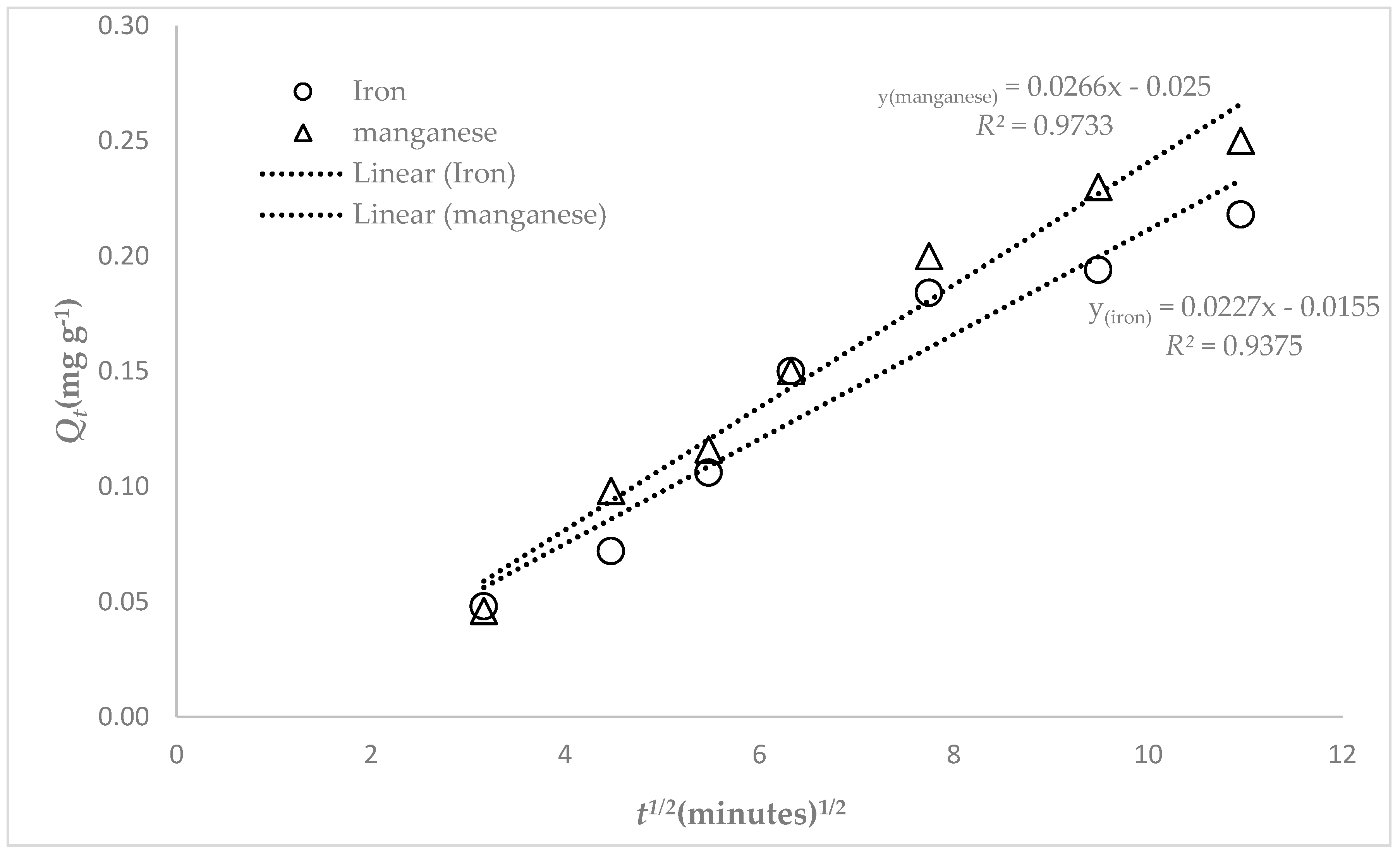
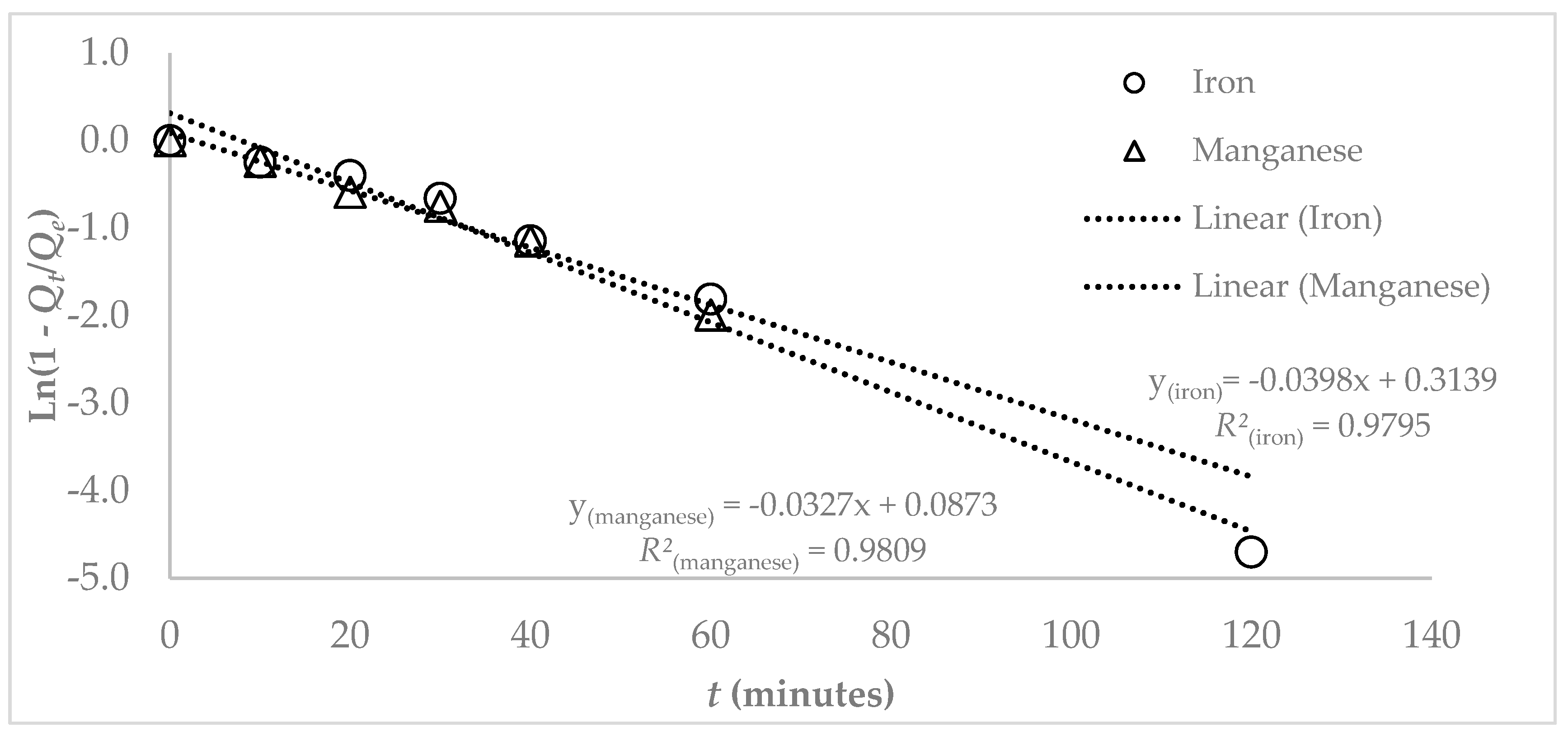
| Fe | 0.221 | 0.026 | 0.963 | 3.8 × 10−5 | 0.235 | 0.858 | 0.009 | 0.967 | 5 × 10−5 | 0.602 |
| Mn | 0.282 | 0.043 | 0.911 | 1.1 × 10−4 | 0.267 | 0.976 | 0.012 | 0.949 | 7 × 10−5 | 0.756 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwakye-Awuah, B.; Sefa-Ntiri, B.; Von-Kiti, E.; Nkrumah, I.; Williams, C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water 2019, 11, 1912. https://doi.org/10.3390/w11091912
Kwakye-Awuah B, Sefa-Ntiri B, Von-Kiti E, Nkrumah I, Williams C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water. 2019; 11(9):1912. https://doi.org/10.3390/w11091912
Chicago/Turabian StyleKwakye-Awuah, Bright, Baah Sefa-Ntiri, Elizabeth Von-Kiti, Isaac Nkrumah, and Craig Williams. 2019. "Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin" Water 11, no. 9: 1912. https://doi.org/10.3390/w11091912
APA StyleKwakye-Awuah, B., Sefa-Ntiri, B., Von-Kiti, E., Nkrumah, I., & Williams, C. (2019). Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water, 11(9), 1912. https://doi.org/10.3390/w11091912






