Organic Contaminants in Zooplankton of Italian Subalpine Lakes: Patterns of Distribution and Seasonal Variations
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sampling and Determination of Biomass
2.3. Chemical Analysis
2.3.1. Perfluoroalkyl Substances
2.3.2. Organochlorine Compounds
2.4. Data Analysis
2.4.1. Statistical Analysis
2.4.2. Spatial Analysis
3. Results and Discussion
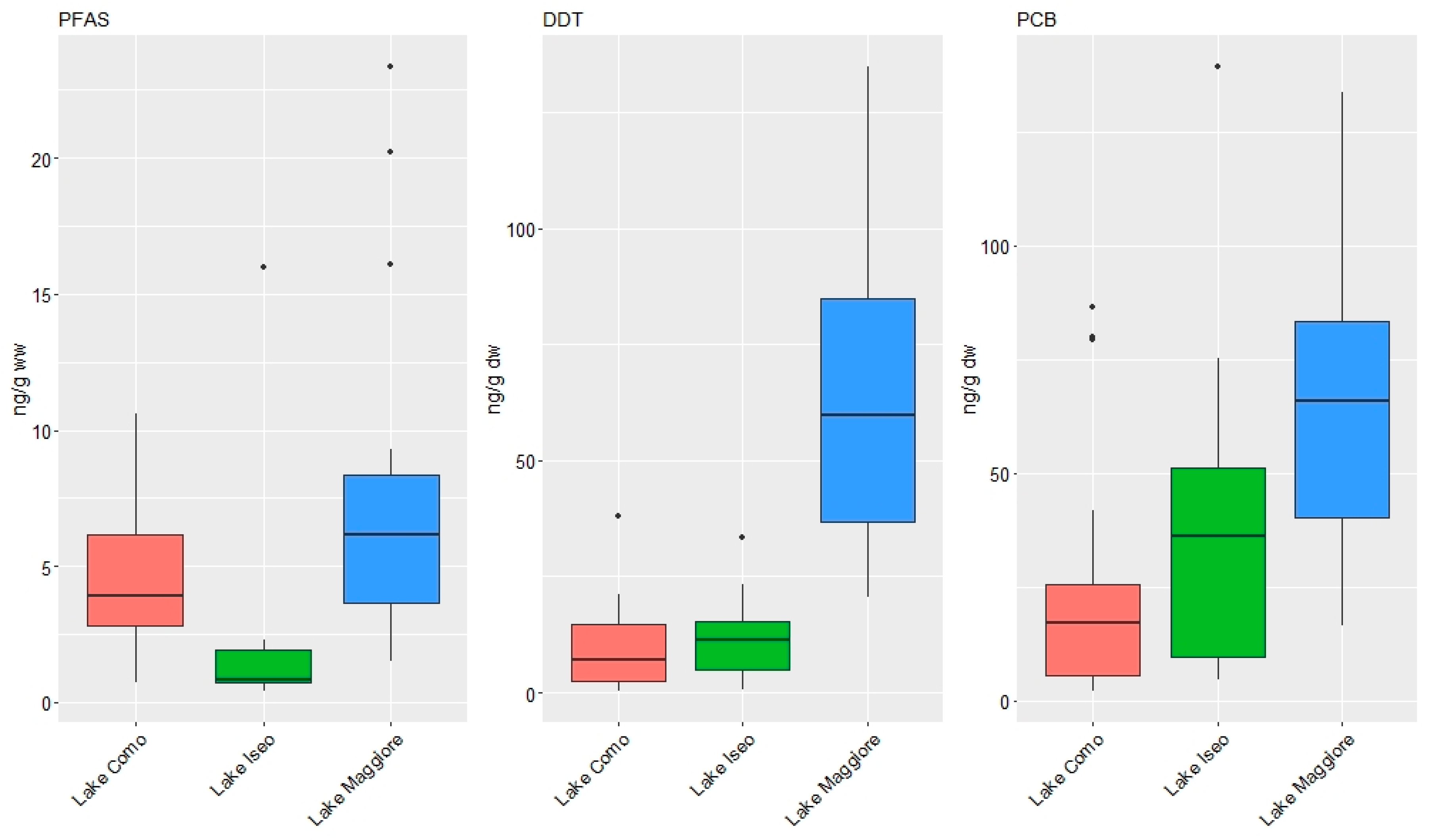
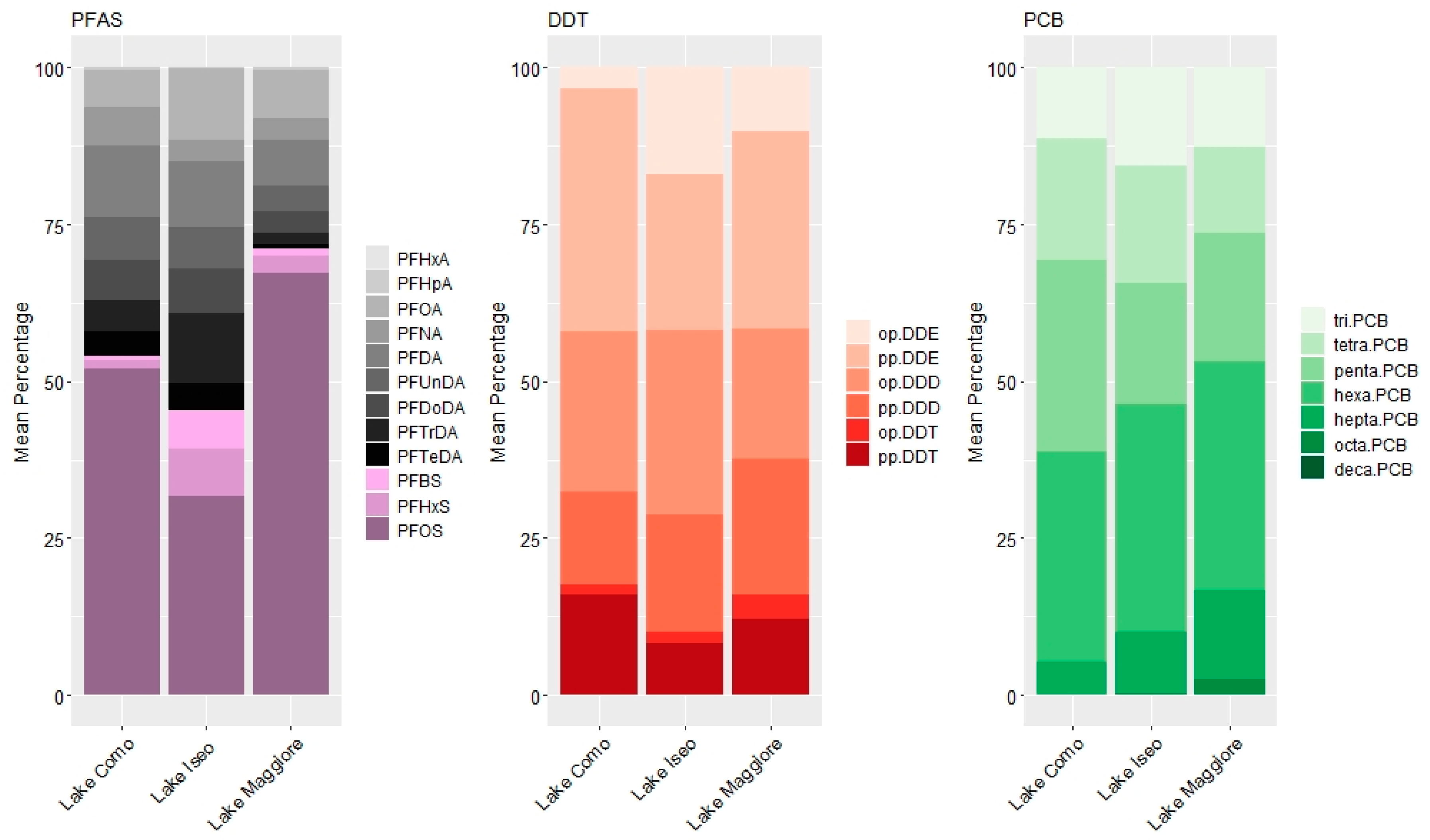
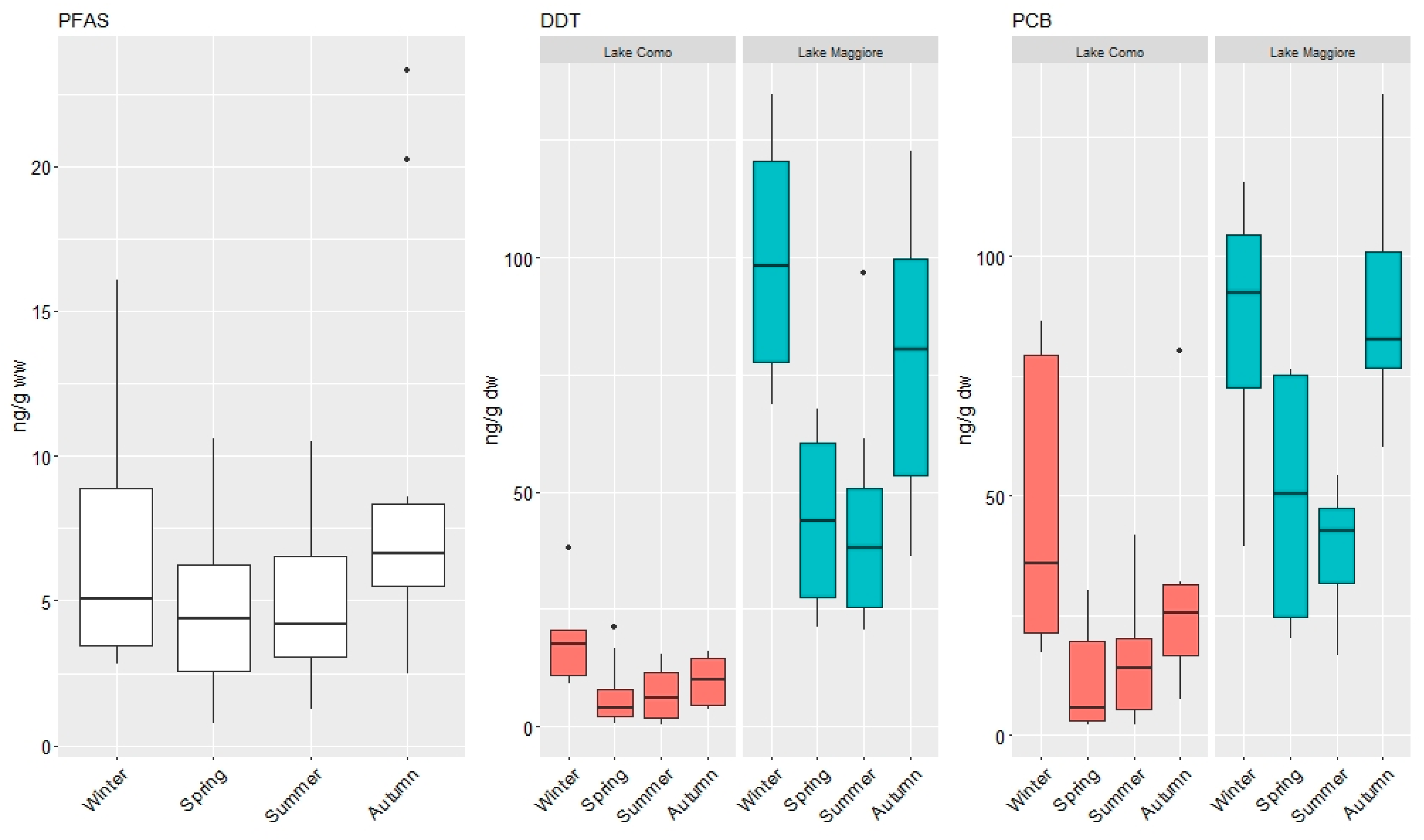
3.1. Concentrations of Organic Contaminants
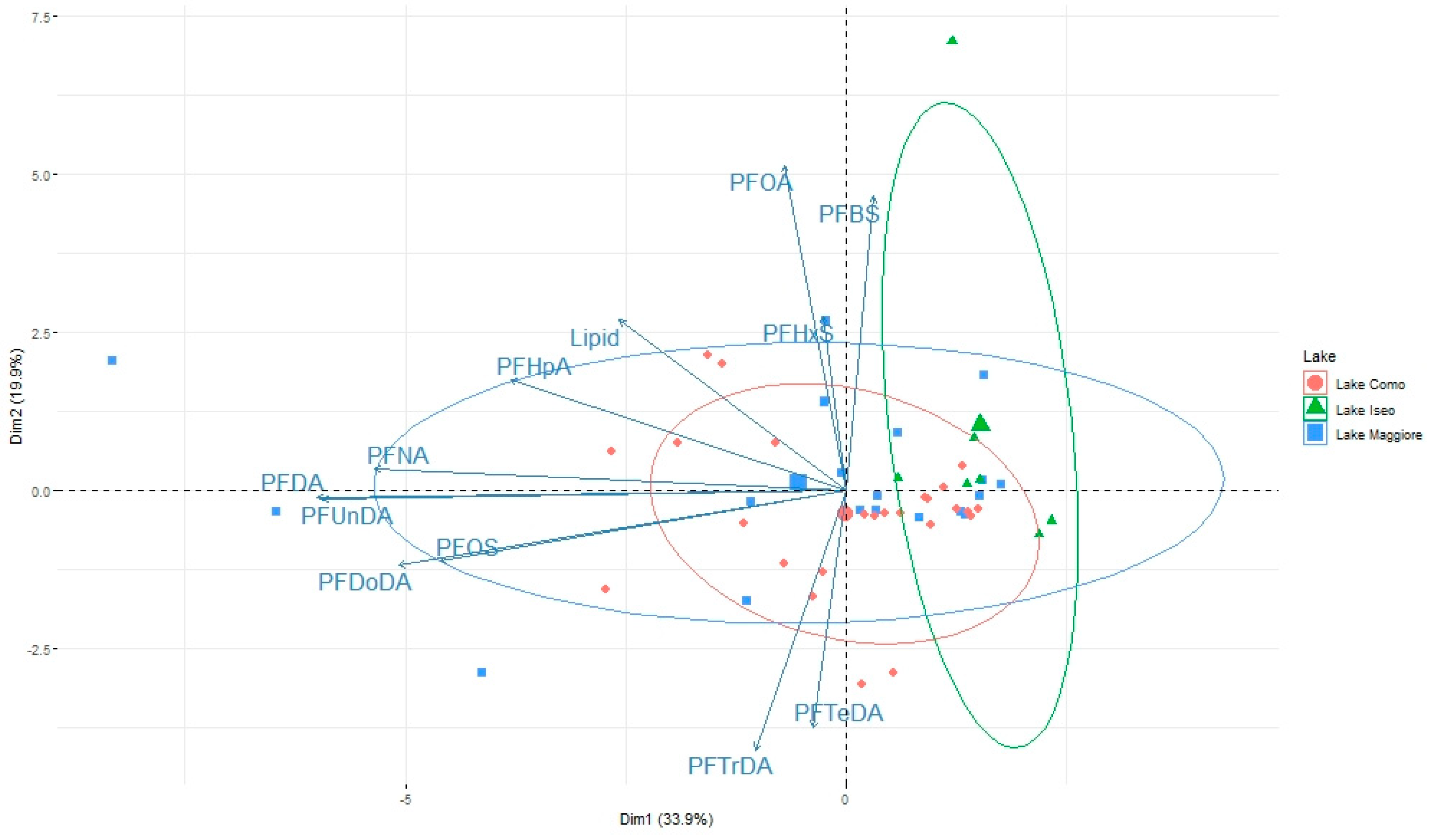
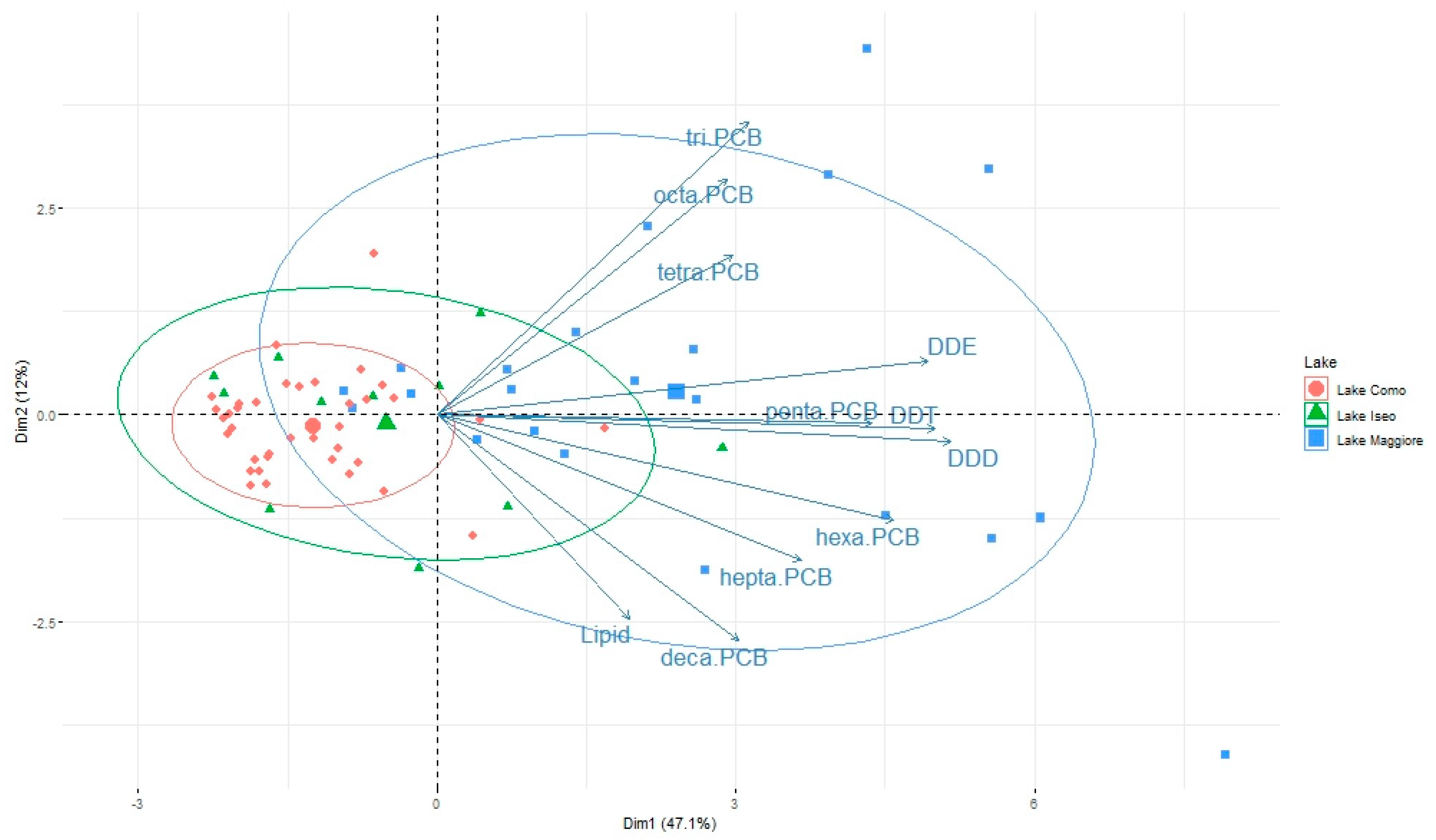
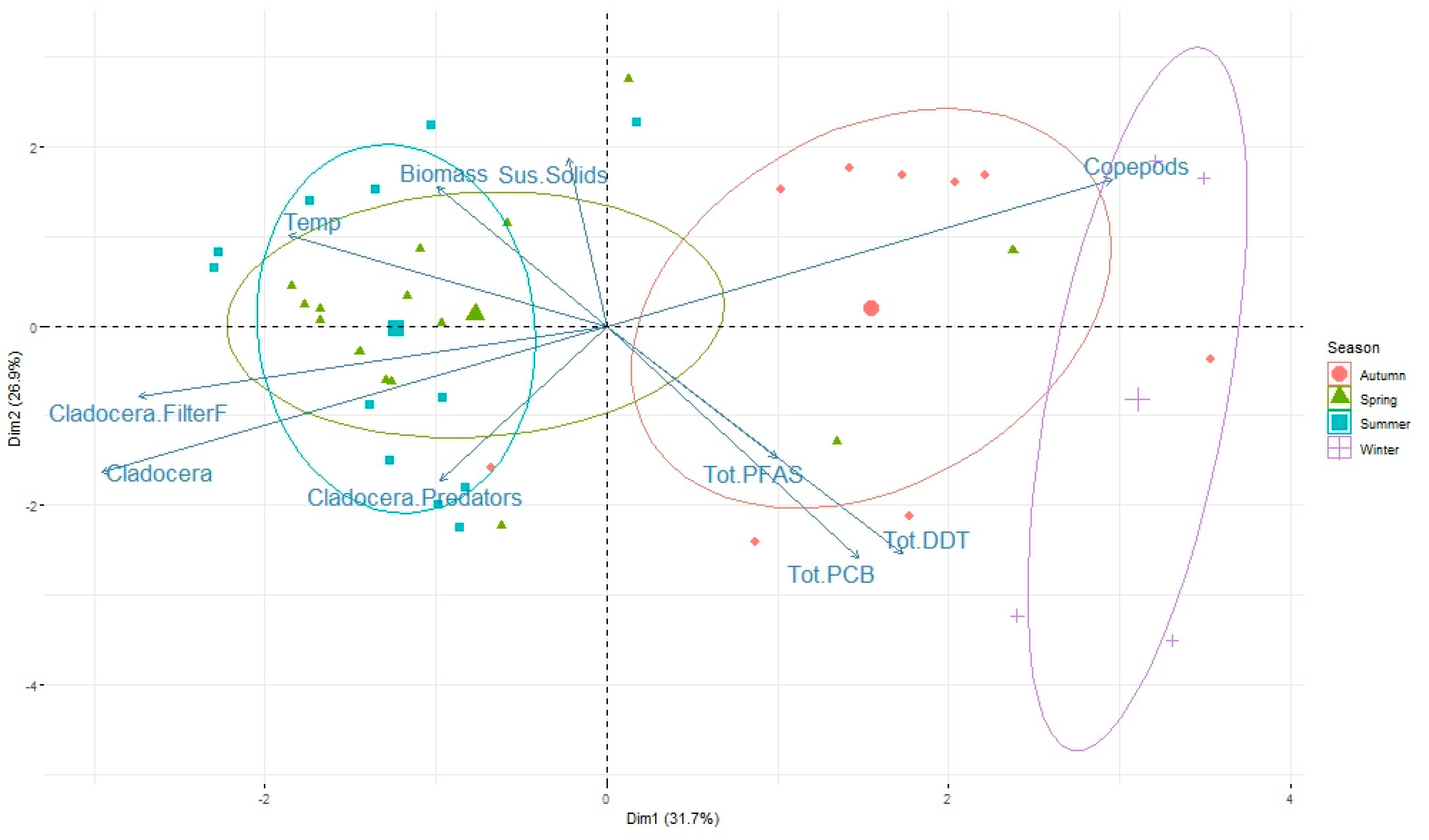
3.2. Pattern of Contamination
3.3. Role of Zooplankton Size and Seasonality on Contaminant Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frederiksen, M.; Edwards, M.; Richardson, A.J.; Halliday, N.C.; Wanless, S. From plankton to top predators: Bottom-up control of a marine food web across four trophic levels. J. Anim. Ecol. 2006, 75, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.; Shin, Y.J.; Jennings, S.; Cury, P. Towards end-to-end models for investigating the effects of climate and fishing in marine ecosystems. Prog. Oceanogr. 2007, 75, 751–770. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Bӑnaru, D.; Dromard, C.R.; Ourgaud, M.; Carlotti, F. Biochemical composition and energy content of size-fractionated zooplankton east of the Kerguelen Islands. Polar Biol. 2019, 42, 603–617. [Google Scholar] [CrossRef]
- Kainz, M.; Mazumder, A. Effect of algal and bacterial diet on methyl mercury concentrations in zooplankton. Environ. Sci. Technol. 2005, 39, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, F.; Jouandet, M.P.; Nowaczyk, A.; Harmelin-Vivien, M.; Lefèvre, D.; Richard, P.; Zhu, Y.; Zhou, M. Mesozooplankton structure and functioning during the onset of the Kerguelen phytoplankton bloom during the KEOPS2 survey. Biogeosciences 2015, 12, 4543–4563. [Google Scholar] [CrossRef]
- Taylor, W.D.; Carey, J.H.; Lean, D.R.S.; McQueen, D.J. Organochlorine concentrations in the plankton of lakes in Southern Ontario and their relationship to plankton biomass. Can. J. Fish. Aquat. Sci. 1991, 48, 1960–1966. [Google Scholar] [CrossRef]
- Back, R.C.; Gorski, P.R.; Cleckner, L.B.; Hurley, J.P. Mercury content and speciation in the plankton and benthos of Lake Superior. Sci. Total Environ. 2003, 304, 349–354. [Google Scholar] [CrossRef]
- Salmaso, N.; Anneville, O.; Straile, D.; Viaroli, P. European Large Perialpine Lakes Under Anthropogenic Pressures and Climate Change: Present Status, Research Gaps and Future Challenges; Springer International Publishing: Cham, Switzerland, 2018; Volume 824, ISBN 0123456789. [Google Scholar]
- CIPAIS. Indagini Su DDT E Sostanze Pericolose nell’ecosistema del Lago Maggiore, Programma 2008-2012, Rapporto Annuale 2009; Commissione internazionale per la protezione delle acque italo-svizzere (CIPAIS): Verbania Pallanza, Italy, 2009. [Google Scholar]
- Corsolini, S.; Sarà, L. The trophic transfer of persistent pollutants (HCB, DDTs, PCBs) within polar marine food webs. Chemosphere 2017, 177, 189–199. [Google Scholar] [CrossRef]
- Bettinetti, R.; Quadroni, S.; Boggio, E.; Galassi, S. Recent DDT and PCB contamination in the sediment and biota of the Como Bay (Lake Como, Italy). Sci. Total Environ. 2016, 542, 404–410. [Google Scholar] [CrossRef]
- Valsecchi, S.; Rusconi, M.; Mazzoni, M.; Viviano, G.; Pagnotta, R.; Zaghi, C.; Serrini, G.; Polesello, S. Occurrence and sources of perfluoroalkyl acids in Italian river basins. Chemosphere 2015, 129, 126–134. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Liang, R.; He, J.; Shi, Y.; Li, Z.; Sarvajayakesavalu, S.; Baninla, Y.; Guo, F.; Chen, J.; Xu, X.; Lu, Y. Effects of Perfluorooctane sulfonate on immobilization, heartbeat, reproductive and biochemical performance of Daphnia magna. Chemosphere 2017, 168, 1613–1618. [Google Scholar] [CrossRef]
- Jeong, T.Y.; Yuk, M.S.; Jeon, J.; Kim, S.D. Multigenerational effect of perfluorooctane sulfonate (PFOS) on the individual fitness and population growth of Daphnia magna. Sci. Total Environ. 2016, 569–570, 1553–1560. [Google Scholar] [CrossRef]
- Houde, M.; Czub, G.; Small, J.M.; Backus, S.; Wang, X.; Alaee, M.; Muir, D.C.G. Fractionation and bioaccumulation of perfluorooctane sulfonate (PFOS) isomers in a lake Ontario food web. Environ. Sci. Technol. 2008, 42, 9397–9403. [Google Scholar] [CrossRef]
- Xu, J.; Guo, C.S.; Zhang, Y.; Meng, W. Bioaccumulation and trophic transfer of perfluorinated compounds in a eutrophic freshwater food web. Environ. Pollut. 2014, 184, 254–261. [Google Scholar] [CrossRef]
- Mosello, R.; Ambrosetti, W.; Arisci, S.; Bettinetti, R.; Buzzi, F.; Calderoni, A.; Carrara, E.; De Bernardi, R.; Galassi, S.; Garibaldi, L.; et al. Evoluzione recente della qualità delle acque dei laghi profondi sudalpini (Maggiore, Lugano, Como, Iseo e Garda) in risposta alle pressioni antropiche e alle variazioni climatiche. Biol. Ambient. 2010, 24, 167–177. [Google Scholar]
- Garibaldi, L.; Mezzanotte, V.; Brizzio, M.C.; Rogora, M.; Mosello, R. The trophic evolution of Lake Iseo as related to its holomixis. J. Limnol. 1999, 58, 10–19. [Google Scholar] [CrossRef][Green Version]
- Mosello, R.; Barbieri, A.; Brizzio, M.C.; Calderoni, A.; Marchetto, A.; Passera, S.; Rogora, M.; Tartari, G. Nitrogen budget of Lago Maggoire: The relative importance of atmospheric deposition and catchment sources. J. Limnol. 2001, 60, 27–40. [Google Scholar] [CrossRef]
- Bettinetti, R.; Quadroni, S.; Manca, M.; Piscia, R.; Volta, P.; Guzzella, L.; Roscioli, C.; Galassi, S. Seasonal fluctuations of DDTs and PCBs in zooplankton and fish of Lake Maggiore (Northern Italy). Chemosphere 2012, 88, 344–351. [Google Scholar] [CrossRef]
- Manca, M.; Comoli, P. Biomass estimates of freshwater zooplankton from length-carbon regression equations. J. Limnol. 2000, 59, 15–18. [Google Scholar] [CrossRef]
- Mazzoni, M.; Polesello, S.; Rusconi, M.; Valsecchi, S. Liquid chromatography mass spectrometry determination of perfluoroalkyl acids in environmental solid extracts after phospholipid removal and on-line turbulent flow chromatography purification. J. Chromatogr. A 2016, 1453, 62–70. [Google Scholar] [CrossRef]
- ARPA Lombardia, Portale Idrologico Geografico. Available online: http://idro.arpalombardia.it/pmapper-4.0/map.phtml (accessed on 10 September 2019).
- Geographical Information Platform of the Swiss Confederation within the Federal Administration. Available online: https://www.geo.admin.ch/ (accessed on 10 September 2019).
- Dijkstra, L.; Poelman, H. A Harmonised Definition of Cities and Rural Areas: The New Degree of Urbanisation; Regional Working Paper WP01/2014; European Commission: Brussel, Belgium, 2014. [Google Scholar]
- Istat—Istituto Nazionale di Statistica. Available online: https://www.istat.it/ (accessed on 10 September 2019).
- Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Geoportale Nazionale Italiano. Available online: http://www.pcn.minambiente.it/mattm/ (accessed on 10 September 2019).
- Regione Lombardia, Geoportale della Lombardia. Available online: http://www.geoportale.regione.lombardia.it/ (accessed on 10 September 2019).
- Regione Piemonte, Geoportale Piemonte. Available online: http://www.geoportale.piemonte.it/ (accessed on 10 September 2019).
- Loos, R.; Carvalho, R.; Antonio, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Mazzoni, M.; Buffo, A.; Cappelli, F.; Pascariello, S.; Polesello, S.; Valsecchi, S.; Volta, P.; Bettinetti, R. Perfluoroalkyl acids in fish of Italian deep lakes: Environmental and human risk assessment. Sci. Total Environ. 2019, 653, 351–358. [Google Scholar] [CrossRef]
- Guzzella, L.M.; Novati, S.; Casatta, N.; Roscioli, C.; Valsecchi, L.; Binelli, A.; Parolini, M.; Solcà, N.; Bettinetti, R.; Manca, M.; et al. Spatial and temporal trends of target organic and inorganic micropollutants in Lake Maggiore and Lake Lugano (Italian-Swiss water bodies): Contamination in sediments and biota. Hydrobiologia 2018. [Google Scholar] [CrossRef]
- Bettinetti, R.; Garibaldi, L.; Leoni, B.; Quadroni, S.; Galassi, S. Zooplankton as an early warning system of persistent organic pollutants contamination in a deep lake (lake Iseo, Northern Italy). J. Limnol. 2012, 71, 335–338. [Google Scholar] [CrossRef]
- Mazzoni, M.; Boggio, E.; Manca, M.; Piscia, R.; Quadroni, S.; Bellasi, A.; Bettinetti, R. Trophic transfer of persistent organic pollutants through a pelagic food web: The case of Lake Como (Northern Italy). Sci. Total Environ. 2018, 640–641. [Google Scholar] [CrossRef]
- Gebbink, W.A.; Bignert, A.; Berger, U. Perfluoroalkyl Acids (PFAAs) and Selected Precursors in the Baltic Sea Environment: Do Precursors Play a Role in Food Web Accumulation of PFAAs? Environ. Sci. Technol. 2016, 50, 6354–6362. [Google Scholar] [CrossRef]
- Munoz, G.; Budzinski, H.; Babut, M.; Drouineau, H.; Lauzent, M.; Menach, K.L.; Lobry, J.; Selleslagh, J.; Simonnet-Laprade, C.; Labadie, P. Evidence for the Trophic Transfer of Perfluoroalkylated Substances in a Temperate Macrotidal Estuary. Environ. Sci. Technol. 2017, 51, 8450–8459. [Google Scholar] [CrossRef]
- Lescord, G.L.; Kidd, K.A.; De Silva, A.O.; Williamson, M.; Spencer, C.; Wang, X.; Muir, D.C.G. Perfluorinated and polyfluorinated compounds in lake food webs from the Canadian High Arctic. Environ. Sci. Technol. 2015, 49, 2694–2702. [Google Scholar] [CrossRef]
- Loi, E.I.H.; Yeung, L.W.Y.; Taniyasu, S.; Lam, P.K.S.; Kannan, K.; Yamashita, N. Trophic Magnification of Poly- and Perfluorinated Compounds in a Subtropical Food Web. Environ. Sci. Technol. 2011, 45, 5506–5513. [Google Scholar] [CrossRef]
- Khairy, M.A.; Noonan, G.O.; Lohmann, R. Uptake of hydrophobic organic compounds, including organochlorine pesticides, polybrominated diphenyl ethers, and perfluoroalkyl acids in fish and blue crabs of the lower Passaic River, New Jersey, USA. Environ. Toxicol. Chem. 2019, 38, 872–882. [Google Scholar] [CrossRef]
- Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C.G. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 196–204. [Google Scholar] [CrossRef]
- Conder, J.M.; Hoke, R.A.; Wolf, W.D.; Russell, M.H.; Buck, R.C. Are PFCAs Bioaccumulative?—A Critical Review and Comparison with Persistent Lipophilic Compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef]
- Kim, S.K.; Oh, J.R.; Shim, W.J.; Lee, D.H.; Yim, U.H.; Hong, S.H.; Shin, Y.B.; Lee, D.S. Geographical distribution and accumulation features of organochlorine residues in bivalves from coastal areas of South Korea. Mar. Pollut. Bull. 2002, 45, 268–279. [Google Scholar] [CrossRef]
- Binelli, A.; Provini, A. The PCB pollution of Lake Iseo (N. Italy) and the role of biomagnification in the pelagic food web. Chemosphere 2003, 53, 143–151. [Google Scholar] [CrossRef]
- Piscia, R.; Mazzoni, M.; Bettinetti, R.; Caroni, R.; Cicala, D.; Manca, M. Stable Isotope Analysis and Persistent Organic Pollutants in Crustacean Zooplankton: The Role of Size and Seasonality. Water 2019, 11, 1490. [Google Scholar] [CrossRef]
- Calizza, E.; Costantini, M.L.; Rossi, D.; Carlino, P.; Rossi, L. Effects of disturbance on an urban river food web. Freshw. Biol. 2012, 57, 2613–2628. [Google Scholar] [CrossRef]
- Campbell, L.M.; Schindler, D.W.; Muir, D.C.; Donald, D.B.; Kidd, K.A. Organochlorine transfer in the food web of subalpine Bow Lake, Banff National Park. Can. J. Fish. Aquat. Sci. 2000, 57, 1258–1269. [Google Scholar] [CrossRef]
- Berrojalbiz, N.; Dachs, J.; Ojeda, M.J.; Valle, M.C.; Castro-Jiménez, J.; Wollgast, J.; Ghiani, M.; Hanke, G.; Zaldivar, J.M. Biogeochemical and physical controls on concentrations of polycyclic aromatic hydrocarbons in water and plankton of the Mediterranean and Black Seas. Global Biogeochem. Cycles 2011, 25, 1–14. [Google Scholar] [CrossRef]
- Munoz, G.; Budzinski, H.; Babut, M.; Lobry, J.; Selleslagh, J.; Tapie, N.; Labadie, P. Temporal variations of perfluoroalkyl substances partitioning between surface water, suspended sediment, and biota in a macrotidal estuary. Chemosphere 2019, 233, 319–326. [Google Scholar] [CrossRef]
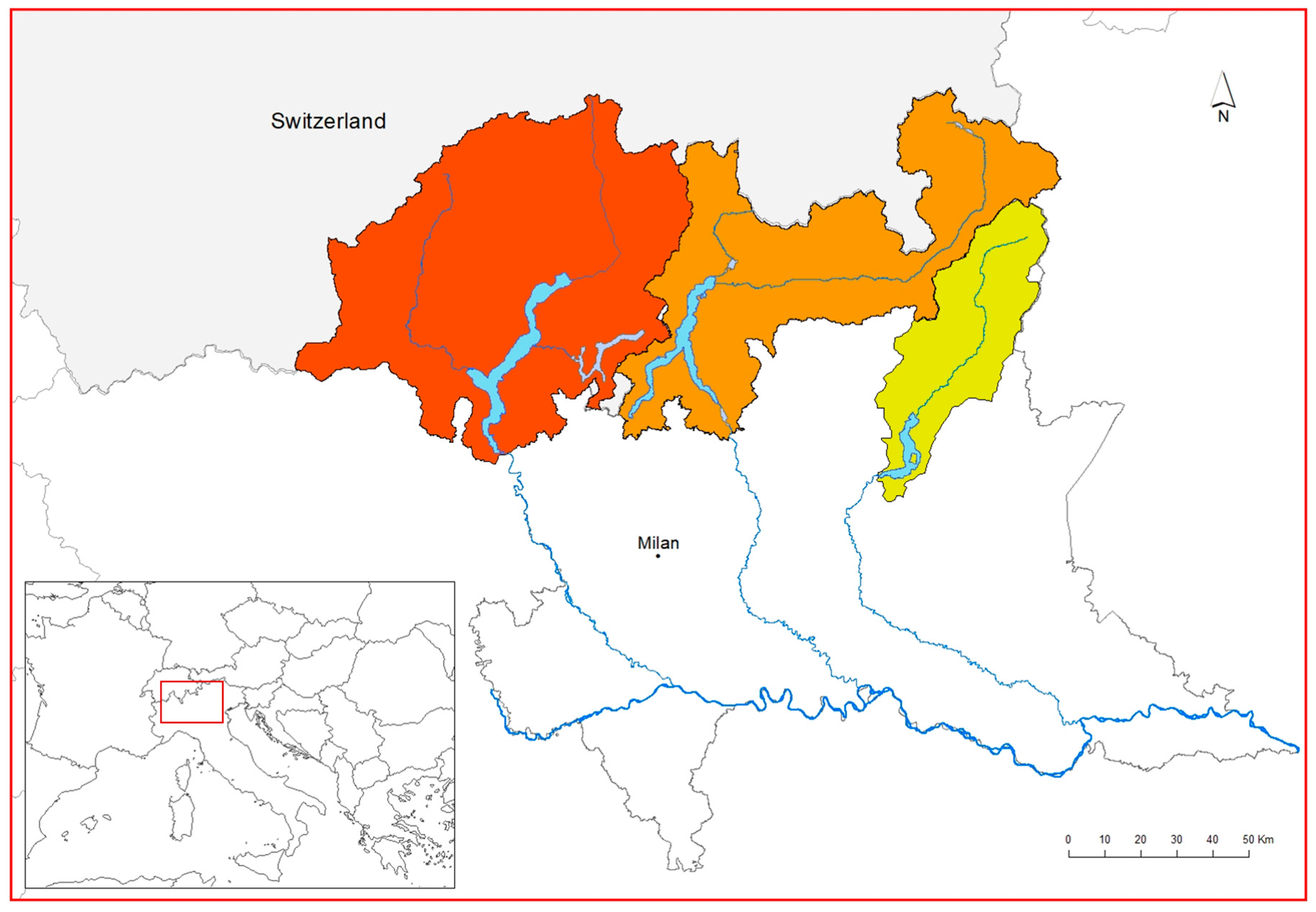
| Lake Basin | DEGURBA | WWTPs | Administrative Data | ||||
|---|---|---|---|---|---|---|---|
| Class | Area % | Total WWTPs | Total Population Equivalent | Total Municipalities | Population (2011) | Area (km2) | |
| Maggiore | 1 | 2.01 | ND | ND | 207 | 923,861 | 6815.64 |
| 2 | 21.42 | ||||||
| 3 | 76.57 | ||||||
| Como | 1 | 1.64 | 143 | 759,461 | 191 | 556,769 | 4611.56 |
| 2 | 18.82 | ||||||
| 3 | 79.53 | ||||||
| Iseo | 1 | 0.00 | 69 | 191,709 | 73 | 191,527 | 1842.48 |
| 2 | 24.20 | ||||||
| 3 | 75.80 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascariello, S.; Mazzoni, M.; Bettinetti, R.; Manca, M.; Patelli, M.; Piscia, R.; Valsecchi, S.; Polesello, S. Organic Contaminants in Zooplankton of Italian Subalpine Lakes: Patterns of Distribution and Seasonal Variations. Water 2019, 11, 1901. https://doi.org/10.3390/w11091901
Pascariello S, Mazzoni M, Bettinetti R, Manca M, Patelli M, Piscia R, Valsecchi S, Polesello S. Organic Contaminants in Zooplankton of Italian Subalpine Lakes: Patterns of Distribution and Seasonal Variations. Water. 2019; 11(9):1901. https://doi.org/10.3390/w11091901
Chicago/Turabian StylePascariello, Simona, Michela Mazzoni, Roberta Bettinetti, Marina Manca, Martina Patelli, Roberta Piscia, Sara Valsecchi, and Stefano Polesello. 2019. "Organic Contaminants in Zooplankton of Italian Subalpine Lakes: Patterns of Distribution and Seasonal Variations" Water 11, no. 9: 1901. https://doi.org/10.3390/w11091901
APA StylePascariello, S., Mazzoni, M., Bettinetti, R., Manca, M., Patelli, M., Piscia, R., Valsecchi, S., & Polesello, S. (2019). Organic Contaminants in Zooplankton of Italian Subalpine Lakes: Patterns of Distribution and Seasonal Variations. Water, 11(9), 1901. https://doi.org/10.3390/w11091901









