Biochar from A Freshwater Macroalga as A Potential Biosorbent for Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Freshwater Macroalga
2.2. Pyrolysis of Freshwater Macroalgae
2.3. Yield of Biochars
2.4. Biosorption of Biochars
2.5. Analytical Techniques
2.5.1. Proximate Analysis
2.5.2. Surface Area Measurements
2.5.3. Scanning Electron Microscopy (SEM) Analysis
2.5.4. FT-IR Analysis
2.5.5. Spectrophotometric Method
2.5.6. Multi-Elemental Composition
3. Results and Discussion
3.1. Yield of Algal Biochar and Its Proximate Analysis
3.2. Mercury Porosimetry
3.3. Multi-elemental Composition of Biochars
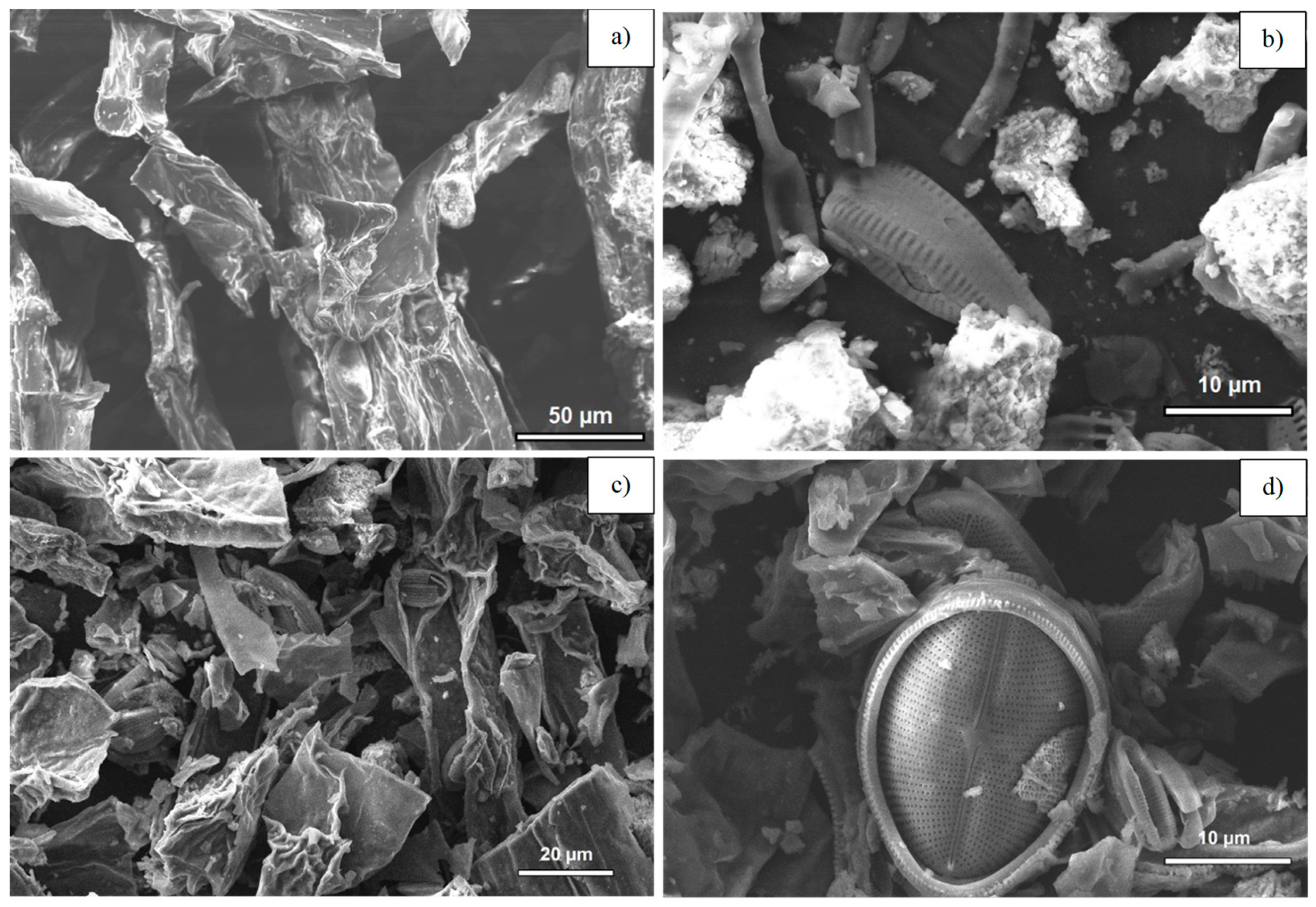
3.4. Scanning Electron Microscopy of a Raw Alga and the Resultant Biochars
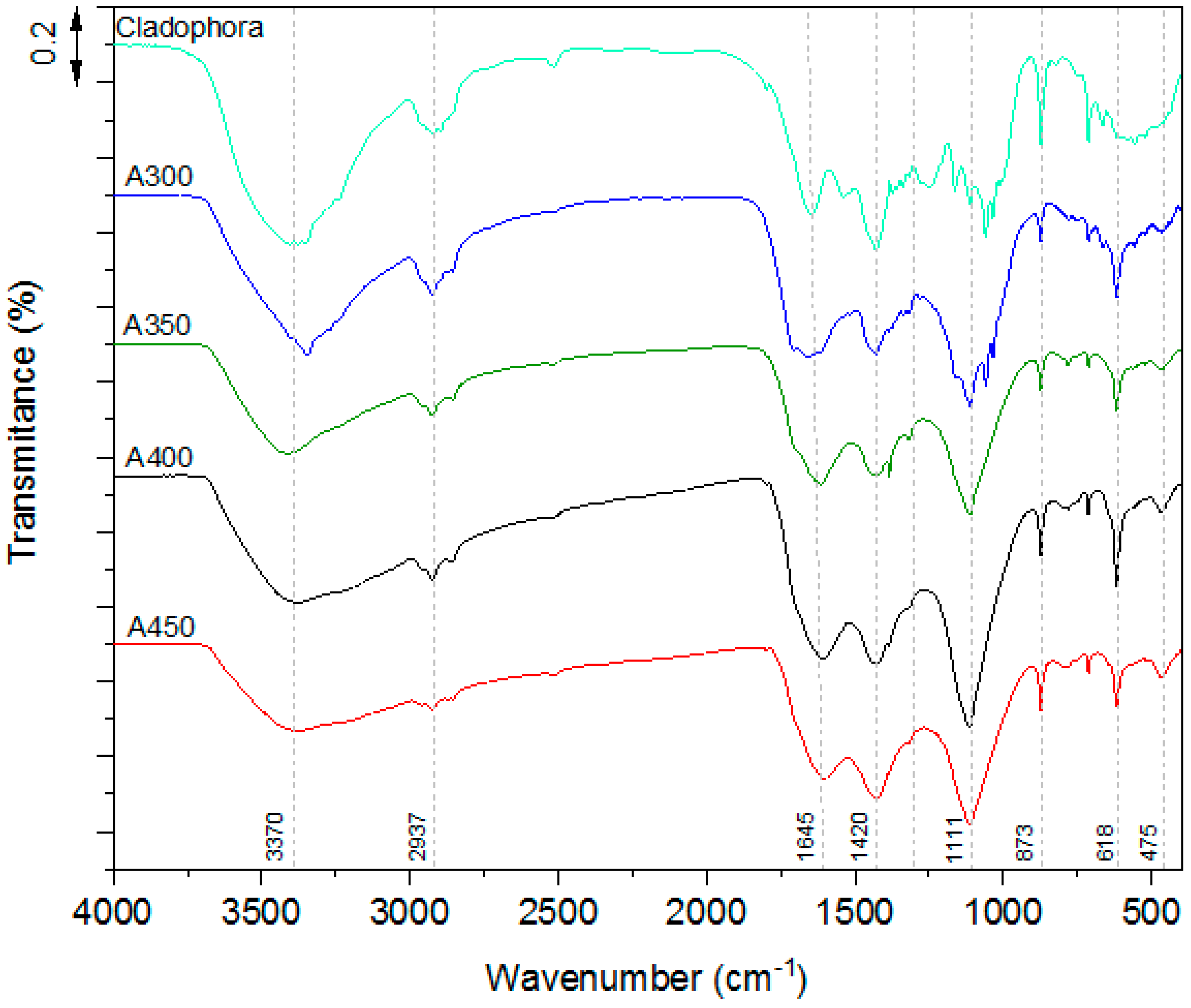
3.5. FT-IR Spectra of a Raw Alga and Produced Biochars
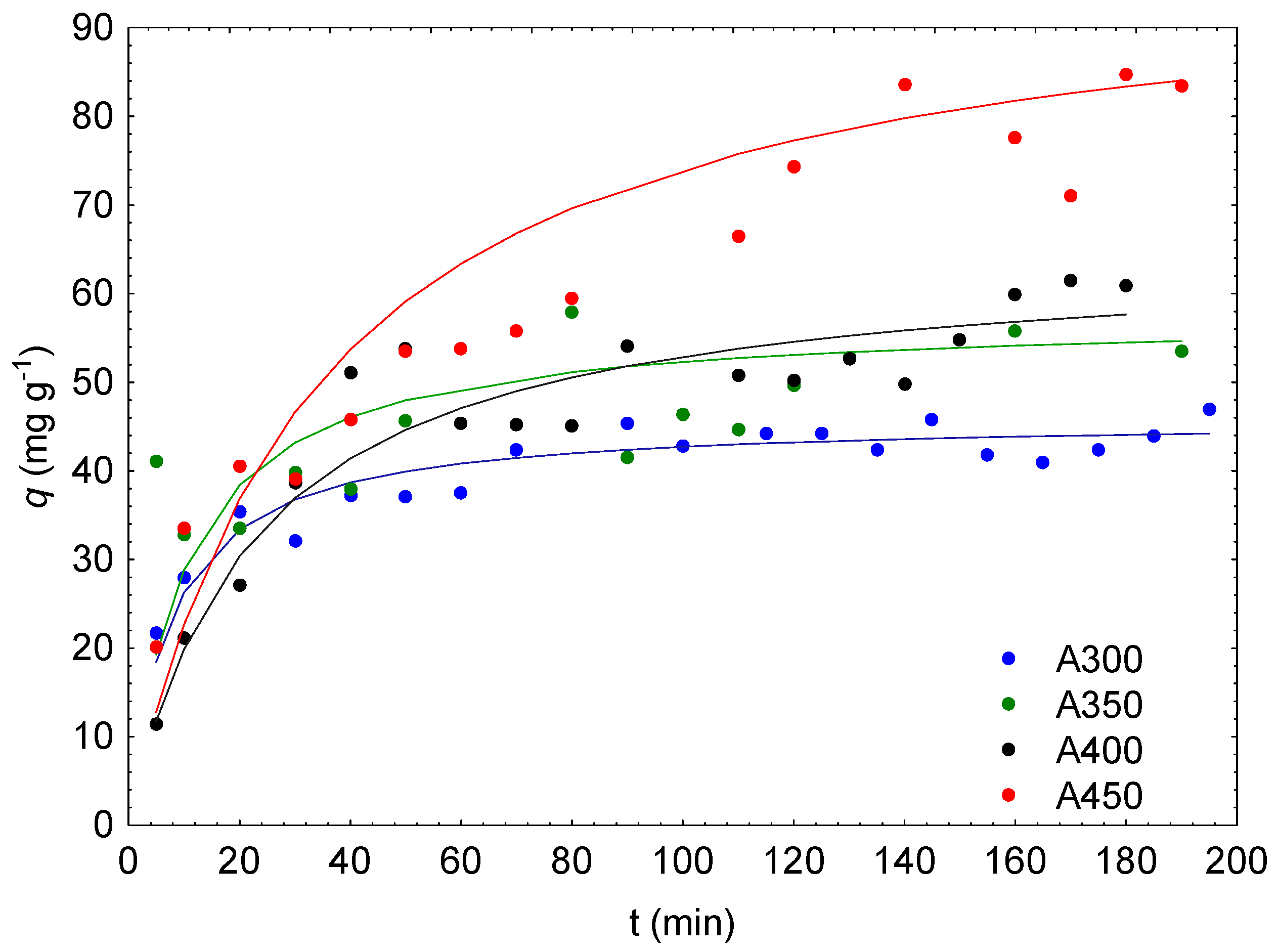
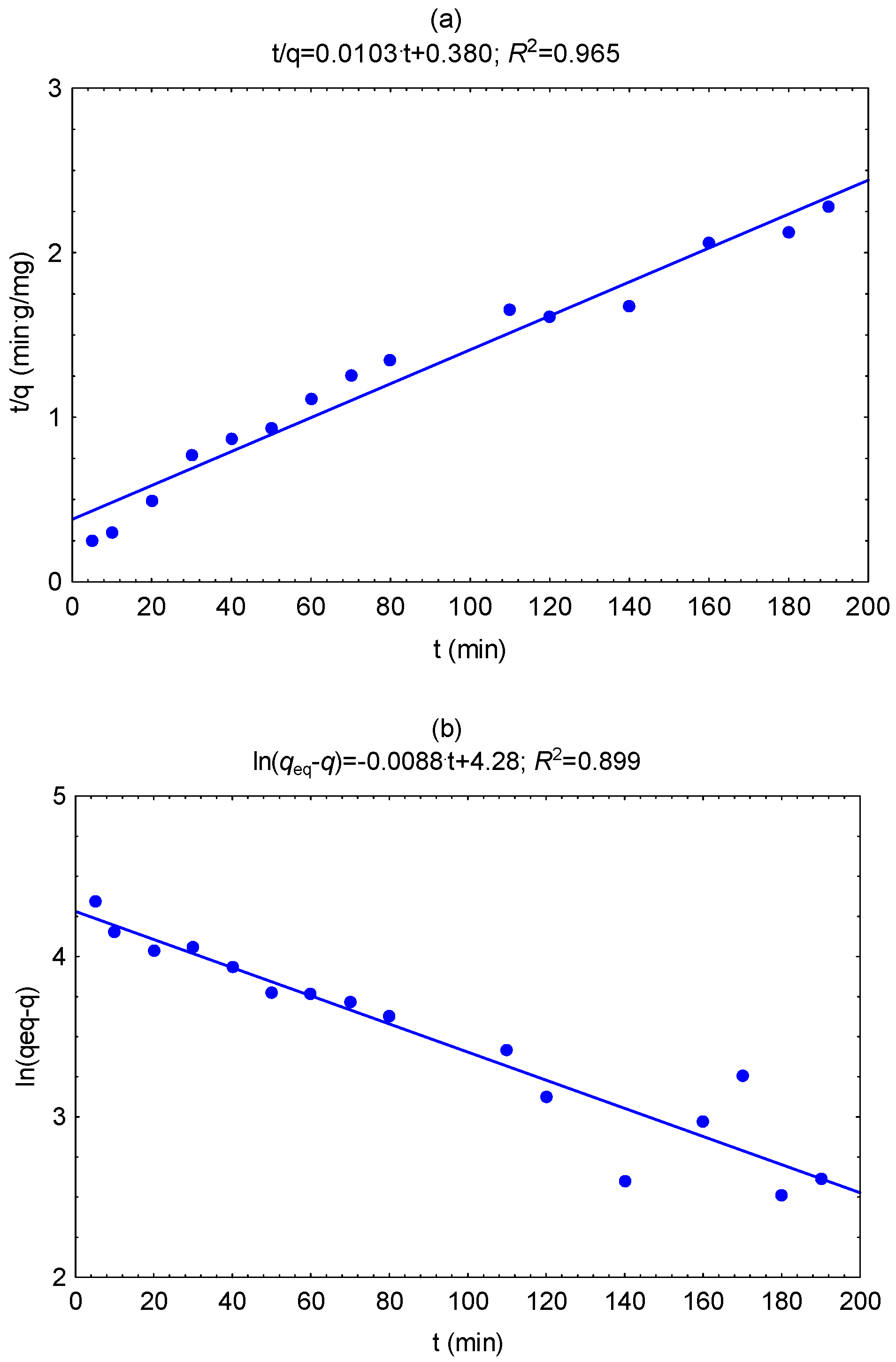
3.6. Biosorption Properties of Biochars
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bird, M.; Wurster, C.M.; de Paula Silva, P.H.; Bass, A.M.; de Nys, R. Algal biochar—production and properties. Bioresour. Technol. 2011, 102, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Mihranyan, A. Cellulose from Cladophorales green algae: From environmental problem to high-tech composite materials. J. Appl. Polymer Sci. 2011, 119, 2449–2460. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Bio/Technol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Chaiwong, K.; Kiatsiriroat, T.; Vorayos, N.; Thararax, C. Biochar production from freshwater algae by slow pyrolysis. Maejo Int. J. Sci. Technol. 2012, 6, 186–195. [Google Scholar]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.H.; Ng, E.P.; Chang, J.S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Poo, K.M.; Son, E.B.; Chan, J.S.; Ren, X.H.; Choi, Y.J.; Chae, K.J. Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefin. 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Son, E.B.; Poo, K.M.; Chang, J.S.; Chae, K.J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef]
- Perez-Ameneiro, M.; Bustos, G.; Vecino, X.; Barbosa-Pereira, L.; Cruz, J.M.; Moldes, A.B. Heterogenous lignocellulosic composites as bio-based adsorbents for wastewater dye removal: A kinetic comparison. Water Air Soil Pollut. 2015, 226, 133. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Villagrasa, S.; Cruz, J.M.; Moldes, A.B. Kinetic and morphology study of alginate-vineyard pruning waste biocomposite vs. non modified vineyard pruning waste for dye removal. J. Environ. Sci. 2015, 38, 158–167. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Paradelo, R.; Igrexas-Soto, A.; Nunez-Delgado, A.; Fernandez-Sanjurjo, M.J.; Alvarez-Rodriguez, E.; Garrote, G.; Novoa-Munoz, J.C.; Arias-Estevez, M. Valorization of biosorbent obtained from a forestry waste: Competitive adsorption, desorption and transport of Cd, Cu, Ni, Pb and Zn. Ecotox. Environ. Saf. 2016, 131, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Cai, L.; Guo, J.; Wang, Y.; Ji, L.; Song, W. Preparation and characterization of macroalgae biochar nanomaterials with highly efficient adsorption and photodegradation ability. Materials 2018, 11, 1709. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Kim, K.; Jeong, T.U.; Ahn, K.H. Influence of pyrolysis temperature on characteristics and phosphate adsorption capability of biochar derived from waste-marine macroalgae (Undaria pinnatifida roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Production and characterization of a value added biochar mix using seaweed, rice husk and pine sawdust: A parametric study. J. Cleaner Prod. 2018, 200, 641–656. [Google Scholar] [CrossRef]
- Cole, A.J.; Paul, N.A.; de Nys, R.; Roberts, D.A. Good for sewage treatment and good for agriculture: Algal based compost and biochar. J. Environ. Manag. 2017, 200, 105–113. [Google Scholar] [CrossRef] [PubMed]
- De Ramon N’Yeurt, A.; Iese, V. The proliferating brown alga Sargassum polycystum in Tuvalu South Pacific: Assessment of the bloom and applications to local agriculture and sustainable energy. J. Appl. Phycol. 2015, 27, 2037–2045. [Google Scholar] [CrossRef]
- Kiran, H. Application of biochar technologies to wastewater treatment. Ph.D Thesis, Massey University, Palmerston North, New Zealand, 2013. Available online: https://muir.massey.ac.nz/bitstream/handle/10179/4288/02_whole.pdf (accessed on 21 September 2017).
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Jiang, Y.; Lei, M.; Duan, L.; Longhurst, P. Integrating phytoremediation with biomass valorisation and critical element recovery: A UK contaminated land perspective. Biomass Bioenerg. 2015, 83, 328–339. [Google Scholar] [CrossRef]
- Kidgell, J.T.; de Nys, R.; Hu, Y.; Paul, N.A.; Roberts, D.A. Bioremediation of a complex industrial effluent by biosorbents derived from freshwater macroalgae. PLoS ONE 2014, 9, e94706. [Google Scholar] [CrossRef]
- Johansson, C.L.; Paul, N.A.; de Nys, R.; Roberts, D.A. Simultaneous biosorption of selenium, arsenic and molybdenum with modified algal-based biochars. J. Environ. Manag. 2016, 165, 117–123. [Google Scholar] [CrossRef]
- Starmach, K. Family: Cladophora Kutzing 1843. Identification Key; Freshwater Biological Association: Windermere, UK, 1975. [Google Scholar]
- Ni, Y.; Chen, S.; Kokot, S. Spectrophotometric determination of metal ions in electroplating solutions in the presence of EDTA with the aid of multivariate calibration and artificial neural networks. Anal. Chim. Acta 2002, 463, 305–316. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. The new application of biosorption properties of Enteromorpha prolifera. Appl. Biochem. Biotechnol. 2010, 160, 1540–1556. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, K.; Marycz, K.; Michalak, I. Freshwater green macroalgae as a biosorbent of Cr(III) ions. Open Chem. 2018, 16, 689–701. [Google Scholar] [CrossRef]
- Order of the Minister of Environment of November 18, 2014 laying down conditions for the introduction of sewage into Water Bodies or Soil and Laying Down the List of Substances Particularly Harmful to Water Environments, J. Laws No. 2014 Item 1800. [Rozporządzenie Ministra Środowiska z dnia 18 listopada 2014 r. w Sprawie Warunków, Jakie Należy Spełnić Przy Wprowadzaniu Ścieków do Wód Lub do Ziemi, Oraz w Sprawie Substancji Szczególnie Szkodliwych dla Środowiska Wodnego, Dz.U. 2014 poz. 1800]; Internetowy System Aktów Prawnych: Warsaw, Poland, 2014.
- Solid Biofuels. Determination of Moisture Content. Oven Dry Method. Part 2: Total Moisture. Simplified Method; PN-EN ISO 18134-2:2017-03; The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warszawa, Poland, 2017.
- Solid Mineral Fuels. Determination of Ash; PN-ISO 1171:2002; The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warszawa, Poland, 2002.
- Solid Biofuels. Determination of The Content of Volatile Matter; PN-EN ISO 18123:2016-01; The Polish Committee for Standardization (Polski Komitet Normalizacyjny—PKN): Warszawa, Poland, 2018.
- Wang, H.; Zhang, M.; Lv, Q. Removal efficiency and mechanism of Cr(VI) from aqueous solution by maize straw biochars derived at different pyrolysis temperatures. Water 2019, 11, 781. [Google Scholar] [CrossRef]
- Greenwood, J.L.; Clason, T.A.; Lowe, R.L.; Belanger, S.E. Examination of endopelic and epilithic algal community structure employing scanning electron microscopy. Freshwater Biol. 1999, 41, 821–828. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Yang, H.; Gentili, F.G.; Söderlind, U.; Wang, X.; Zhang, W.; Chen, H. Hydrothermal carbonization of natural microalgae containing a high ash content. Fuel 2019, 249, 441–448. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process—a review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Mironiuk, M.; Marycz, K. A comprehensive analysis of biosorption of metal ions by macroalgae using ICP-OES, SEM-EDX and FTIR techniques. PLoS ONE 2018, 13, e0205590. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Luo, L.; Xu, C.; Chen, Z.; Zhang, S. Properties of biomass-derived biochars: Combined effects of operating conditions and biomass types. Bioresour. Technol. 2015, 192, 83–89. [Google Scholar] [CrossRef]
- Gokulan, R.; Prabhu, G.G.; Jegan, J. Remediation of complex remazol effluent using biochar derived from green seaweed biomass. Int. J. Phytoremed. 2019. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetic of adsorption on carbon from solutions. J. Sanit. Eng. Div. ASCE 1963, 89, 31–60. [Google Scholar]
- Erhan, D.; Kobya, M.; Ehf, S.; Ozkan, T. Adsorption kinetics for Cr (VI) removal from aqueous solution on activated carbon prepared from Agro wastes. J. Water 2004, 30, 533–541. [Google Scholar]
- Thivya, J.; Vijayaraghavan, J. Single and binary sorption of reactive dyes onto red seaweed-derived biochar: Multi-component isotherm and modelling. Desal. Water Treat. 2019, 156, 87–95. [Google Scholar] [CrossRef]

| Sample | Moisture (Ma) | Ash (Ad) | Volatile matter (VMdaf) | Yield of bio-char (Y) |
|---|---|---|---|---|
| (wt.%) | (wt.%) | (wt.%) | (wt.%) | |
| Cladophora glomerata | 6.1 ± 0.1 | 19.7 ± 0.2 | 84.5 ± 0.8 | - |
| A300 | 1.5 ± 0.0 | 30.3 ± 0.3 | 68.9 ± 0.7 | 63 ± 2 |
| A350 | 1.7 ± 0.0 | 35.6 ± 0.4 | 60.1 ± 0.6 | 56 ± 1 |
| A400 | 1.9 ± 0.0 | 39.1 ± 0.4 | 53.0 ± 0.5 | 50 ± 1 |
| A450 | 1.5 ± 0.0 | 40.1 ± 0.4 | 50.0 ± 0.5 | 47 ± 1 |
| Sample | Total Intrusion Volume | Total Pore Area | Apparent (Skeletal) Density | Median Pore Diameter (Area) | Average Pore Diameter (4V/A) | Porosity |
|---|---|---|---|---|---|---|
| - | cm3 g−1 | m² g−1 | g cm−3 | nm | nm | % |
| A300 | 0.557 ± 0.028 | 20 ± 1 | 1.64 ± 0.08 | 7.6 ± 0.2 | 110 ± 3 | 48 ± 2 |
| A350 | 0.727 ± 0.036 | 19 ± 1 | 1.66 ± 0.08 | 7.7 ± 0.2 | 156 ± 4 | 55 ± 3 |
| A400 | 0.806 ± 0.040 | 19 ± 1 | 1.54 ± 0.08 | 7.9 ± 0.2 | 166 ± 4 | 55 ± 3 |
| A450 | 0.773 ± 0.039 | 21 ± 1 | 1.57 ± 0.08 | 7.3 ± 0.2 | 148 ± 4 | 55 ± 3 |
| Element | Wavelength (nm) | Biochar (mg kg−1 dry basis) | |||
|---|---|---|---|---|---|
| A300 | A350 | A400 | A450 | ||
| Al | 308.21 | 133 ± 20 | 126 ± 19 | 160 ± 24 | 217 ± 33 |
| Ca | 315.89 | 13,577 ± 2715 | 11,654 ± 2331 | 14,610 ± 2922 | 17,977 ± 3595 |
| Cr | 267.72 | 4.85 ± 0.73 | 1.30 ± 0.19 | 1.90 ± 0.28 | 1.03 ± 0.15 |
| Cu | 324.75 | 4.95 ± 0.74 | 0.277 ± 0.042 | 1.15 ± 0.17 | 4.24 ± 0.64 |
| Fe | 259.94 | 471 ± 71 | 455 ± 68 | 57.0 ± 8.6 | 750 ± 112 |
| K | 766.49 | 6218 ± 1243 | 6435 ± 1287 | 7835 ± 1567 | 10,511 ± 2102 |
| Mg | 285.21 | 812 ± 122 | 728 ± 109 | 887 ± 133 | 1158 ± 232 |
| Mn | 257.61 | 182 ± 27 | 202 ± 30 | 248 ± 37 | 310 ± 46 |
| Na | 588.99 | 619 ± 93 | 442 ± 66 | 463 ± 69 | 705 ± 106 |
| P | 213.62 | 761 ± 114 | 752 ± 113 | 906 ± 136 | 1252 ± 250 |
| Pb | 220.35 | <LOD | <LOD | <LOD | 2.29 ± 0.30 |
| Si | 251.61 | 25.8 ± 3.9 | 14.5 ± 2.2 | 16.6 ± 2.5 | 6.73 ± 1.01 |
| Zn | 213.86 | 7.86 ± 1.18 | 3.28 ± 0.49 | 4.42 ± 0.66 | 8.08 ± 1.21 |
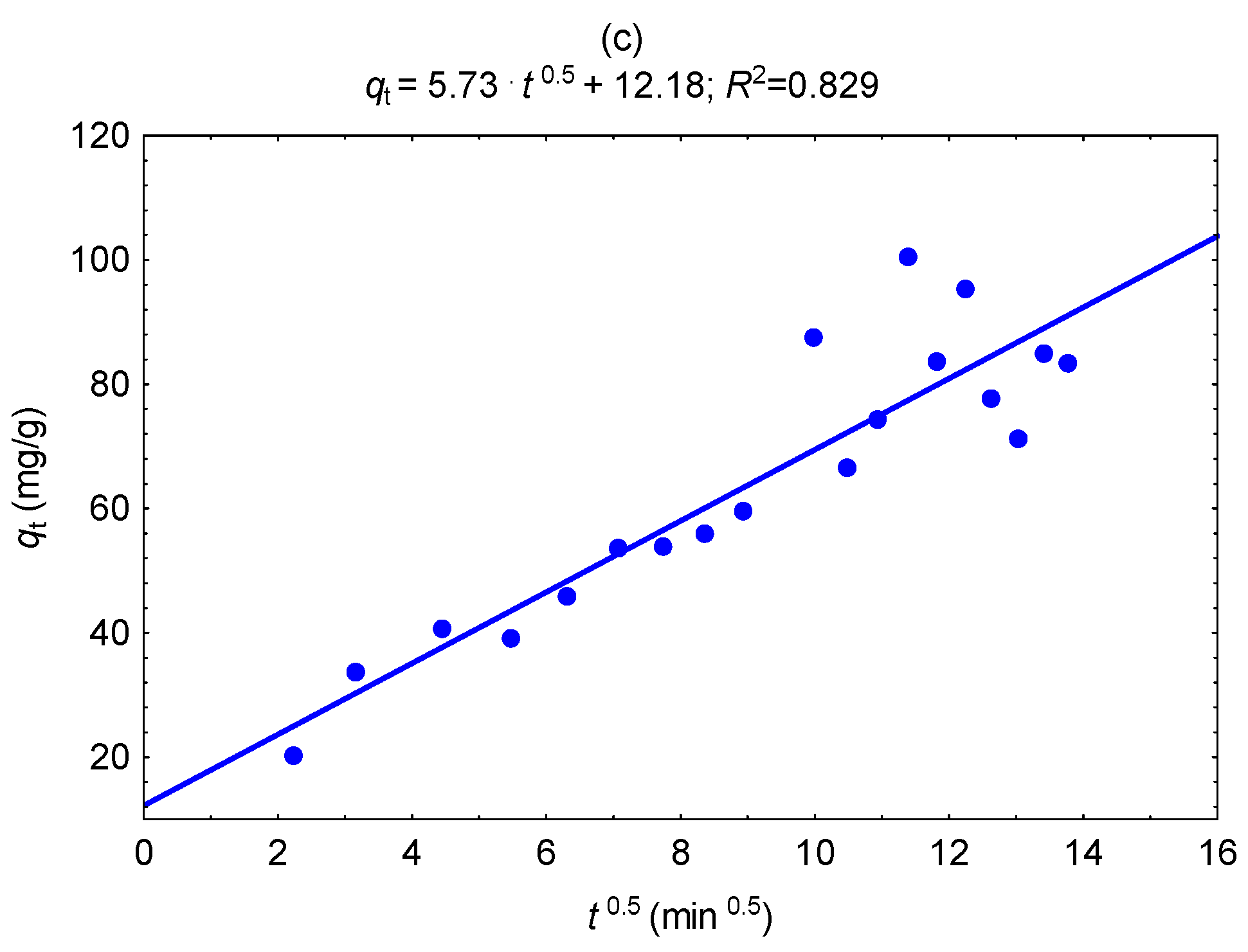
| Biochar | Pseudo-second Order Model | Weber and Morris Diffusion | ||||
|---|---|---|---|---|---|---|
| qeq2 (mg g−1) | k2 (g mg−1 min−1) | R2 | kp (mg g−1 min−0.5) | C (mg g−1) | R2 | |
| A300 | 45.9 | 0.00292 | 0.991 | 1.30 | 28.5 | 0.680 |
| A350 | 57.5 | 0.00175 | 0.925 | 2.09 | 26.2 | 0.866 |
| A400 | 64.9 | 0.000678 | 0.965 | 3.57 | 15.4 | 0.756 |
| A450 | 87.1 | 0.000279 | 0.965 | 5.73 | 12.2 | 0.829 |
| Element | Wavelength (nm) | Concentration of Metal Ions (mg L−1) in Wastewater: | |
|---|---|---|---|
| Before Biosorption | After Biosorption | ||
| Ca | 315.89 | <LOD | 10.8 ± 1.6 |
| Cr | 267.72 | 1.02 ± 0.15 | 0.104 ± 0.016 |
| Cu | 324.75 | 0.412 ± 0.062 | 0.014 ± 0.003 |
| K | 766.49 | 1.67 ± 0.25 | 29.7 ± 4.5 |
| Mg | 285.21 | <LOD | 1.57 ± 0.24 |
| Na | 588.99 | 6.18 ± 0.93 | 7.38 ± 1.11 |
| Zn | 213.85 | 3.72 ± 0.56 | 0.234 ± 0.035 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, I.; Baśladyńska, S.; Mokrzycki, J.; Rutkowski, P. Biochar from A Freshwater Macroalga as A Potential Biosorbent for Wastewater Treatment. Water 2019, 11, 1390. https://doi.org/10.3390/w11071390
Michalak I, Baśladyńska S, Mokrzycki J, Rutkowski P. Biochar from A Freshwater Macroalga as A Potential Biosorbent for Wastewater Treatment. Water. 2019; 11(7):1390. https://doi.org/10.3390/w11071390
Chicago/Turabian StyleMichalak, Izabela, Sylwia Baśladyńska, Jakub Mokrzycki, and Piotr Rutkowski. 2019. "Biochar from A Freshwater Macroalga as A Potential Biosorbent for Wastewater Treatment" Water 11, no. 7: 1390. https://doi.org/10.3390/w11071390
APA StyleMichalak, I., Baśladyńska, S., Mokrzycki, J., & Rutkowski, P. (2019). Biochar from A Freshwater Macroalga as A Potential Biosorbent for Wastewater Treatment. Water, 11(7), 1390. https://doi.org/10.3390/w11071390







