Comparison of Otolith Readability and Reproducibility of Counts of Translucent Zones Using Different Otolith Preparation Methods for Four Endemic Labeobarbus Species in Lake Tana, Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
2.3. Otolith Preparation Methods
2.3.1. Whole Otolith Submerged in Glycerol and Water
2.3.2. Sectioned and Stained Otoliths
2.3.3. Preliminary Evaluation of Otolith Preparation Techniques
3. Statistical Analysis
Translucent Zone Count Precision and Bias
4. Results
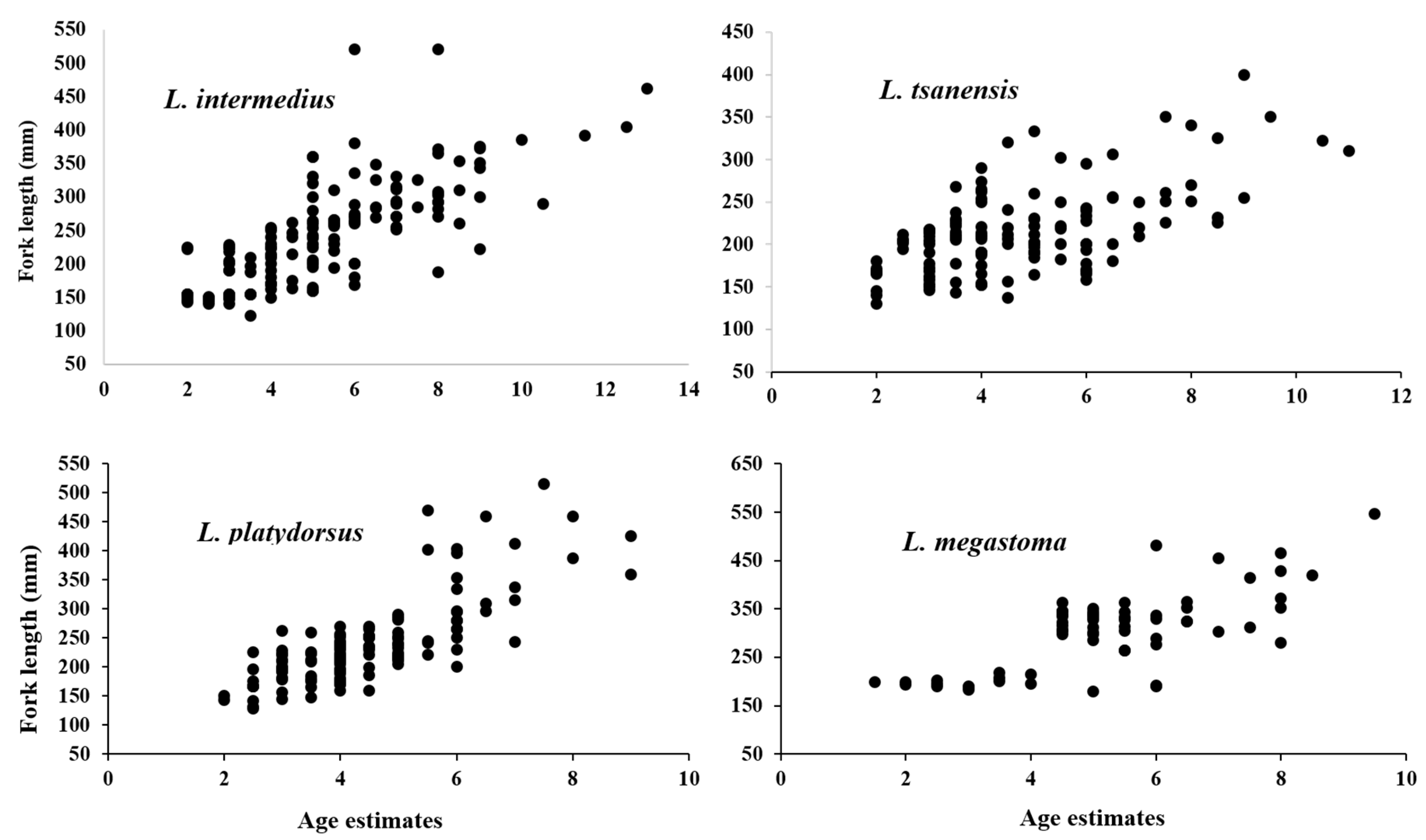
4.1. Length Frequency
4.2. Otolith Readability
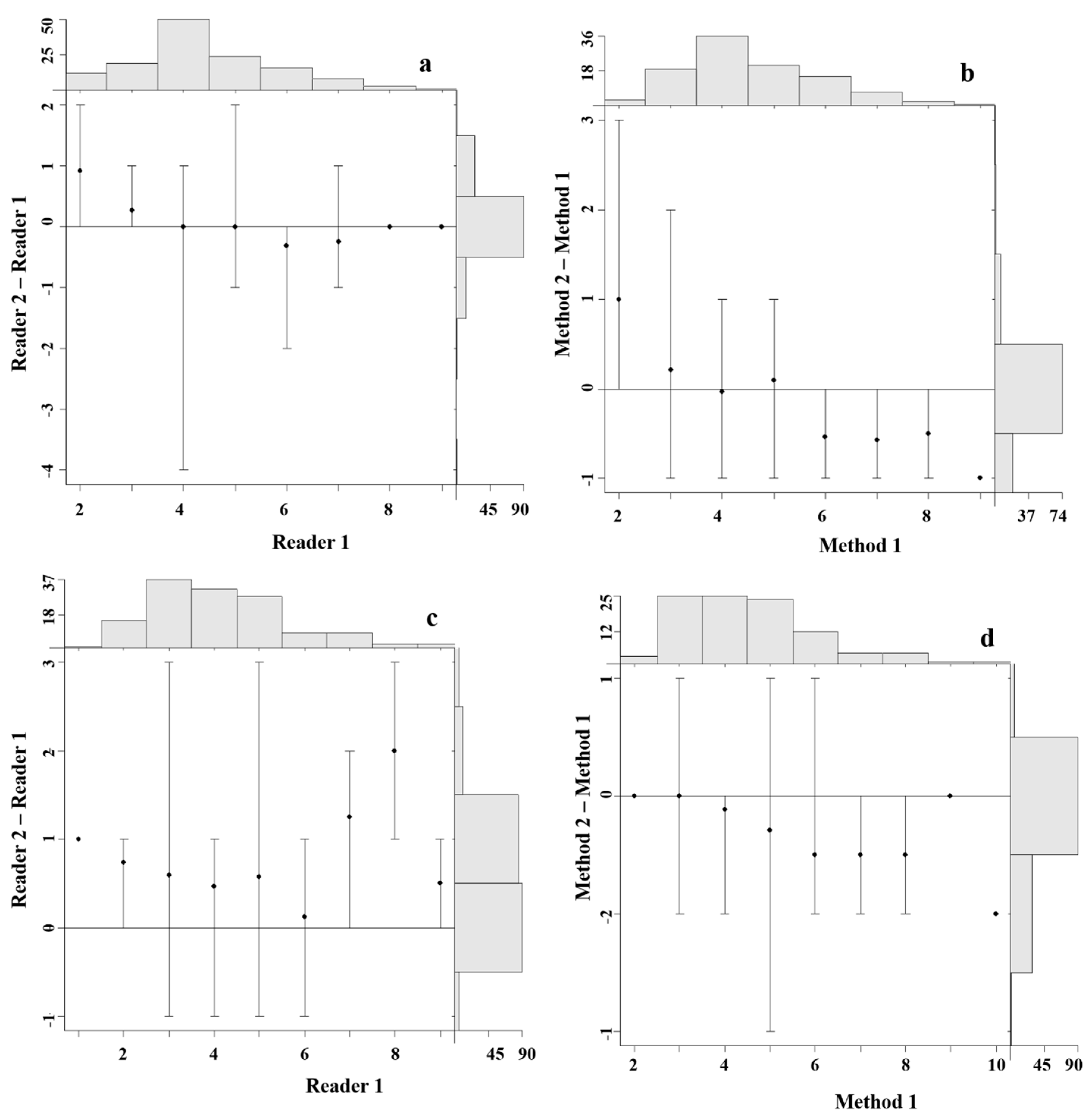
4.3. Precision and Bias between Readers
4.4. Precision and Bias among Aging Methods
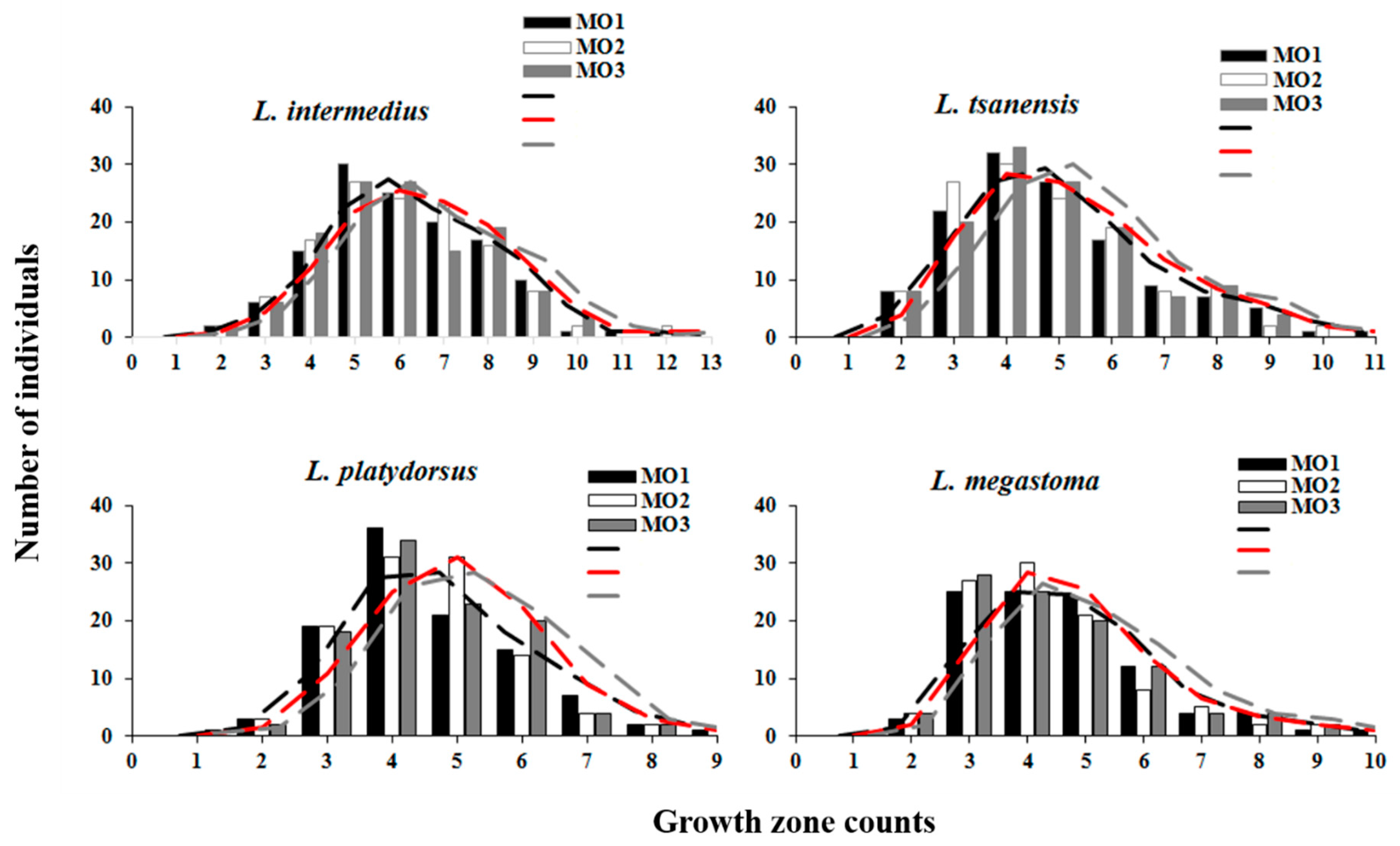
4.5. Statistical Comparisons of Growth Zones Counts between Readers and Methods
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

Appendix C
| Statistics | Readers and Methods Pairs | |||||
|---|---|---|---|---|---|---|
| Reader 1 vs. Reader 2 (MO1) | Reader1 vs. Reader 2 (MO2) | Reader 1 vs. Reader 2 (MO3) | MO1 vs. MO2 | MO1 vs. MO3 | MO2 vs. MO3 | |
| L. intermedius | ||||||
| Regression | ||||||
| Slope | 0.97 ± 0.03 | 0.92 ± 0.04 | 0.95 ± 0.04 | 0.99 ± 0.02 | 1.00 ± 0.03 | 0.92 ± 0.02 |
| p | 0.392 | 0.033 | 0.248 | 0.660 | 0.856 | 0.000 |
| Intercept | 0.31 ± 0.20 | 0.84 ± 0.23 | 0.57 ± 0.26 | 0.03 ± 0.15 | −0.01 ± 0.21 | 0.42 ± 0.15 |
| p | 0.137 | 0.000 | 0.029 | 0.826 | 0.979 | 0.005 |
| Paired t-test | ||||||
| Mean difference | −0.139 | −0.345 | −0.286 | 0.031 | −0.031 | −0.063 |
| p | 0.027 | 0.000 | 0.000 | 0.482 | 0.608 | 0.184 |
| L. tsanensis | ||||||
| Regression | ||||||
| Slope | 0.92 ± 0.03 | 0.89 ± 0.04 | 0.87 ± 0.04 | 0.90 ± 0.03 | 0.90 ± 0.04 | 0.91 ± 0.03 |
| p | 0.012 | 0.007 | 0.000 | 0.002 | 0.009 | 0.002 |
| Intercept | 0.56 ± 0.16 | 0.72 ± 0.19 | 0.68 ± 0.18 | 0.39 ± 0.17 | 0.54 ± 0.21 | 0.29 ± 0.15 |
| p | 0.000 | 0.000 | 0.000 | 0.021 | 0.010 | 0.060 |
| Paired t-test | ||||||
| Mean difference | −0.175 | −0.231 | −0.063 | −0.116 | 0.031 | 0.147 |
| p | 0.006 | 0.002 | 0.372 | 0.063 | 0.682 | 0.007 |
| L. platydorsus | ||||||
| Regression | ||||||
| Slope | 0.83 ± 0.04 | 0.81 ± 0.56 | 0.87 ± 0.05 | 0.79 ± 0.04 | 0.82 ± 0.05 | 0.90 ± 0.03 |
| p | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 | 0.002 |
| Intercept | 0.09 ± 0.17 | 1.02 ± 0.24 | 0.73 ± 0.20 | 0.91 ± 0.20 | 0.81 ± 0.22 | 0.43 ± 0.15 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.006 |
| Paired t-test | ||||||
| Mean difference | −0.068 | −0.235 | −0.163 | 0.058 | 0.019 | −0.038 |
| p | 0.273 | 0.001 | 0.012 | 0.368 | 0.774 | 0.374 |
| L. megastoma | ||||||
| Regression | ||||||
| Slope | 1.04 ± 0.04 | 1.17 ± 0.06 | 1.12 ± 0.05 | 0.89 ± 0.03 | 0.95 ± 0.03 | 0.88 ± 0.03 |
| p | 0.378 | 0.006 | 0.018 | 0.000 | 0.145 | 0.000 |
| Intercept | 0.45 ± 0.19 | 0.12 ± 0.25 | 0.18 ± 0.21 | 0.30 ± 0.15 | 0.12 ± 0.16 | 0.41 ± 0.15 |
| p | 0.020 | 0.618 | 0.391 | 0.048 | 0.460 | 0.007 |
| Paired t-test | ||||||
| Mean difference | −0.609 | −0.760 | −0.658 | 0.212 | 0.101 | −0.111 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.049 | 0.033 |
References
- Nagelkerke, L.A.; Sibbing, F.A.; van den Boogaart, J.G.; Lammens, E.H.; Osse, J.W. The barbs (Barbus spp.) of Lake Tana: A forgotten species flock? Environ. Biol. Fishes 1994, 39, 1–22. [Google Scholar] [CrossRef]
- Anteneh, W.; Getahun, A.; Dejen, E.; Sibbing, F.A.; Nagelkerke, L.A.J.; De Graaf, M.; Wudneh, T.; Vijverberg, J.; Palstra, A.P. Spawning migrations of the endemic Labeobarbus (Cyprinidae, Teleostei) species of Lake Tana, Ethiopia: Status and threats. J. Fish Biol. 2012, 81, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, F.A.; Nagelkerke, L.A.J. Resource partitioning by Lake Tana barbs predicted from fish morphometrics and prey characteristics. Rev. Fish Biol. Fish. 2001, 10, 393–437. [Google Scholar] [CrossRef]
- de Graaf, M.; Machiels, M.A.M.; Wudneh, T.; Sibbing, F.A. Declining stocks of Lake Tana’s endemic Barbus species flock (Pisces, Cyprinidae): Natural variation or human impact? Biol. Conserv. 2004, 116, 277–287. [Google Scholar] [CrossRef]
- de Graaf, M.; van Zwieten, P.A.M.; Machiels, M.A.M.; Lemma, E.; Wudneh, T.; Dejen, E.; Sibbing, F.A. Vulnerability to a small-scale commercial fishery of Lake Tana’s (Ethiopia) endemic Labeobarbus compared with African catfish and Nile tilapia: An example of recruitment-overfishing? Fish. Res. 2006, 82, 304–318. [Google Scholar] [CrossRef]
- Dejen, E.; Anteneh, W.; Vijverberg, J. The Decline of the Lake Tana (Ethiopia) Fisheries: Causes and Possible Solutions. Land Degrad. Dev. 2017, 28, 1842–1851. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Getahun, A.; Anteneh, W.; Bruneel, S.; Goethals, P. A Drivers-Pressure-State-Impact-Responses Framework to Support the Sustainability of Fish and Fisheries in Lake Tana, Ethiopia. Sustainability 2018, 10, 2957. [Google Scholar] [CrossRef]
- Getahun, A. Labeobarbus species. In The IUCN Red List of Threatened Species 2010; International Union for Conservation of Nature and Natural Resources: Gland, Switsersland, 2010. [Google Scholar]
- Snoeks, J.; Laleye, P.; Getahun, A.; Contreras-MacBeath, T. Labeobarbus macrophtalmus. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switsersland, 2010. [Google Scholar]
- Nagelkerke, L.A.; Mina, M.V.; Wudneh, T.; Sibbing, F.A.; Osse, J.W. In Lake Tana, a unique fish fauna needs protection. Bioscience 1995, 45, 772–775. [Google Scholar]
- Nagelkerke, L.A.; Sibbing, F.A. The large barbs (Barbus spp., Cyprinidae, Teleostei) of Lake Tana (Ethiopia), with a description of a new species, Barbus osseensis. Neth. J. Zool. 2000, 50, 179–214. [Google Scholar] [CrossRef]
- de Graaf, M.; Machiels, M.; Wudneh, T.; Sibbing, F. Length at maturity and gillnet selectivity of Lake Tana’s Barbus species (Ethiopia): Implications for management and conservation. Aquat. Ecosyst. Health Manag. 2003, 6, 325–336. [Google Scholar] [CrossRef]
- de Graaf, M. Lake Tana’s Piscivorous Barbus (Cyprinidae, Ethiopia) Ecology-Evolution-Exploitation; WIAS: Berlin, Germany, 2003. [Google Scholar]
- Palstra, A.P.; de Graaf, M.; Sibbing, F.A. Riverine spawning and reproductive segregation in a lacustrine cyprinid species flock, facilitated by homing? Anim. Biol. 2004, 54, 393–415. [Google Scholar]
- Anteneh, W.; Getahun, A.; Dejen, E. Spawning migrations of Lake Tana Labeobarbus spp. (Teleostei: Cyprinidae) in the Ribb River, Ethiopia. Afr. J. Aquat. Sci. 2013, 38, 61–68. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Mingist, M.; Getahun, A.; Anteneh, W. Spawning migration of Labeobarbus spp. (Pisces: Cyprinidae) of Lake Tana to Arno-Garno river, Lake Tana sub-basin, Ethiopia. SINET Ethiop. J. Sci. 2012, 35, 95–106. [Google Scholar]
- Gebremedhin, S.; Getahun, A.; Anteneh, W.; Gedif, B.; Gashu, B.; Tefera, B.; Berhanie, Z.; Alemaw, D. Effect of large weirs on abundance and diversity of migratory Labeobarbus species in tributaries of Lake Tana, Ethiopia. Afr. J. Aquat. Sci. 2017, 42, 367–373. [Google Scholar]
- Dwyer, K.S.; Walsh, S.J.; Campana, S.E. Age determination, validation and growth of Grand Bank yellowtail flounder (Limanda ferruginea). ICES J. Mar. Sci. 2003, 60, 1123–1138. [Google Scholar] [CrossRef]
- Campana, S. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Beamish, R.J.; McFarlane, G. The forgotten requirement for age validation in fisheries biology. Trans. Am. Fish. Soc. 1983, 112, 735–743. [Google Scholar] [CrossRef]
- Marriott, R.J.; Mapstone, B.D. Consequences of inappropriate criteria for accepting age estimates from otoliths, with a case study for a long-lived tropical reef fish. Can. J. Fish. Aquat. Sci. 2006, 63, 2259–2274. [Google Scholar] [CrossRef]
- Braaten, P.J.; Doeringsfeld, M.R.; Guy, C.S. Comparison of age and growth estimates for river carpsuckers using scales and dorsal fin ray sections. N. Am. J. Fish. Manag. 1999, 19, 786–792. [Google Scholar] [CrossRef]
- Maceina, M.; Sammons, S. An evaluation of different structures to age freshwater fish from a northeastern US river. Fish. Manag. Ecol. 2006, 13, 237–242. [Google Scholar] [CrossRef]
- Kanwal, B.; Pathani, S. Age-growth, length-weight and condition factor of a hill stream fish, Gara lamta (Hamilton Buchanan) of Kumaun Himalaya. Nat. Sci. 2011, 9, 199–206. [Google Scholar]
- Khan, S.; Khan, M.A.; Miyan, K. Comparison of age estimates from otoliths, vertebrae, and pectoral spines in African sharptooth catfish, Clarias gariepinus (Burchell). Estonian J. Ecol. 2011, 60. [Google Scholar] [CrossRef]
- Steward, C.A.; DeMaria, K.D.; Shenker, J.M. Using otolith morphometrics to quickly and inexpensively predict age in the gray angelfish (Pomacanthus arcuatus). Fish. Res. 2009, 99, 123–129. [Google Scholar] [CrossRef]
- Dejen, E.; Vijverberg, J.; Nagelkerke, L.A.; Sibbing, F.A. Temporal and spatial distribution of microcrustacean zooplankton in relation to turbidity and other environmental factors in a large tropical lake (L.; Tana, Ethiopia). Hydrobiologia 2004, 513, 39–49. [Google Scholar] [CrossRef]
- Nagelkerke, L. The Barbs of Lake Tana, Ethiopia: Morphological Diversity and Its Implications for Taxonomy, Trophic Resource Partitioning, and Fisheries; Wageningen Agricultural University: Wageningen, The Netherlands, 1997. [Google Scholar]
- Brown, P.; Green, C.; Sivakumaran, K.; Stoessel, D.; Giles, A. Validating otolith annuli for annual age determination of common carp. Trans. Am. Fish. Soc. 2004, 133, 190–196. [Google Scholar] [CrossRef]
- Phelps, Q.E.; Edwards, K.R.; Willis, D.W. Precision of five structures for estimating age of common carp. N. Am. J. Fish. Manag. 2007, 27, 103–105. [Google Scholar] [CrossRef]
- Weyl, O.L.; Stadtlander, T.; Booth, A.J. Establishment of translocated populations of smallmouth yellowfish, Labeobarbus aeneus (Pisces: Cyprinidae), in lentic and lotic habitats in the Great Fish River system, South Africa. Afr. Zool. 2009, 44, 93–105. [Google Scholar] [CrossRef]
- Beamish, R.; Fournier, D. A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- Chang, W.Y. A statistical method for evaluating the reproducibility of age determination. Can. J. Fish. Aquat. Sci. 1982, 39, 1208–1210. [Google Scholar] [CrossRef]
- Ogle, D. Modified Age Bias Plot. Available online: http://derekogle.com/fishR/2017-04-14-Modified_AgeBiasPlot (accessed on 3 April 2019).
- Hoenig, J.; Morgan, M.; Brown, C. Analysing differences between two age determination methods by tests of symmetry. Can. J. Fish. Aquat. Sci. 1995, 52, 364–368. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Bekaert, K.; Getahun, A.; Bruneel, S.; Anteneh, W.; Torreele, E.; Goethals, P. Comparison of Otolith Readability and Reproducibility of Counts of Translucent Zones Using Different Otolith Preparation Methods for Four Endemic Labeobarbus Species in Lake Tana, Ethiopia. Water 2019, 11, 1336. [Google Scholar] [CrossRef]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Ogle, D. FishR Vignette. Von Bertalanffy Growth Models; Northland College: Ashland, WI, USA, 2013; Volume 54. [Google Scholar]
- Chilton, D.E.; Beamish, R.J. Age Determination Methods for Fishes Studied by the Groundfish Program at the Pacific Biological Station; Department of Fisheries and Oceans: Ottawa, ON, Canada, 1982.
- Campana, S.E. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef]
- Zhu, X.; Rick, W.; Leonard, D.; Howland, K.; Mann, S.; Carmichael, T.J.; Tallman, R.F. Comparison of scales, pectoral fin rays, and otoliths for estimating age, growth, and mortality of lake whitefish, coregonus clupeaformis. In Great Slave Lake; Canadian Science Advisory Secretariat: Ottawa, ON, Canada, 2017. [Google Scholar]
- Winker, H.; Ellender, B.R.; Weyl, O.L.; Booth, A.J. Validation of growth zone deposition in otoliths of two large endemic cyprinids in Lake Gariep, South Africa. Afr. Zool. 2010, 45, 133–138. [Google Scholar] [CrossRef]
- Winker, H.; Weyl, O.; Booth, A.; Ellender, B. Validating and corroborating the deposition of two annual growth zones in asteriscus otoliths of common carp Cyprinus carpio from South Africa’s largest impoundment. J. Fish. Biol. 2010, 77, 2210–2228. [Google Scholar] [CrossRef] [PubMed]
- Ellender, B.; Weyl, O.; Winker, H. Age and growth and maturity of southern Africa’s largest cyprinid fish, the largemouth yellowfish Labeobarbus kimberleyensis. J. Fish. Biol. 2012, 81, 1271–1284. [Google Scholar] [CrossRef]
- Winker, H.; Weyl, O.L.; Booth, A.J.; Ellender, B.R. Life history strategy and population characteristics of an unexploited riverine cyprinid, Labeo capensis, in the largest impoundment in the Orange River Basin. Afr. Zool. 2012, 47, 85–99. [Google Scholar] [CrossRef]
- Taylor, G.C.; Peel, R.A.; Hay, C.J.; Weyl, O.L. Validation of the periodicity of growth zone formation in the otoliths of four fish species from the Upper Zambezi ecoregion, southern Africa. Afr. Zool. 2016, 51, 153–159. [Google Scholar] [CrossRef]
- Vilizzi, L.; Walker, K.F. Age and growth of the common carp, Cyprinus carpio, in the River Murray, Australia: Validation, consistency of age interpretation, and growth models. Environ. Biol. Fishes 1999, 54, 77–106. [Google Scholar] [CrossRef]



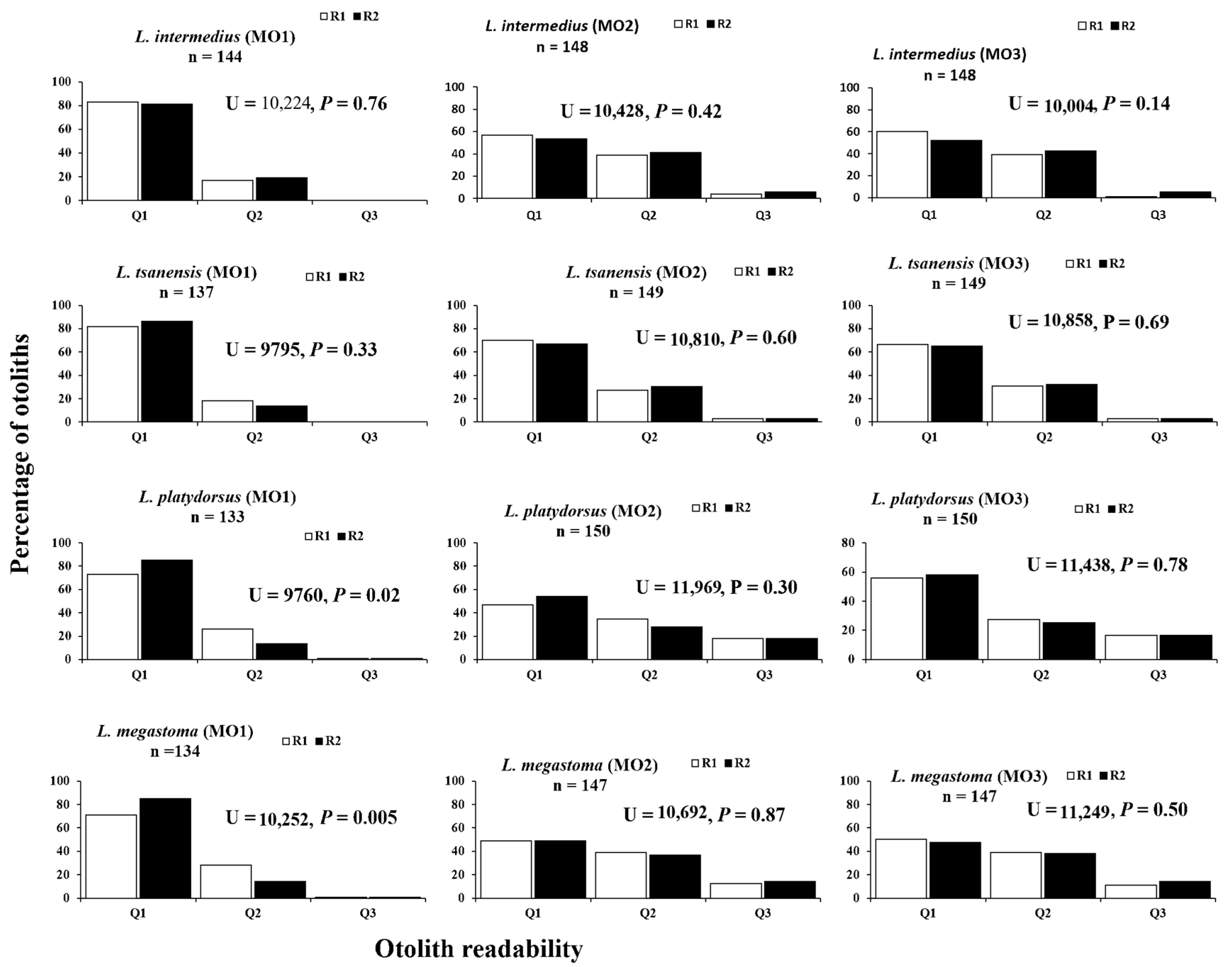

| Aging Methods | Readers’ Confidence | Kruskal–Wallis Rank Sum Test | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Chi-Square | df | p-Value | |
| L. intermedius | ||||||
| MO1a | 119 | 25 | 0 | 26.25 | 2 | 0.000 |
| MO2b | 85 | 58 | 5 | |||
| MO3b | 88 | 58 | 2 | |||
| L. tsanensis | ||||||
| MO1a | 112 | 25 | 0 | 9.69 | 2 | 0.008 |
| MO2b | 104 | 41 | 4 | |||
| MO3b | 99 | 46 | 4 | |||
| L. platydorsus | ||||||
| MO1a | 97 | 35 | 1 | 27.10 | 2 | 0.000 |
| MO2b | 70 | 52 | 28 | |||
| MO3b | 84 | 41 | 25 | |||
| L. megastoma | ||||||
| MO1a | 95 | 38 | 1 | 23.97 | 2 | 0.000 |
| MO2b | 72 | 57 | 18 | |||
| MO3b | 74 | 57 | 16 | |||
| Between Readers | |||||||||||||||
| Species | MO1 | MO2 | MO3 | ||||||||||||
| n | R | CV | APE | PA | n | R | CV | APE | PA | n | R | CV | APE | PA | |
| L. intermedius | 144 | 2 | 4 | 3 | 69 | 139 | 2 | 7 | 5 | 57 | 144 | 2 | 6 | 4 | 64 |
| L. tsanensis | 137 | 2 | 7 | 5 | 60 | 143 | 2 | 9 | 6 | 56 | 143 | 2 | 9 | 6 | 56 |
| L. platydorsus | 133 | 2 | 7 | 5 | 68 | 119 | 2 | 9 | 6 | 55 | 123 | 2 | 9 | 6 | 62 |
| L. megastoma | 133 | 2 | 11 | 8 | 45 | 125 | 2 | 13 | 9 | 42 | 123 | 2 | 11 | 8 | 47 |
| Between Methods | |||||||||||||||
| MO1 vs MO2 | MO1 vs MO3 | MO2 vs MO3 | |||||||||||||
| L. intermedius | 128 | 2 | 3 | 2 | 77 | 128 | 2 | 4 | 3 | 67 | 128 | 2 | 3 | 2 | 77 |
| L. tsanensis | 129 | 2 | 4 | 3 | 73 | 129 | 2 | 5 | 4 | 71 | 129 | 2 | 4 | 3 | 78 |
| L. platydorsus | 104 | 2 | 5 | 4 | 71 | 104 | 2 | 5 | 4 | 75 | 104 | 2 | 3 | 2 | 81 |
| L. megastoma | 99 | 2 | 4 | 3 | 71 | 99 | 2 | 4 | 3 | 77 | 99 | 2 | 4 | 3 | 79 |
| Methods | Reader | t-Value | df | p-Value | 95% Confidence Intervals | |
|---|---|---|---|---|---|---|
| R1(Mean) | R2 (Mean) | |||||
| L. intermedius | ||||||
| MO1 | 5.9 | 6.1 | −0.61 | 285 | 0.54 | −0.59–0.31 |
| MO2 | 5.8 | 6.1 | −1.45 | 276 | 0.15 | −0.81–0.12 |
| MO3 | 5.8 | 6.1 | −1.22 | 277 | 0.22 | −0.75–0.17 |
| L. tsanensis | ||||||
| MO1 | 4.7 | 4.9 | −0.75 | 272 | 0.46 | −0.64–0.29 |
| MO2 | 4.5 | 4.8 | −1.06 | 284 | 0.29 | −0.66–0.20 |
| MO3 | 4.8 | 4.8 | −0.28 | 284 | 0.78 | −0.50–0.38 |
| L. platydorsus | ||||||
| MO1 | 4.4 | 4.5 | −0.39 | 263 | 0.70 | −0.41–0.27 |
| MO2 | 4.1 | 4.3 | −1.45 | 236 | 0.15 | −0.56–0.08 |
| MO3 | 4.2 | 4.3 | −0.94 | 244 | 0.35 | −0.50–0.18 |
| L. megastoma | ||||||
| MO1 | 4.1 | 4.7 | −2.97 | 259 | 0.003 | −1.01–(−0.20) |
| MO2 | 3.8 | 4.6 | −3.42 | 229 | 0.000 | −1.20–(−0.32) |
| MO3 | 4.0 | 4.6 | −3.03 | 233 | 0.003 | −1.09–(−0.23) |
| Between Readers | |||||||||
| Species | MO1 | MO2 | MO3 | ||||||
| df | Chi-sq | p | df | Chi-sq | p | df | Chi-sq | p | |
| L. intermedius | 17 | 19 | ns | 19 | 28 | ** | 15 | 24 | ns |
| L. tsanensis | 14 | 22 | ns | 15 | 30 | * | 15 | 21 | ns |
| L. platydorsus | 10 | 19 | ns | 10 | 20 | * | 10 | 15 | ns |
| L. megastoma | 15 | 58 | ** | 17 | 59 | ** | 13 | 58 | ** |
| Between Methods | |||||||||
| MO1 vs. MO2 | MO1 vs. MO3 | MO2 vs. MO3 | |||||||
| L. intermedius | 9 | 7 | ns | 10 | 8 | ns | 10 | 9 | ns |
| L. tsanensis | 13 | 14 | ns | 16 | 12 | ns | 13 | 15 | ns |
| L. platydorsus | 9 | 15 | ns | 11 | 10 | ns | 4 | 6 | ns |
| L. megastoma | 8 | 16 | * | 7 | 11 | ns | 7 | 9 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebremedhin, S.; Bekaert, K.; Getahun, A.; Bruneel, S.; Anteneh, W.; Goethals, P.; Torreele, E. Comparison of Otolith Readability and Reproducibility of Counts of Translucent Zones Using Different Otolith Preparation Methods for Four Endemic Labeobarbus Species in Lake Tana, Ethiopia. Water 2019, 11, 1336. https://doi.org/10.3390/w11071336
Gebremedhin S, Bekaert K, Getahun A, Bruneel S, Anteneh W, Goethals P, Torreele E. Comparison of Otolith Readability and Reproducibility of Counts of Translucent Zones Using Different Otolith Preparation Methods for Four Endemic Labeobarbus Species in Lake Tana, Ethiopia. Water. 2019; 11(7):1336. https://doi.org/10.3390/w11071336
Chicago/Turabian StyleGebremedhin, Shewit, Karen Bekaert, Abebe Getahun, Stijn Bruneel, Wassie Anteneh, Peter Goethals, and Els Torreele. 2019. "Comparison of Otolith Readability and Reproducibility of Counts of Translucent Zones Using Different Otolith Preparation Methods for Four Endemic Labeobarbus Species in Lake Tana, Ethiopia" Water 11, no. 7: 1336. https://doi.org/10.3390/w11071336
APA StyleGebremedhin, S., Bekaert, K., Getahun, A., Bruneel, S., Anteneh, W., Goethals, P., & Torreele, E. (2019). Comparison of Otolith Readability and Reproducibility of Counts of Translucent Zones Using Different Otolith Preparation Methods for Four Endemic Labeobarbus Species in Lake Tana, Ethiopia. Water, 11(7), 1336. https://doi.org/10.3390/w11071336






