Dissolved Carbon Transport and Processing in North America’s Largest Swamp River Entering the Northern Gulf of Mexico
Abstract
1. Introduction
2. Methods
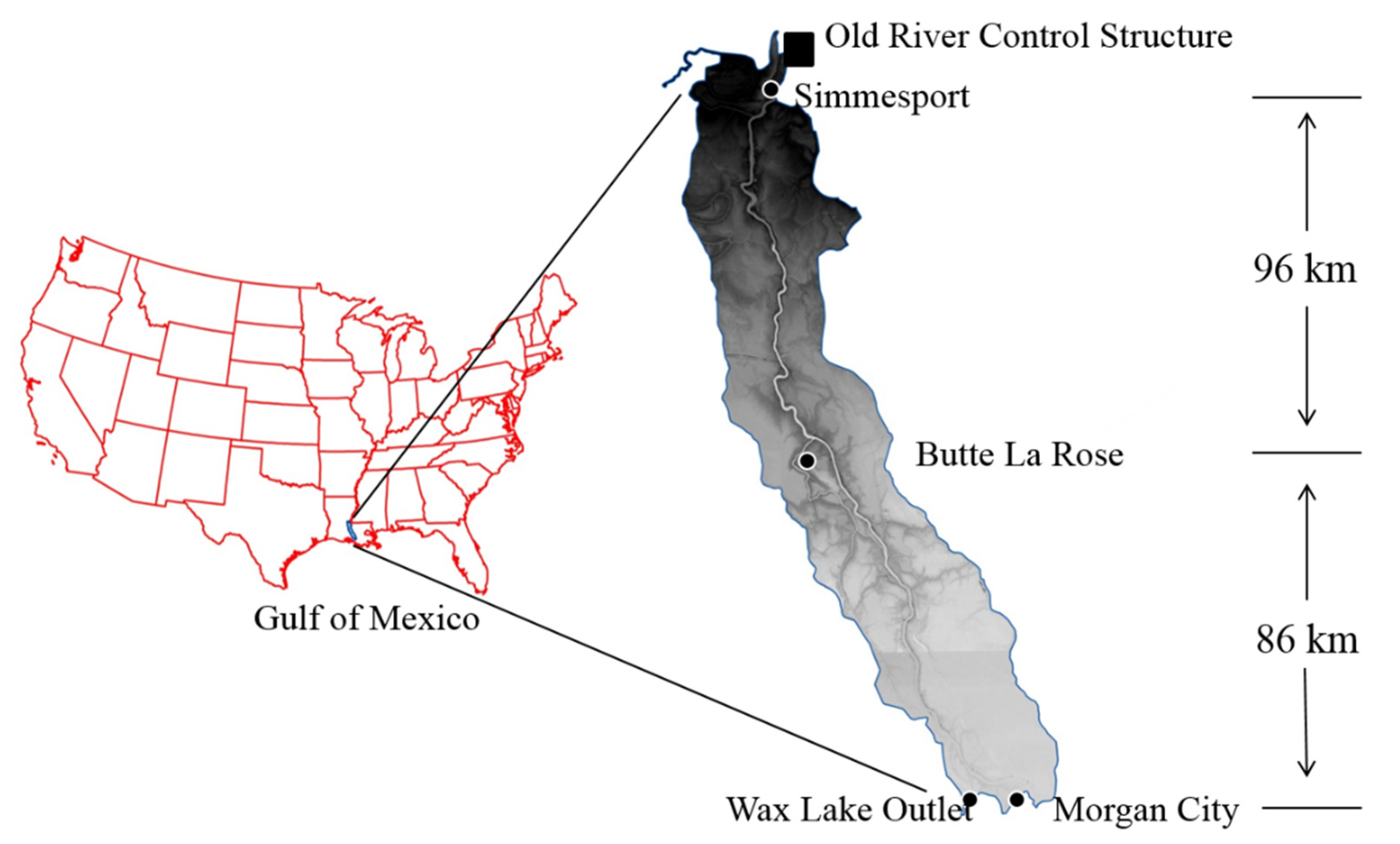
2.1. Study Area
2.2. River Water Measurement and Sampling
2.3. Water Sample Analysis
2.4. Mass Flux Calculation, pCO2 Calculation, and Data Analysis
3. Results
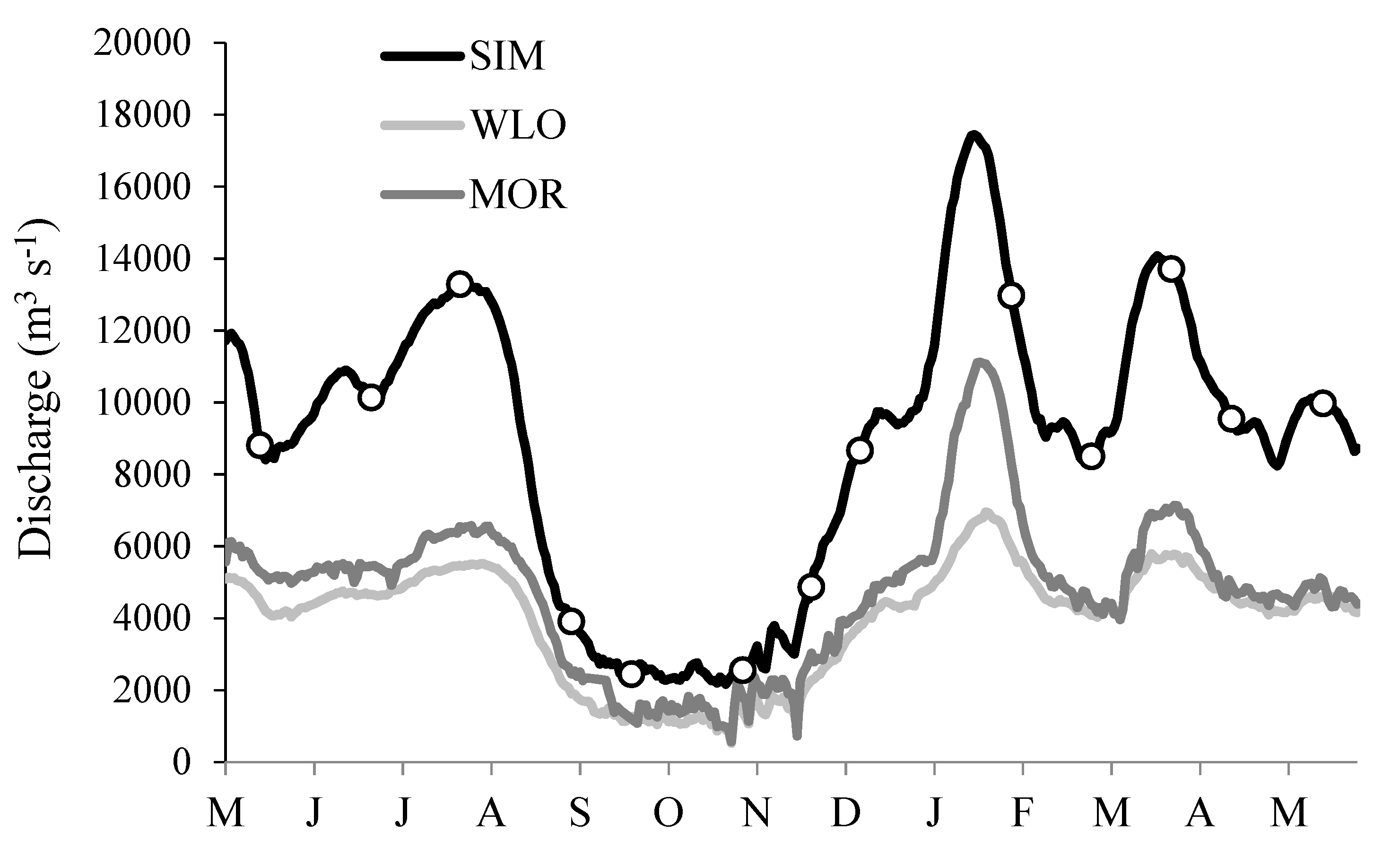
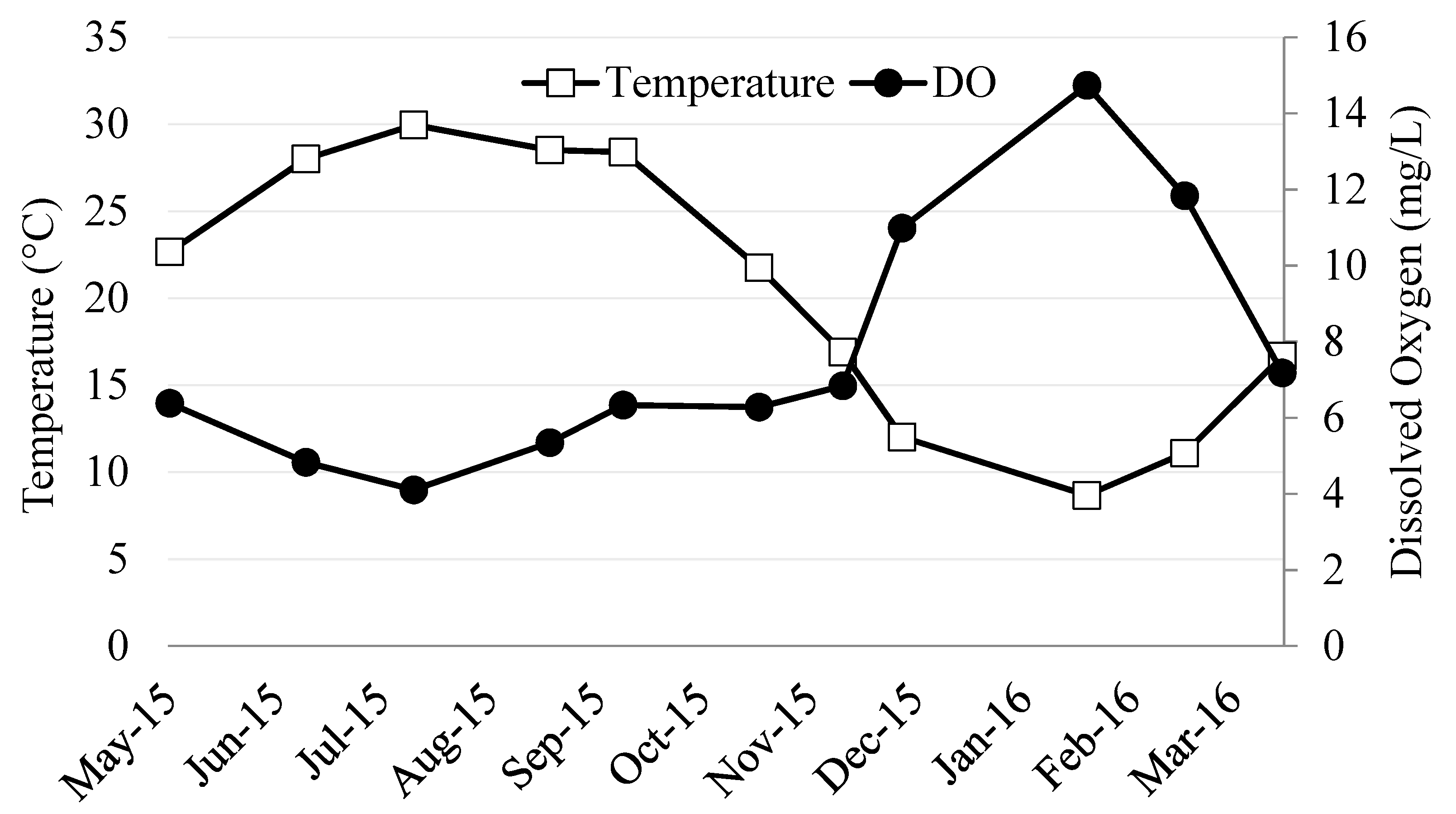
3.1. River Ambient Conditions
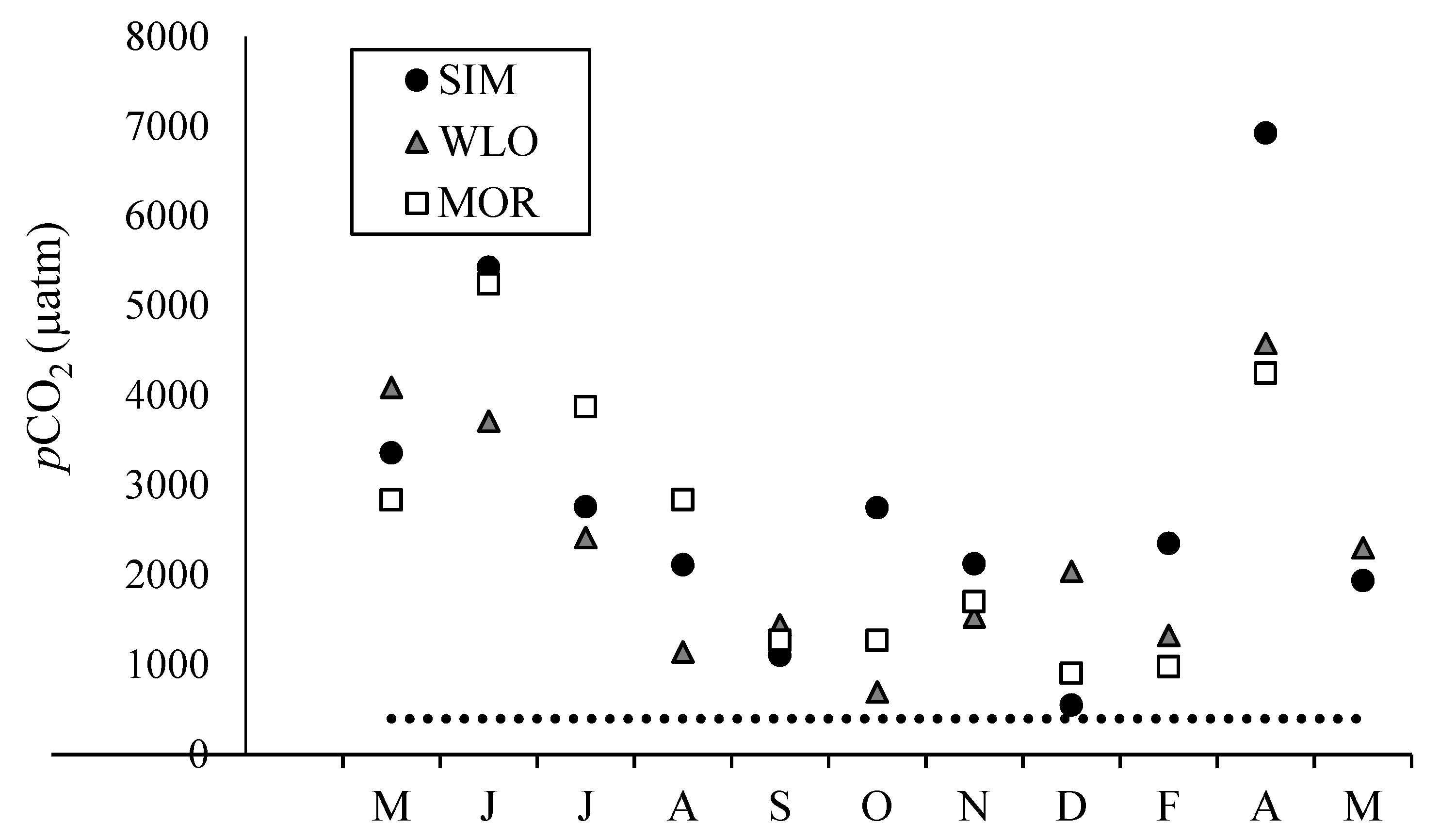
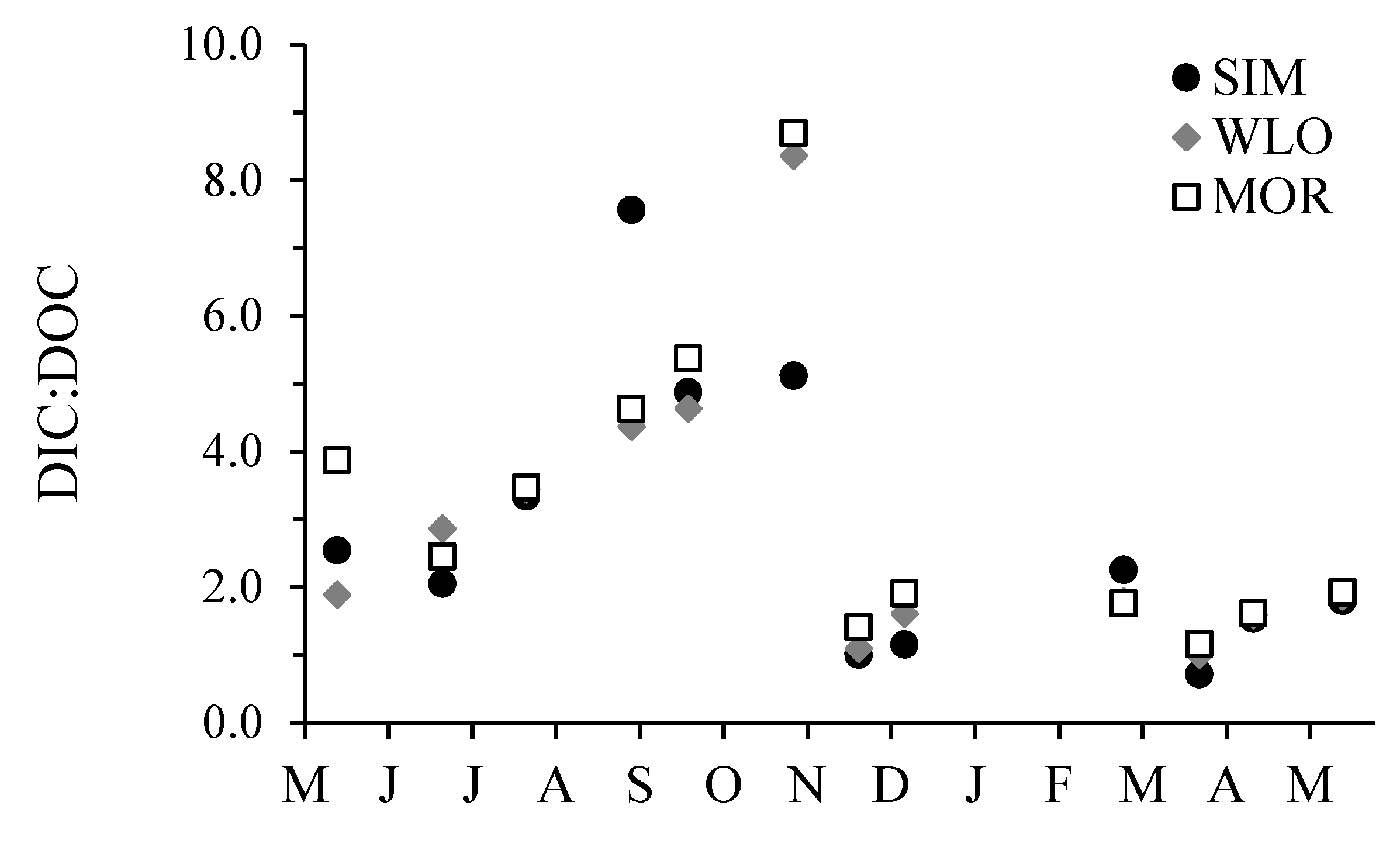
3.2. Dissolved Inorganic Carbon Concentrations, pCO2, and δ13CDIC
3.3. Dissolved Organic Carbon Concentrations and δ13CDOC
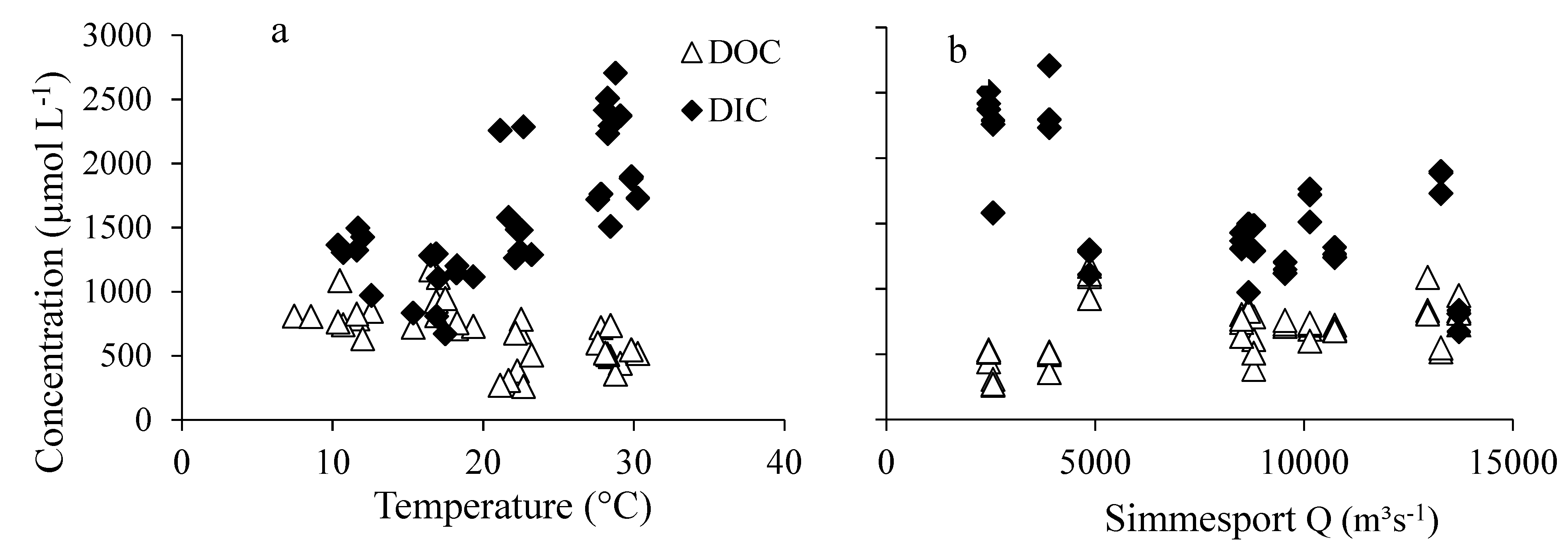
3.4. Relations of DIC and DOC Concentrations with Water Temperature and Discharge
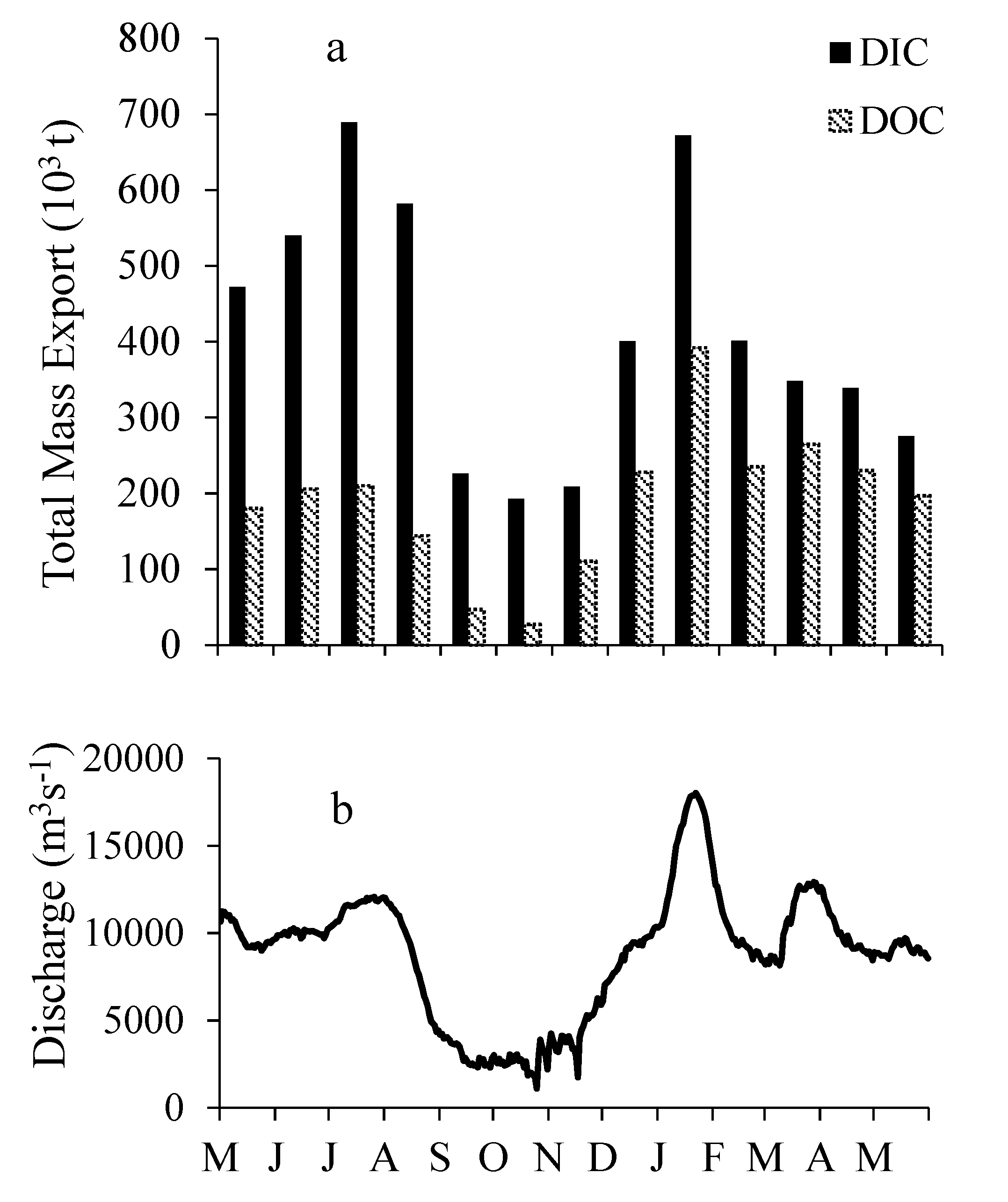
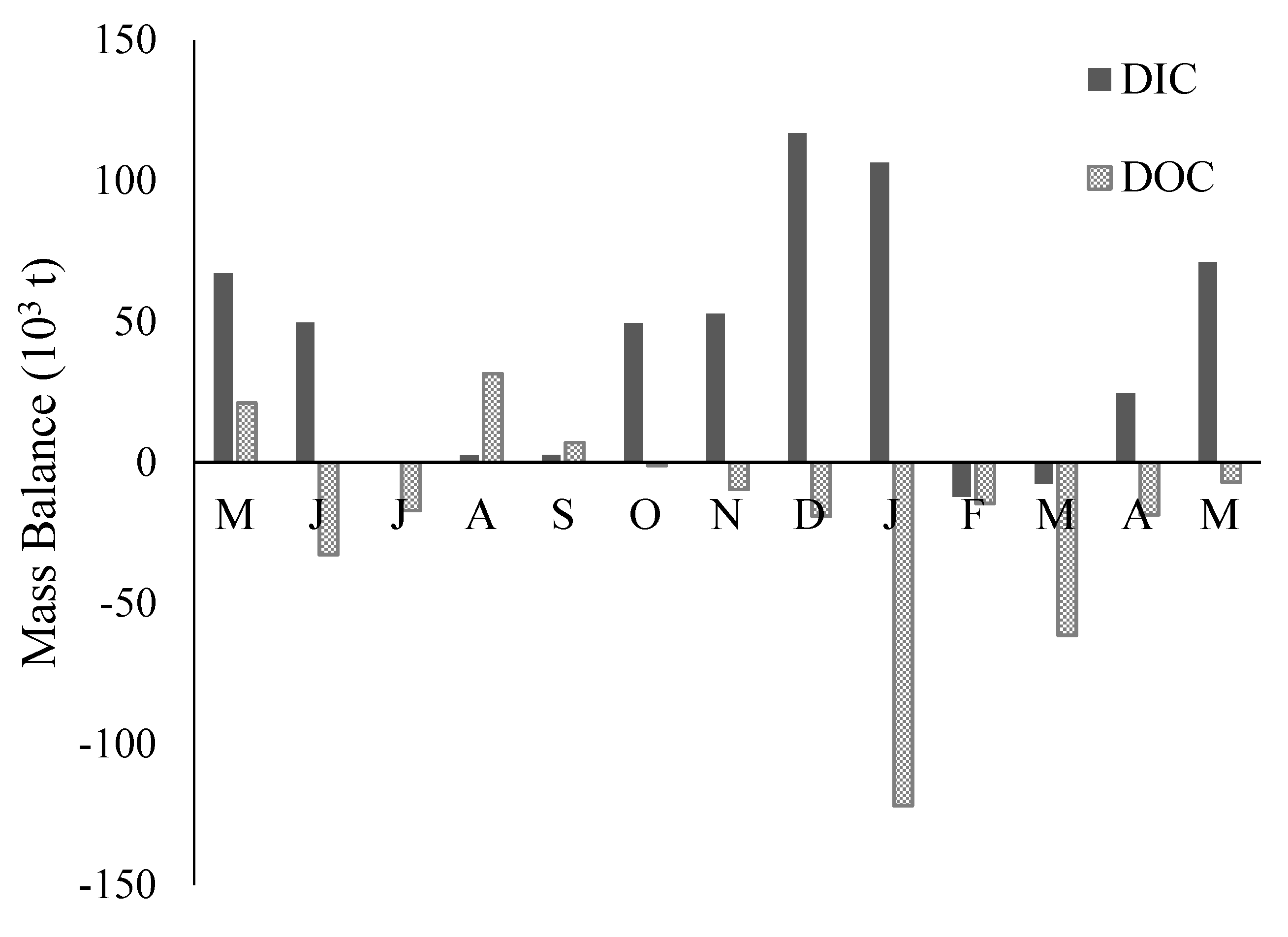
3.5. DIC and DOC Mass Flux and Mass Balance
4. Discussion
4.1. Dissolved Inorganic Carbon Dynamics
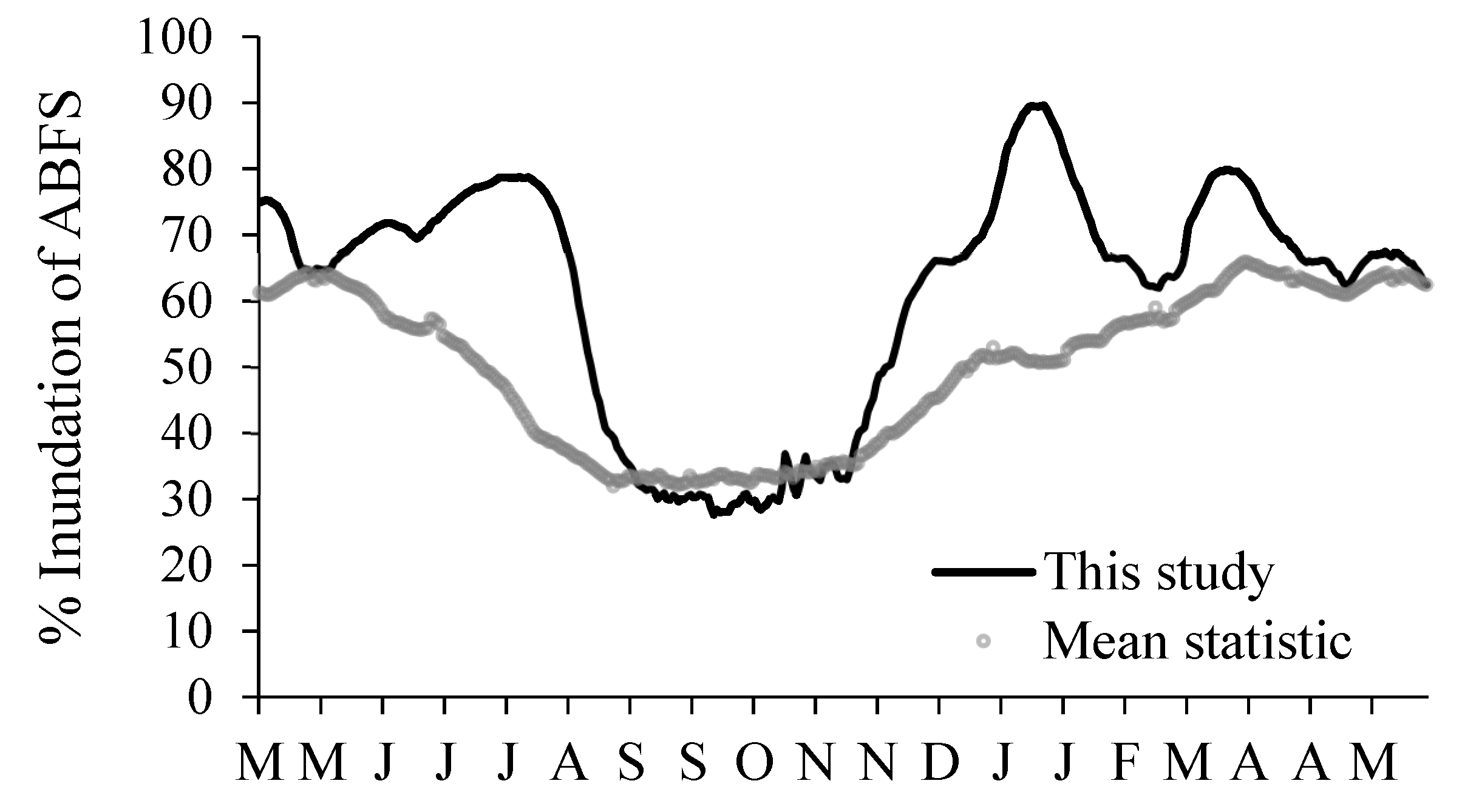
4.2. Impacts of River Corridor and Floodplain Wetlands
4.3. Dissolved Organic Carbon Dynamics
4.4. Annual DOC and DIC Mass Load Estimates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butman, D.; Raymond, P.A. Significant efflux of carbon dioxide from streams and rivers in the United States. Nat. Geosci. 2011, 4, 839–842. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef]
- Hope, D.; Billett, M.F.; Cresser, M.S. A review of the export of carbon in river water: Fluxes and processes. Environ. Pollut. 1994, 84, 301–324. [Google Scholar] [CrossRef]
- Abril, G.; Martinez, J.M.; Artigas, L.F.; Moreira-Turcq, P.; Benedetti, M.F.; Vidal, L.; Meziane, T.; Kim, J.H.; Bernardes, M.C.; Savoye, N.; et al. Amazon River carbon dioxide outgassing fuelled by wetlands. Nature 2014, 505, 395. [Google Scholar] [CrossRef] [PubMed]
- Teodoru, C.R.; Nyoni, F.C.; Borges, A.V.; Darchambeau, F.; Nyambe, I.; Bouillon, S. Dynamics of greenhouse gases (CO2, CH4, N2O) along the Zambezi River and major tributaries, and their importance in the riverine carbon budget. Biogeosciences 2015, 12, 2431–2453. [Google Scholar] [CrossRef]
- Briggs, S.V.; Maher, M.T.; Tongway, D.J. Dissolved and particulate organic carbon in two wetlands in southwestern New South Wales, Australia. Hydrobiologia 1993, 264, 13–19. [Google Scholar] [CrossRef]
- Cai, Y.; Shim, M.J.; Guo, L.; Shiller, A. Floodplain influence on carbon speciation and fluxes from the lower Pearl River, Mississippi. Geochim. Cosmochim. Acta 2016, 186, 189–206. [Google Scholar] [CrossRef]
- Tockner, K.; Pennetzdorfer, D.; Reiner, N.; Schiemer, F.; Ward, J.V. Hydrological connectivity, and the exchange of organic matter and nutrients in a dynamic river–floodplain system (Danube, Austria). Freshw. Biol. 1999, 41, 521–535. [Google Scholar] [CrossRef]
- Aitkenhead, J.A.; McDowell, W.H. Soil C:N ratio as a predictor of annual riverine DOC flux at local and global scales. Glob. Biogeochem. Cycles 2000, 14, 127–138. [Google Scholar] [CrossRef]
- Harrison, J.A.; Caraco, N.; Seitzinger, S.P. Global patterns and sources of dissolved organic matter export to the coastal zone: Results from a spatially explicit, global model. Glob. Biogeochem. Cycles 2005, 19, GB4S04. [Google Scholar] [CrossRef]
- Ludwig, W.; Probst, J.L.; Kempe, S. Predicting the oceanic input of organic carbon by continental erosion. Glob. Biogeochem. Cycles 1996, 10, 23–41. [Google Scholar] [CrossRef]
- Mantoura, R.F.C.; Woodward, E.M.S. Conservative behaviour of riverine dissolved organic carbon in the Severn Estuary: Chemical and geochemical implications. Geochim. Cosmochim. Acta 1983, 47, 1293–1309. [Google Scholar] [CrossRef]
- Meybeck, M. Carbon, nitrogen, and phosphorus transport by world rivers. Am. J. Sci. 1982, 282, 401–450. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, L.; Douglas, T.A. Temporal variation in organic carbon species and fluxes from the Chena River, Alaska. Limnol. Oceanogr. 2008, 53, 1408–1419. [Google Scholar] [CrossRef]
- Kempe, S.; Pettine, M.; Cauwet, G. Biogeochemistry of European Rivers in Biogeochemistry of Major World Rivers, SCOPE 42; Degens, E.T., Kempe, S., Richey, J.E., Eds.; Scientific Committee on Problems of the Environment: Paris, France, 1991; pp. 169–212. [Google Scholar]
- Lerman, A.; Wu, L.; Mackenzie, F.T. CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar. Chem. 2007, 106, 326–350. [Google Scholar] [CrossRef]
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R.; Aalto, R.E.; Yoo, K. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front. Ecol. Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef]
- Regnier, P.; Friedlingstein, P.; Ciais, P.; Mackenzie, F.T.; Gruber, N.; Janssens, I.A.; Laruelle, G.G.; Lauerwald, R.; Luyssaert, S.; Andersson, A.J.; et al. Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat. Geosci. 2013, 6, 597–607. [Google Scholar] [CrossRef]
- Richey, J.E.; Melack, J.M.; Aufdenkampe, A.K.; Ballester, V.M.; Hess, L.L. Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2. Nature 2002, 416, 617–620. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Joshi, S.; Xu, Y.J. Assessment of suspended sand availability under different flow conditions of the Lowermost Mississippi River at Tarbert Landing during 1973–2013. Water 2015, 7, 7022–7044. [Google Scholar] [CrossRef]
- Rosen, T.; Xu, Y.J. Estimation of sedimentation rates in the distributary basin of the Mississippi River, the Atchafalaya River Basin, USA. Hydrol. Res. 2015, 46, 244–257. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, L.; Wang, X.; Aiken, G. Abundance, stable isotopic composition, and export fluxes of DOC, POC, and DIC from the Lower Mississippi River during 2006–2008. J. Geophys. Res. Biogeosci. 2015, 120, 2273–2288. [Google Scholar] [CrossRef]
- Dubois, K.D.; Lee, D.; Veizer, J. Isotopic constraints on alkalinity, dissolved organic carbon, and atmospheric carbon dioxide fluxes in the Mississippi River. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef]
- Leenheer, J. United States Geological Survey data information service. Transp. Carbon Miner. Major World Rivers 1982, 1, 355–356. [Google Scholar]
- Cai, W.J. Riverine inorganic carbon flux and rate of biological uptake in the Mississippi River plume. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Tian, H.; Ren, W.; Yang, J.; Tao, B.; Cai, W.J.; Lohrenz, S.E.; Hopkinson, C.S.; Liu, M.; Yang, Q.; Lu, C.; et al. Climate extremes dominating seasonal and interannual variations in carbon export from the Mississippi River basin. Glob. Biogeochem. Cycles 2015, 29, 1333–1347. [Google Scholar] [CrossRef]
- Shen, Y.; Fichot, C.G.; Benner, R. Floodplain influence on dissolved organic matter composition and export from the Mississippi—Atchafalaya River system to the Gulf of Mexico. Limnol. Oceanogr. 2012, 57, 1149–1160. [Google Scholar] [CrossRef]
- Butman, D.E.; Wilson, H.F.; Barnes, R.T.; Xenopoulos, M.A.; Raymond, P.A. Increased mobilization of aged carbon to rivers by human disturbance. Nature Geosci. 2015, 8, 112–116. [Google Scholar] [CrossRef]
- Fisher, S.G.; Likens, G.E. Stream Ecosystem: Organic Energy Budget. Biosciences 1972, 22, 33–35. [Google Scholar] [CrossRef]
- Wipfli, M.S.; Richardson, J.S.; Naiman, R.J. Ecological linkages between headwaters and downstream ecosystems: Transport of organic matter, invertebrates, and wood down headwater channels. J. Am. Water Res. Assoc. 2007, 43, 72–85. [Google Scholar] [CrossRef]
- Xu, Y.J. Long-term sediment transport and delivery of the largest distributary of the Mississippi River, the Atchafalaya, USA. Sediment Dyn. Chang. Future 2010, 337, 282–290. [Google Scholar]
- Hupp, C.R.; Demas, C.R.; Kroes, D.E.; Day, R.H.; Doyle, T.W. Recent sedimentation patterns within the central Atchafalaya Basin, Louisiana. Wetlands 2008, 28, 125–140. [Google Scholar] [CrossRef]
- Roberts, H.H. Delta switching: Early responses to the Atchafalaya River diversion. J. Coast. Res. 1998, 14, 882–899. [Google Scholar]
- Xu, Y.J. Total nitrogen inflow and outflow from a large river swamp basin to the Gulf of Mexico. Hydrol. Sci. J. 2006, 51, 531–542. [Google Scholar] [CrossRef]
- Xu, Y.J. Transport and Retention of Nitrogen, Phosphorus and Carbon in North America’s Largest River Swamp Basin, the Atchafalaya River Basin. Water 2013, 5, 379–393. [Google Scholar] [CrossRef]
- Deines, P. The isotopic composition of reduced organic carbon. Handb. Environ. Iost. Geochem. 1980, 329–406. [Google Scholar]
- Ehleringer, J.R.; Cerling, T.E. C3 and C4 photosynthesis. Encycl. Glob. Environ. Chang. 2002, 2, 186–190. [Google Scholar]
- Mook, W.G.; Koopmans, M.; Carter, A.F.; Keeling, C.D. Seasonal, latitudinal, and secular variations in the abundance and isotopic ratios of atmospheric carbon dioxide: 1. Results from land stations. J. Geophys. Res. Oceans 1983, 88, 10915–10933. [Google Scholar] [CrossRef]
- Vogel, J.C. Variability of Carbon Isotope Fractionation during Photosynthesis. Stable Isotopes and Plant Carbon-Water Relations; Academic Press: Cambridge, MA, USA, 2012; pp. 29–38. [Google Scholar]
- Baird, M.E.; Emsley, S.M.; McGlade, J.M. Using a phytoplankton growth model to predict the fractionation of stable carbon isotopes. J. Plankton Res. 2001, 23, 841–848. [Google Scholar] [CrossRef]
- Allen, Y.C.; Constant, G.C.; Couvillion, B.R. Preliminary Classification of Water Areas within the Atchafalaya Basin Floodway System by Using Landsat Imagery. United States; U.S. Geological Survey: Reston, VA, USA, 2008.
- Lambou, V.W.; Hern, S.C. Transport of organic carbon in the Atchafalaya Basin Louisiana. Hydrobiologia 1983, 98, 25–34. [Google Scholar] [CrossRef]
- Department of the Interior. Endrin Pollution in the Lower Mississippi River Basin; Federal Water Pollution Control Administration: Dallas, TX, USA, 1969; p. 213.
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Wang, Y. The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers, Georgia. Limnol. Oceanogr. 1998, 43, 657–668. [Google Scholar] [CrossRef]
- Weiss, R.F. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Harned, H.S.; Davis, R. The Ionization Constant of Carbonic Acid in Water and the Solubility of Carbon Dioxide in Water and Aqueous Salt Solutions from 0 to 50°. J. Am. Chem. Soc. 1943, 65, 2030–2037. [Google Scholar] [CrossRef]
- Harned, H.S.; Scholes, S.R., Jr. The Ionization Constant of HCO3− from 0 to 50°. J. Am. Chem. Soc. 1941, 63, 1706–1709. [Google Scholar] [CrossRef]
- Cole, J.J.; Cole, J.J.; Caraco, N.F.; Caraco, N.F. Carbon in catchments: Connecting terrestrial carbon losses with aquatic metabolism. Mar. Freshw. Res. 2001, 52, 101–110. [Google Scholar] [CrossRef]
- Gupta, G.V.M.; Sarma, V.V.S.S.; Robin, R.S.; Raman, A.V.; Jai Kumar, M.; Rakesh, M.; Subramanian, B.R. Influence of net ecosystem metabolism in transferring riverine organic carbon to atmospheric CO2 in a tropical coastal lagoon (Chilka Lake, India). Biogeochemistry 2008, 87, 265–285. [Google Scholar] [CrossRef]
- Khadka, M.B.; Martin, J.B.; Jin, J. Transport of dissolved carbon and CO2 degassing from a river system in a mixed silicate and carbonate catchment. J. Hydrol. 2014, 513, 391–402. [Google Scholar] [CrossRef]
- Wang, X.; Veizer, J. Respiration–photosynthesis balance of terrestrial aquatic ecosystems, Ottawa area, Canada. Geochim. Cosmochim. Acta 2000, 66, 3775–3786. [Google Scholar] [CrossRef]
- Roberts, B.J.; Doty, S.M. Spatial and Temporal Patterns of Benthic Respiration and Net Nutrient Fluxes in the Atchafalaya River Delta Estuary. Estuaries Coast. 2015, 38, 1918–1936. [Google Scholar] [CrossRef]
- Raymond, P.A.; Caraco, N.F.; Cole, J.J. Carbon dioxide concentration and atmospheric flux in the Hudson River. Estuaries 1997, 20, 381–390. [Google Scholar] [CrossRef]
- Reiman, J.H.; Xu, Y.J. Diel variability of pCO2 and CO2 outgassing from the Lower Mississippi River: Implications for riverine CO2 outgassing estimation. Water 2019, 11, 43. [Google Scholar] [CrossRef]
- Geldern, R.; Schulte, P.; Mader, M.; Baier, A.; Barth, J.A. Spatial and temporal variations of pCO2, dissolved inorganic carbon and stable isotopes along a temperate karstic watercourse. Hydrol. Process. 2015, 29, 3423–3440. [Google Scholar] [CrossRef]
- Devol, A.H.; Forsberg, B.R.; Richey, J.E.; Pimentel, T.P. Seasonal variation in chemical distributions in the Amazon (Solimões) River: A multiyear time series. Glob. Biogeochem. Cycles 1995, 9, 307–328. [Google Scholar] [CrossRef]
- Ellis, E.E.; Richey, J.E.; Aufdenkampe, A.K.; Krusche, A.V.; Quay, P.D.; Salimon, C.; da Cunha, H.B. Factors controlling water-column respiration in rivers of the central and southwestern Amazon Basin. Limnol. Oceanogr. 2012, 57, 527–540. [Google Scholar] [CrossRef]
- Cerling, T.E.; Solomon, D.K.; Quade, J.; Bowman, J.R. On the isotopic composition of carbon in soil carbon dioxide. Geochim. Cosmochim. Acta 1991, 55, 3403–3405. [Google Scholar] [CrossRef]
- Coleman, D.C. Compartmental Analysis of “Total Soil Respiration”: An Exploratory Study. Oikos 1973, 24, 361–366. [Google Scholar] [CrossRef]
- Pulliam, W.M. Carbon Dioxide and Methane Exports from a Southeastern Floodplain Swamp. Ecol. Monogr. 1993, 63, 29–53. [Google Scholar] [CrossRef]
- Jones, J.J.B.; Mulholland, P.J. Carbon Dioxide Variation in a Hardwood Forest Stream: An Integrative Measure of Whole Catchment Soil Respiration. Ecosystems 1998, 1, 183–196. [Google Scholar]
- Johnson, M.S.; Lehmann, J.; Riha, S.J.; Krusche, A.V.; Richey, J.E.; Ometto, J.P.H.B.; Couto, E.G. CO2 efflux from Amazonian headwater streams represents a significant fate for deep soil respiration. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.V.; Abril, G.; Darchambeau, F.; Teodoru, C.R.; Deborde, J.; Vidal, L.O.; Lambert, T.; Bouillon, S. Divergent biophysical controls of aquatic CO2 and CH4 in the World’s two largest rivers. Sci. Rep. 2015, 5, 15614. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, E.; Aufdenkampe, A.K.; Masiello, C.A.; Krusche, A.V.; Hedges, J.I.; Quay, P.D.; Richey, J.E.; Brown, T.A. Young organic matter as a source of carbon dioxide outgassing from Amazonian rivers. Nature 2005, 436, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Song, C.C.; Guo, Y.D. The spatiotemporal distribution of dissolved carbon in the main stems and their tributaries along the lower reaches of Heilongjiang River Basin, Northeast China. Environ. Sci. Pollut. Res. 2016, 23, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Bianchi, T.S.; Sampere, T.P. Temporal variability in the composition and abundance of terrestriallyderived dissolved organic matter in the lower Mississippi and Pearl Rivers. Mar. Chem. 2007, 103, 172–184. [Google Scholar] [CrossRef]
- Xu, Y.J.; DelDuco, E. Unravelling the Relative Contribution of Dissolved Carbon by the Red River to the Atchafalaya River. Water 2017, 9, 871. [Google Scholar] [CrossRef]
- Cummins, K.; Klug, J.; Wetzel, R.; Petersen, R.; Suberkropp, K.; Manny, B.; Wuycheck, J.; Howard, F. Organic enrichment with leaf leachate in experimental lotic ecosystems. Bioscience 1972, 22, 719–722. [Google Scholar] [CrossRef]
- Lush, D.L.; Hynes, H.B.N. The formation of particles in freshwater leachates of dead leaves. Limnol. Oceanogr. 1973, 18, 968–977. [Google Scholar] [CrossRef][Green Version]
- Otsuki, A.; Hanya, T. Production of dissolved organic matter from dead green algal cells. I. Aerobic microbial decomposition. Limnol. Oceanogr. 1972, 17, 248–257. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Sabater, F. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 2008, 1, 95–100. [Google Scholar] [CrossRef]
- Hern, S.; Lambou, V.W. Productivity Responses to Changes in Hydrological Regimes in the Atchafalaya Basin, Louisiana; Water and Land Quality Branch, Environmental Monitoring and Support Laboratory, US Environmental Protection Agency: Washington, DC, USA, 1978.
- Benner, R.; Opsahl, S. Molecular indicators of the sources and transformations of dissolved organic matter in the Mississippi River plume. Org. Geochem. 2001, 32, 597–611. [Google Scholar] [CrossRef]
- Kiffney, P.M.; Richardson, J.S.; Feller, M.C. Fluvial and epilithic organic matter dynamics in headwater streams of southwestern British Columbia, Canada. Arch. Hydrobiol. 2000, 149, 109–129. [Google Scholar] [CrossRef]
- Wallace, J.B.; Whiles, M.R.; Eggert, S.; Cuffney, T.F.; Lugthart, G.J.; Chung, K. Long-Term Dynamics of Coarse Particulate Organic Matter in Three Appalachian Mountain Streams. North Am. Benthol. Soc. 1995, 14, 217–232. [Google Scholar] [CrossRef]
- Raymond, P.A.; Oh, N.H.; Turner, R.E.; Broussard, W. Anthropogenically enhanced fluxes of water and carbon from the Mississippi River. Nature 2008, 451, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Raymond, P.A. Dissolved organic matter export from a forested watershed during Hurricane Irene. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Mata, L.J.; Arnell, N.W.; Döll, P.; Jimenez, B.; Miller, K.; Oki, T.; Şen, Z.; Shiklomanov, I. The implications of projected climate change for freshwater resources and their management. Hydrol. Sci. J. 2008, 53, 3–10. [Google Scholar] [CrossRef]
- Ren, W.; Tian, H.; Tao, B.; Yang, J.; Pan, S.; Cai, W.J.; Lohrenz, S.E.; He, R.; Hopkinson, C.S. Large increase in dissolved inorganic carbon flux from the Mississippi River to Gulf of Mexico due to climatic and anthropogenic changes over the 21st century. J. Geophys. Res. Biogeosci. 2015, 120, 724–736. [Google Scholar] [CrossRef]
- Tao, B.; Tian, H.Q.; Ren, W.; Yang, J.; Yang, Q.C.; He, R.Y.; Cai, W.J.; Lohrenz, S. Increasing Mississippi river discharge throughout the 21st century influenced by changes in climate, land use, and atmospheric CO2. Geophys. Res. Lett. 2014, 41, 4978–4986. [Google Scholar] [CrossRef]
| Sampling Event | Simmesport | Wax Lake Outlet | Morgan City Outlet | |||
|---|---|---|---|---|---|---|
| DIC | δ13CDIC | DIC | δ13CDIC | DIC | δ13CDIC | |
| 5/13/2015 | 1289 | −13.12 | 1480 | −13.20 | 1485 | −13.37 |
| 6/21/2015 | 1510 | −14.58 | 1719 | −13.85 | 1763 | −14.26 |
| 7/22/2015 | 1730 | −14.11 | 1884 | −13.89 | 1898 | −14.55 |
| 8/30/2015 | 2706 | −11.56 | 2232 | −11.12 | 2294 | −12.51 |
| 9/20/2015 | 2508 | −10.06 | 2416 | −10.26 | 2372 | −10.13 |
| 10/29/2015 | 1580 | −12.19 | 2258 | −12.05 | 2286 | −12.10 |
| 11/22/2015 | 1107 | −14.16 | 1283 | −14.06 | 1297 | −13.74 |
| 12/9/2015 | 972 | −14.78 | 1326 | −12.95 | 1497 | −13.47 |
| 1/31/2016 | n/a | n/a | n/a | n/a | n/a | n/a |
| 2/28/2016 | 1428 | −13.18 | 1366 | −13.61 | 1307 | −13.26 |
| 3/27/2016 | 672 | −14.86 | 811 | −14.37 | 835 | −14.96 |
| 4/17/2016 | 1118 | −16.19 | 1203 | −15.54 | 1147 | −15.60 |
| 5/19/2016 | 1239 | −14.65 | 1264 | −13.99 | 1317 | −14.55 |
| Sampling Event | Simmesport | Wax Lake Outlet | Morgan City Outlet | |||
|---|---|---|---|---|---|---|
| DOC | δ13CDOC | DOC | δ13CDOC | DOC | δ13CDOC | |
| 5/13/2015 | 508 | −27.67 | 786 | −28.98 | 384 | −26.76 |
| 6/21/2015 | 738 | −28.43 | 601 | −27.81 | 720 | −28.10 |
| 7/22/2015 | 519 | −27.68 | 548 | −27.81 | 548 | −27.93 |
| 8/30/2015 | 358 | −28.15 | 511 | −28.33 | 496 | −28.02 |
| 9/20/2015 | 515 | −28.79 | 522 | −28.25 | 442 | −29.41 |
| 10/29/2015 | 309 | −27.67 | 270 | −27.12 | 263 | −26.99 |
| 11/22/2015 | 1111 | −27.43 | 1172 | −27.62 | 925 | −27.67 |
| 12/9/2015 | 847 | −27.78 | 828 | −28.54 | 788 | −28.59 |
| 1/31/2016 | 1087 | −28.91 | 807 | −28.34 | 810 | −28.15 |
| 2/28/2016 | 635 | −27.85 | 766 | −27.90 | 743 | −28.48 |
| 3/27/2016 | 949 | −28.77 | 814 | −28.85 | 724 | −28.47 |
| 4/17/2016 | 731 | −28.15 | 759 | −28.08 | 712 | −27.90 |
| 5/19/2016 | 692 | −27.90 | 679 | −28.16 | 687 | −28.21 |
| Mean | 692 | −28.09 | 697 | −28.14 | 634 | −28.05 |
| Location | Q (km3) | DIC Load (Tg) | DOC Load (Tg) | |
|---|---|---|---|---|
| Input | Simmesport | 305.342 | 4.822 | 2.717 |
| Output | Wax Lake | 136.619 | 2.440 | 1.167 |
| Morgan City | 163.694 | 2.905 | 1.307 | |
| Combined | 300.313 | 5.346 | 2.473 | |
| Balance | (Output–Input) | −5.030 | 0.524 | −0.243 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DelDuco, E.M.; Xu, Y.J. Dissolved Carbon Transport and Processing in North America’s Largest Swamp River Entering the Northern Gulf of Mexico. Water 2019, 11, 1395. https://doi.org/10.3390/w11071395
DelDuco EM, Xu YJ. Dissolved Carbon Transport and Processing in North America’s Largest Swamp River Entering the Northern Gulf of Mexico. Water. 2019; 11(7):1395. https://doi.org/10.3390/w11071395
Chicago/Turabian StyleDelDuco, Emily M., and Y. Jun Xu. 2019. "Dissolved Carbon Transport and Processing in North America’s Largest Swamp River Entering the Northern Gulf of Mexico" Water 11, no. 7: 1395. https://doi.org/10.3390/w11071395
APA StyleDelDuco, E. M., & Xu, Y. J. (2019). Dissolved Carbon Transport and Processing in North America’s Largest Swamp River Entering the Northern Gulf of Mexico. Water, 11(7), 1395. https://doi.org/10.3390/w11071395






