Effects of Artificial LED Light on the Growth of Three Submerged Macrophyte Species during the Low-Growth Winter Season: Implications for Macrophyte Restoration in Small Eutrophic Lakes
Abstract
1. Introduction
2. Materials and Methods
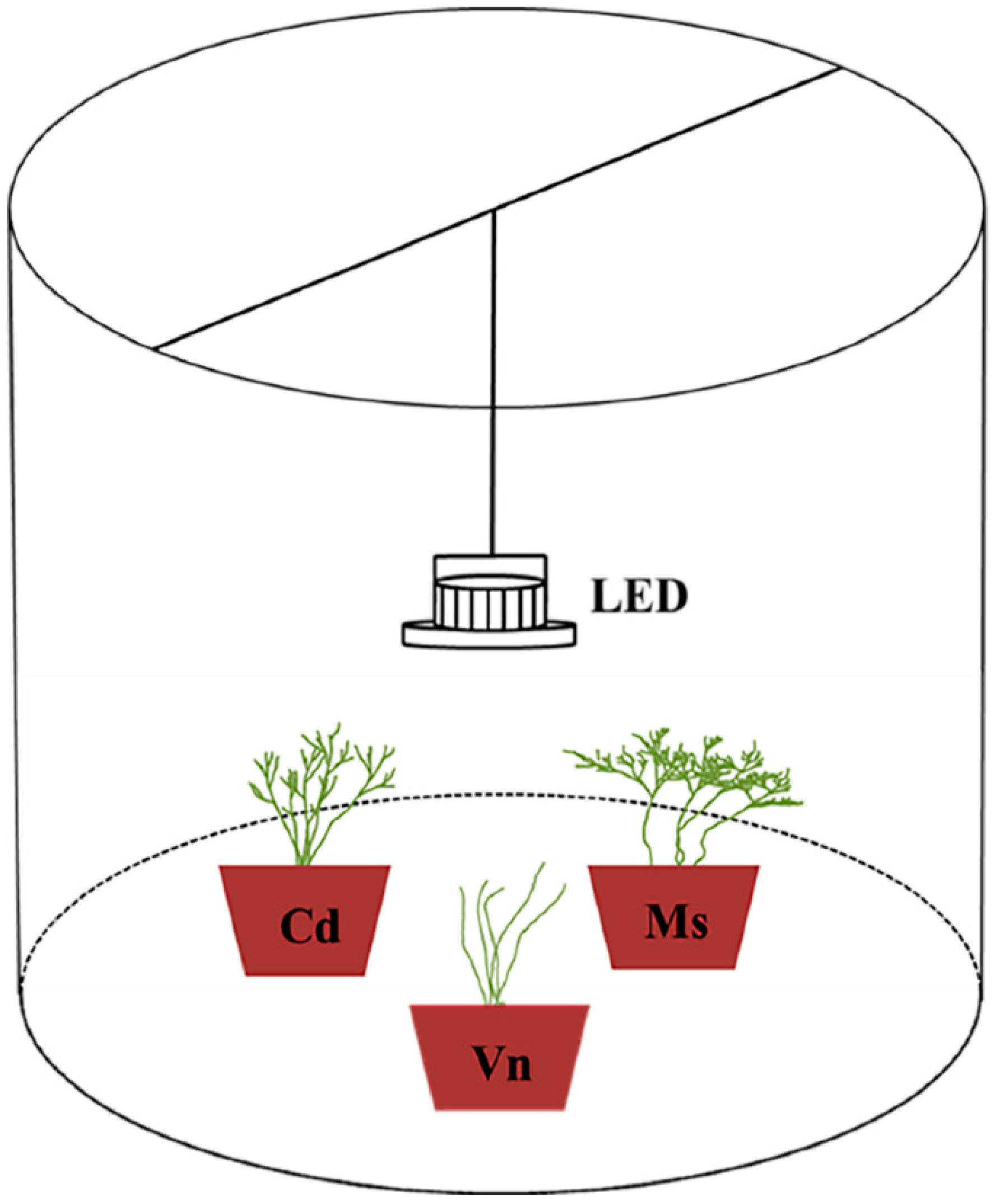
2.1. Study Area and Experimental System
2.2. Experimental Treatments
2.3. Sampling and Measurement
2.4. Calculation and Statistical Analyses
3. Results
3.1. Environmental Variables
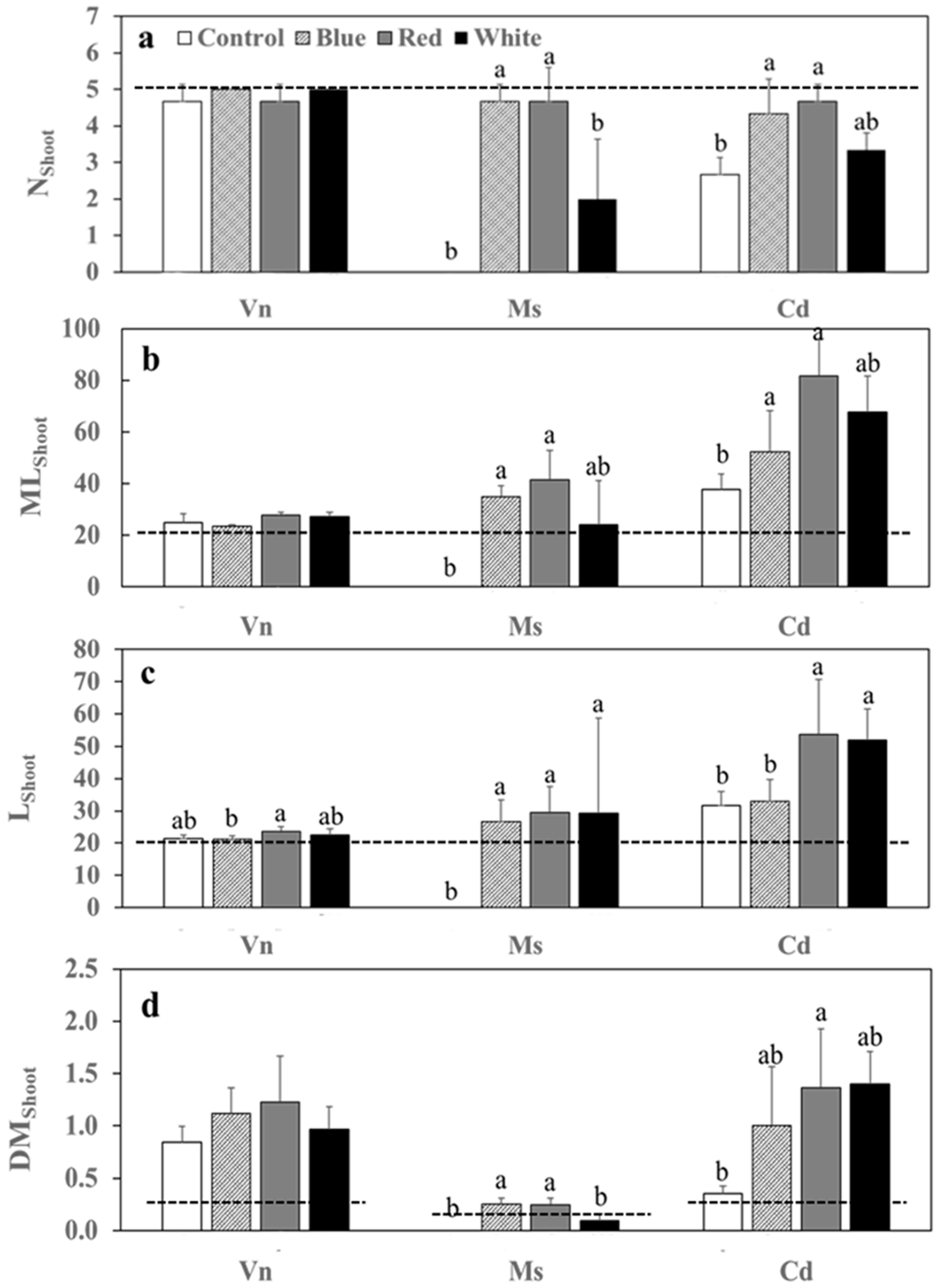
3.2. Growth Variables of Plants
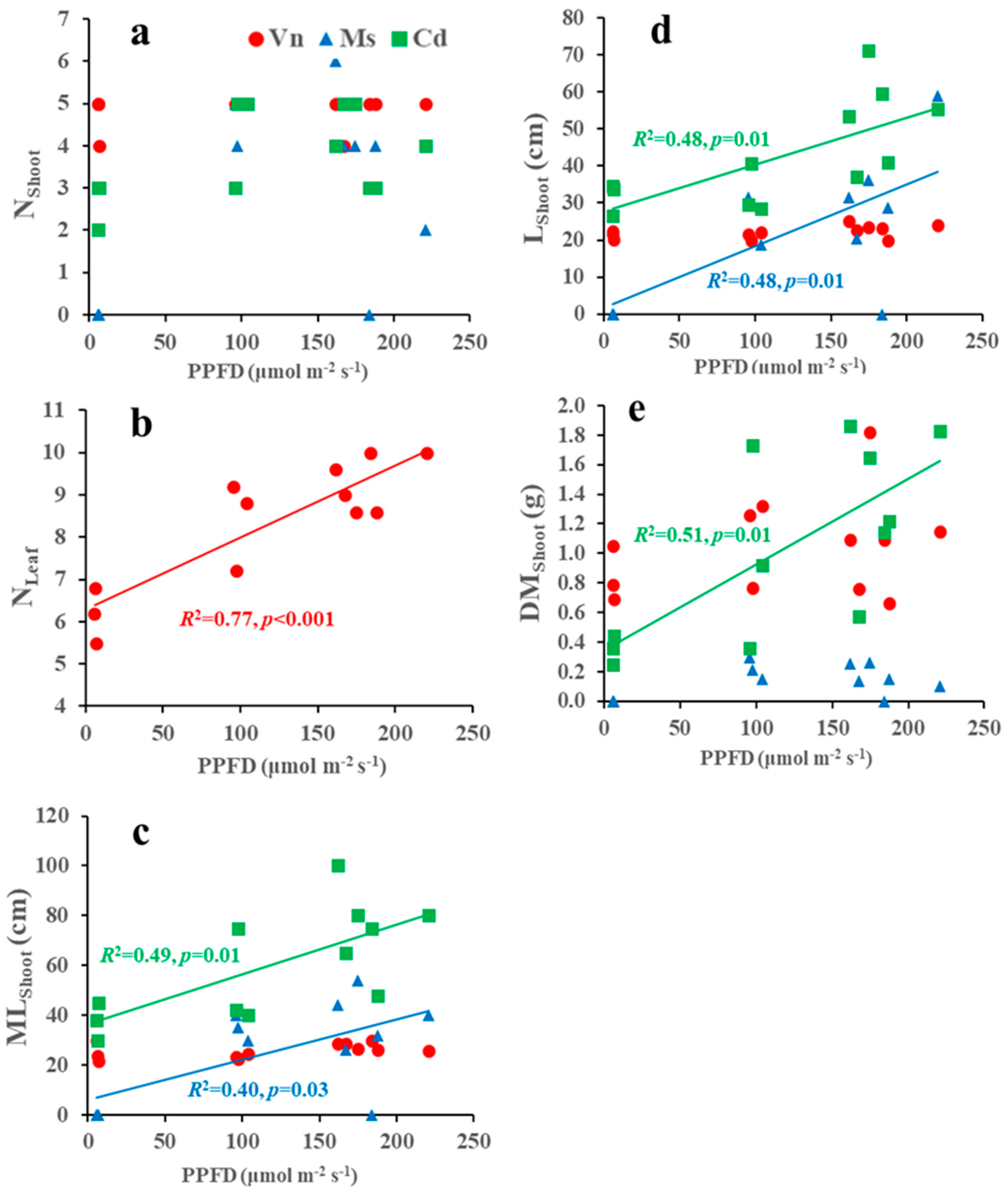
3.3. Relationships between Growth Variables and Environments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeppesen, E.; Søndergaard, M.; Søndergaard, M.; Christoffersen, K. The Structuring Role of Submerged Macrophytes in Lakes; Springer: New York, NY, USA, 1998. [Google Scholar]
- Scheffer, M. Ecology of Shallow Lakes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Moore, K.A.; Wetzel, R.L. Seasonal variations in eelgrass (Zostera marina L.) responses to nutrient enrichment and reduced light availability in experimental ecosystems. J. Exp. Mar. Biol. Ecol. 2000, 244, 1–28. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, H.Z.; Liang, X.M.; Pan, B.Z.; Kosten, S. Macrophyte species strongly affects changes in C, N, and P stocks in shallow lakes after a regime shift from macrophyte to phytoplankton dominance. Inland Waters 2016, 6, 449–460. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, Q.; Gao, Y.; Gu, Q.; Li, G.; Song, L. Long-term effects of temperature and nutrient concentrations on the phytoplankton biomass in three lakes with differing trophic statuses on the Yungui Plateau, China. Ann. Limnol.-Int. J. Limnol. 2018, 54, 9. [Google Scholar] [CrossRef]
- Bachmann, R.W.; Horsburgh, C.A.; Hoyer, M.V.; Mataraza, L.K.; Canfield, D.E. Relations between trophic state indicators and plant biomass in Florida lakes. Hydrobiologia 2002, 470, 219–234. [Google Scholar] [CrossRef]
- Wang, H.J.; Liang, X.M.; Jiang, P.H.; Wang, J.; Wu, S.K.; Wang, H.Z. TN:TP ratio and planktivorous fish do not affect nutrient-chlorophyll relationships in shallow lakes. Freshw. Biol. 2008, 53, 935–944. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, H.Z.; Liang, X.M.; Wu, S.K. Total phosphorus thresholds for regime shifts are nearly equal in subtropical and temperate shallow lakes with moderate depths and areas. Freshw. Biol. 2014, 59, 1659–1671. [Google Scholar] [CrossRef]
- Sagrario, G.; María, A.; Jeppesen, E.; Goma, J.; Sondergaard, M.; Jensen, J.P.; Lauridsen, T.; Landkildehus, F. Does high nitrogen loading prevent clear-water conditions in shallow lakes at moderately high phosphorus concentrations? Freshw. Biol. 2005, 50, 27–41. [Google Scholar] [CrossRef]
- Kosten, S.; Kamarainen, A.; Jeppesen, E.; van Nes, E.H.; Peeters, E.T.H.M.; Mazzeo, N.; Sass, L.; Hauxwell, J.; Hansel-Welch, N.; Lauridsen, T.L.; et al. Climate-related differences in the dominance of submerged macrophytes in shallow lakes. Glob. Chang. Biol. 2009, 15, 2503–2517. [Google Scholar] [CrossRef]
- Barker, T.; Hatton, K.; O’Connor, M.; Connor, L.; Moss, B. Effects of nitrate load on submerged plant biomass and species richness: Results of a mesocosm experiment. Fundam. Appl. Limnol. 2008, 173, 89–100. [Google Scholar] [CrossRef]
- Sayer, C.D.; Burgess, A.; Kari, K.; Davidson, T.A.; Peglar, S.; Yang, H.; Rose, N. Long-term dynamics of submerged macrophytes and algae in a small and shallow, eutrophic lake: Implications for the stability of macrophyte-dominance. Freshw. Biol. 2010, 55, 565–583. [Google Scholar] [CrossRef]
- Lauridsen, T.L.; Jensen, J.P.; Jeppesen, E.; Søndergaard, M. Response of submerged macrophytes in Danish lakes to nutrient loading reductions and biomanipulation. Hydrobiologia 2003, 506, 641–649. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Seasonal response of nutrients to reduced phosphorus loading in 12 Danish lakes. Freshw. Biol. 2005, 50, 1605–1615. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Jacobsen, B.A.; Hansen, R.S.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.L.; Mazzeo, N.; Branco, C.W.C. Restoration of shallow lakes by nutrient control and biomanipulation-the successful strategy varies with lake size and climate. Hydrobiologia 2007, 581, 269–285. [Google Scholar] [CrossRef]
- Jeppesen, E.; Mehner, T.; Winfield, I.J.; Kangur, K.; Sarvala, J.; Gerdeaux, D.; Rask, M.; Malmquist, H.J.; Holmgren, K.; Volta, P. Impacts of climate warming on the long-term dynamics of key fish species in 24 European lakes. Hydrobiologia 2012, 694, 1–39. [Google Scholar] [CrossRef]
- Hellsten, S.; Riihimaki, J. Effects of lake water level regulation on the dynamics of littoral vegetation in northern Finland. Hydrobiologia 1996, 340, 85–92. [Google Scholar] [CrossRef]
- Wang, H.Z.; Wang, H.J.; Liang, X.M.; Ni, L.Y.; Liu, X.Q.; Cui, Y.D. Empirical modelling of submersed macrophytes in Yangtze lakes. Ecol. Model. 2005, 188, 483–491. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Su, W.H.; Zhang, G.F.; Zhang, Y.S.; Xiao, H.; Xia, F. The phptosynthetic characteristics of five submerged aquatic plants. Acta Hydrobiol. Sin. 2004, 28, 391–395. (In Chinese) [Google Scholar]
- Wen, J.; Bao, S.S.; Yang, Q.C.; Cui, H.X. Influence of R/B ratio in LED lighting on physiology and quality of lettuce. Chin. J. Agrometeorol. 2009, 30, 413–416. (In Chinese) [Google Scholar]
- Yang, J.F.; Du, M.Y.; Wen, C.J.; Bai, L.; Guo, W.W.; Guo, J.L.; Liu, J.Y. Effect of high power LED on the growth of aquatic animals and plants. China Illum. Eng. J. 2012, 23, 47–51. (In Chinese) [Google Scholar]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef]
- Hölker, F.; Wurzbacher, C.; Weissenborn, C.; Monaghan, M.T.; Holzhauer, S.I.J.; Premke, K. Microbial diversity and community respiration in freshwater sediments influenced by artificial light at night. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140130. [Google Scholar] [CrossRef]
- Grubisic, M.; Singer, G.; Bruno, M.C.; Grunsven, R.H.A.V.; Manfrin, A.; Monaghan, M.T.; Hölker, F. Artificial light at night decreases biomass and alters community composition of benthic primary producers in a sub-alpine stream. Limnol. Oceanogr. 2017, 62, 2799–2810. [Google Scholar] [CrossRef]
- Grubisic, M.; Singer, G.; Bruno, M.C.; Grunsven, R.H.A.V.; Manfrin, A.; Monaghan, M.T.; Hölker, F. A pigment composition analysis reveals community changes in pre-established stream periphyton under low-level artificial light at night. Limnologica 2017, 69, 55–58. [Google Scholar] [CrossRef]
- Grubisic, M.; Grunsven, R.H.A.V.; Manfrin, A.; Monaghan, M.T.; Hölker, F. A transition to white LED increases ecological impacts of nocturnal illumination on aquatic primary producers in a lowland agricultural drainage ditch. Environ. Pollut. 2018, 240, 630–638. [Google Scholar] [CrossRef]
- Huang, X.F.; Chen, W.M.; Cai, Q.M. Standard Methods for Observation and Analysis in Chinese Ecosystem Research Network-survey, Observation and Analysis of Lake Ecology; Standards Press of China: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Zhang, X.Q.; Yuan, Z.F.; Du, M.Y.; Zhang, Y.; Wang, G.X. Impact of artificial lights on submerged plants’ decolorization and enzyme activity. Environ. Eng. 2015, 33, 7–11. (In Chinese) [Google Scholar]
- Yang, S.G.; Li, X.Y. The action and influence of the color light of LEDs during on the growth of water creature. China Illum. Eng. J. 2003, 14, 35–38. (In Chinese) [Google Scholar]
| Treatment | TN | TP | WT | Turb | Chl a | PPFD |
|---|---|---|---|---|---|---|
| Control 1 | 2.17 ± 1.21 | 0.19 ± 0.11 | 12.33 ± 3.59 | 1.47 ± 1.00 | 0.62 ± 0.36 | 4.20 ± 4.87 |
| Control 2 | 2.38 ± 1.13 | 0.21 ± 0.11 | 12.30 ± 3.60 | 1.33 ± 1.08 | 0.36 ± 0.26 | 5.08 ± 6.03 |
| Control 3 | 2.57 ± 1.11 | 0.19 ± 0.07 | 12.23 ± 3.62 | 1.01 ± 0.73 | 0.45 ± 0.46 | 4.60 ± 5.31 |
| Blue 1 | 2.07 ± 0.87 | 0.18 ± 0.09 | 12.20 ± 3.67 | 1.56 ± 1.72 | 0.55 ± 0.39 | 91.75 ± 18.43 |
| Blue 2 | 2.26 ± 1.02 | 0.19 ± 0.08 | 12.40 ± 3.57 | 1.43 ± 1.00 | 0.45 ± 0.26 | 94.75 ± 21.69 |
| Blue 3 | 2.43 ± 1.16 | 0.16 ± 0.09 | 12.33 ± 3.59 | 1.45 ± 1.06 | 0.27 ± 0.22 | 92.25 ± 24.09 |
| Red 1 | 1.90 ± 1.01 | 0.18 ± 0.10 | 12.23 ± 3.72 | 1.66 ± 1.51 | 2.18 ± 1.39 | 159.25 ± 22.17 |
| Red 2 | 2.27 ± 1.16 | 0.16 ± 0.08 | 12.40 ± 3.57 | 1.21 ± 0.90 | 0.45 ± 0.46 | 159.75 ± 29.88 |
| Red 3 | 1.94 ± 1.17 | 0.13 ± 0.07 | 12.27 ± 3.71 | 1.03 ± 0.71 | 0.18 ± 0.26 | 156.75 ± 19.82 |
| White 1 | 1.88 ± 1.14 | 0.14 ± 0.09 | 12.27 ± 3.71 | 1.55 ± 0.87 | 1.00 ± 0.34 | 177.50 ± 35.80 |
| White 2 | 1.52 ± 0.92 | 0.11 ± 0.07 | 12.20 ± 3.73 | 1.82 ± 1.29 | 0.64 ± 0.72 | 173.25 ± 35.38 |
| White 3 | 2.35 ± 1.03 | 0.19 ± 0.10 | 12.40 ± 3.70 | 1.08 ± 0.94 | 0.27 ± 0.22 | 205.50 ± 42.85 |
| Mean ± SD | 2.14 ± 0.28 | 0.17 ± 0.03 | 12.30 ± 0.07 | 1.39 ± 0.25 | 0.62 ± 0.51 |
| TN | TP | Turb | Chl a | PPFD | |
|---|---|---|---|---|---|
| TN | 0.63 * | −0.69 * | −0.63 * | −0.46 | |
| TP | 0.03 | −0.34 | −0.16 | −0.53 | |
| Turb | 0.02 | 0.29 | 0.77 ** | 0.15 | |
| Chl a | 0.03 | 0.62 | 0.003 | 0.1 | |
| PPFD | 0.13 | 0.08 | 0.64 | 0.76 |
| TN | TP | Turb | Chl a | PPFD | ||
|---|---|---|---|---|---|---|
| Vn | NShoot | 0.00 | −0.36 | −0.19 | −0.20 | 0.13 |
| Nleaf | −0.29 | −0.49 | −0.03 | −0.15 | 0.77 ** | |
| MLShoot | −0.63 * | −0.43 | −0.22 | −0.07 | 0.40 | |
| LShoot | −0.08 | −0.17 | −0.43 | −0.27 | 0.48 | |
| DMShoot | 0.34 | −0.04 | −0.46 | −0.44 | 0.22 | |
| Ms | NShoot | −0.16 | −0.48 | 0.03 | −0.35 | 0.20 |
| MLShoot | 0.01 | −0.47 | −0.21 | −0.51 | 0.43 | |
| LShoot | 0.07 | −0.36 | −0.15 | −0.47 | 0.51 | |
| DMShoot | −0.01 | −0.51 | 0.01 | −0.37 | 0.19 | |
| Cd | NShoot | −0.27 | −0.06 | 0.16 | 0.05 | 0.30 |
| MLShoot | −0.39 | −0.49 | −0.08 | −0.20 | 0.69 * | |
| LShoot | −0.51 | −0.55 | 0.10 | 0.10 | 0.75 ** | |
| DMShoot | −0.45 | −0.44 | −0.04 | −0.21 | 0.64 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Wang, H.-J.; Yu, Q.; Wang, H.-Z.; Liang, X.-M.; Liu, M.; Jeppesen, E. Effects of Artificial LED Light on the Growth of Three Submerged Macrophyte Species during the Low-Growth Winter Season: Implications for Macrophyte Restoration in Small Eutrophic Lakes. Water 2019, 11, 1512. https://doi.org/10.3390/w11071512
Xu C, Wang H-J, Yu Q, Wang H-Z, Liang X-M, Liu M, Jeppesen E. Effects of Artificial LED Light on the Growth of Three Submerged Macrophyte Species during the Low-Growth Winter Season: Implications for Macrophyte Restoration in Small Eutrophic Lakes. Water. 2019; 11(7):1512. https://doi.org/10.3390/w11071512
Chicago/Turabian StyleXu, Chao, Hai-Jun Wang, Qing Yu, Hong-Zhu Wang, Xiao-Min Liang, Miao Liu, and Erik Jeppesen. 2019. "Effects of Artificial LED Light on the Growth of Three Submerged Macrophyte Species during the Low-Growth Winter Season: Implications for Macrophyte Restoration in Small Eutrophic Lakes" Water 11, no. 7: 1512. https://doi.org/10.3390/w11071512
APA StyleXu, C., Wang, H.-J., Yu, Q., Wang, H.-Z., Liang, X.-M., Liu, M., & Jeppesen, E. (2019). Effects of Artificial LED Light on the Growth of Three Submerged Macrophyte Species during the Low-Growth Winter Season: Implications for Macrophyte Restoration in Small Eutrophic Lakes. Water, 11(7), 1512. https://doi.org/10.3390/w11071512







