Analysis of Environmental Factors Associated with Cyanobacterial Dominance after River Weir Installation
Abstract
:1. Introduction
2. Materials and Methods
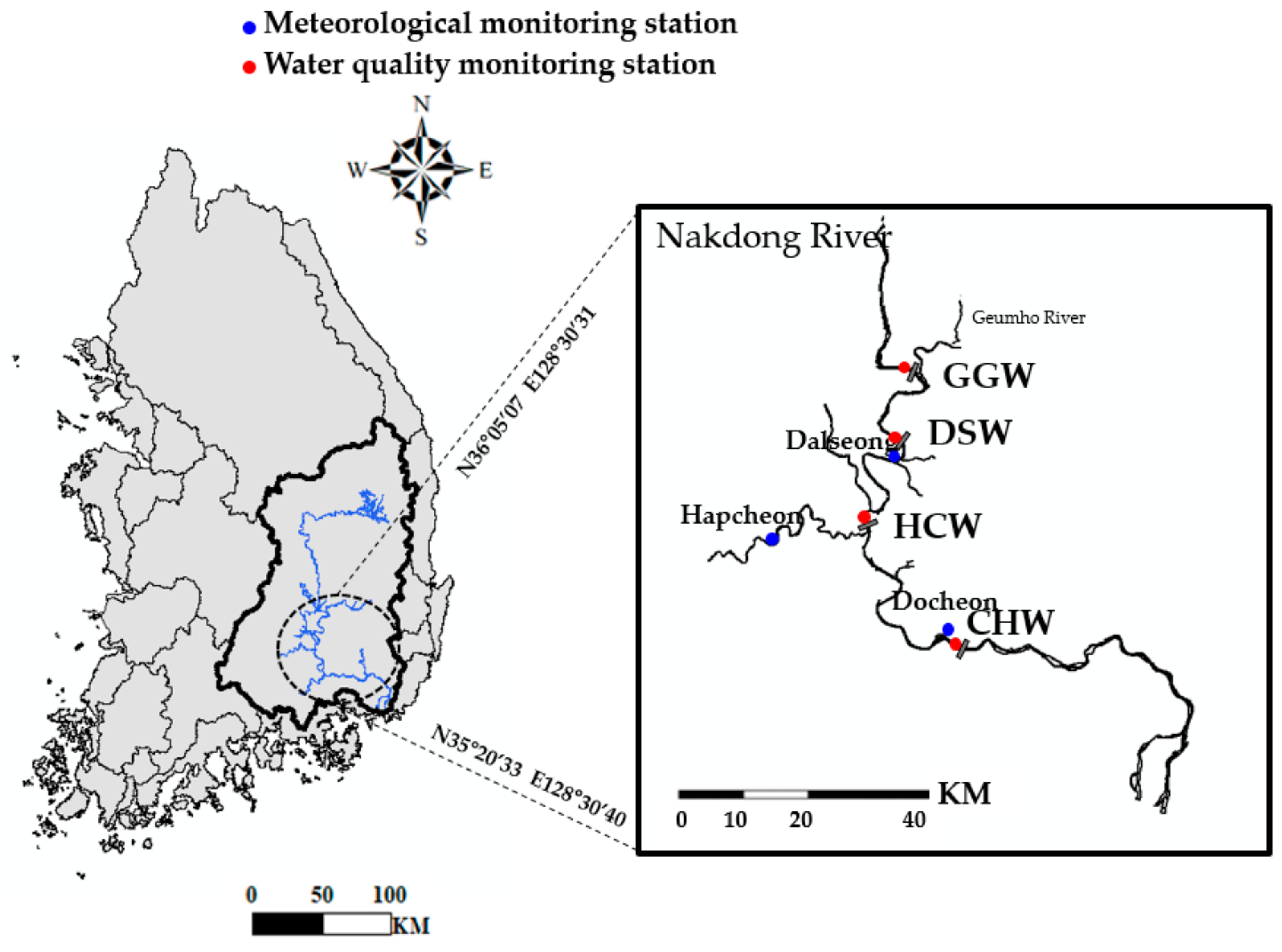
2.1. Site Description
2.2. Sampling and Analysis
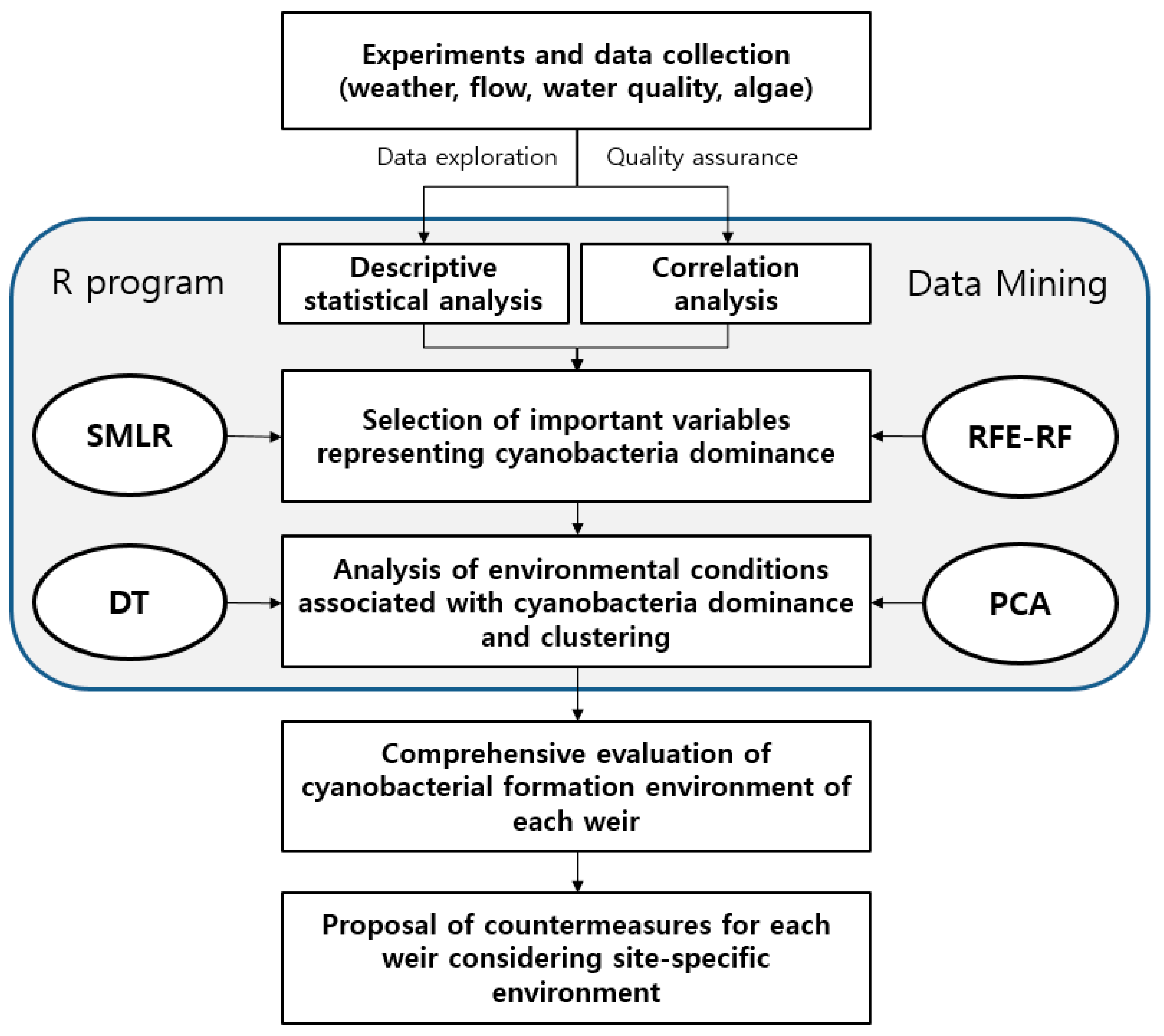
2.3. Statistical Analyses
3. Results and Discussion
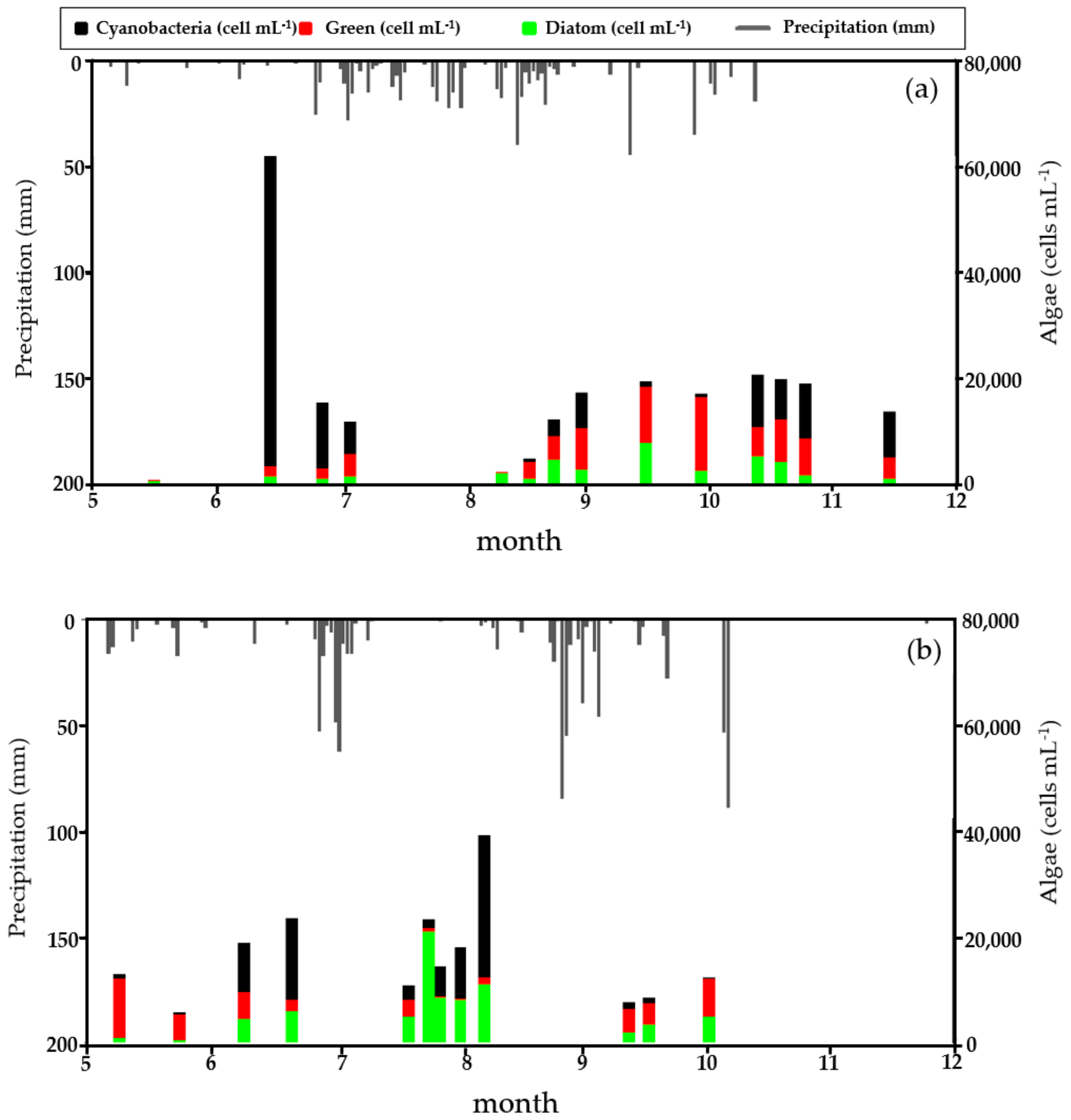
3.1. Descriptive Statistics of Collected Data
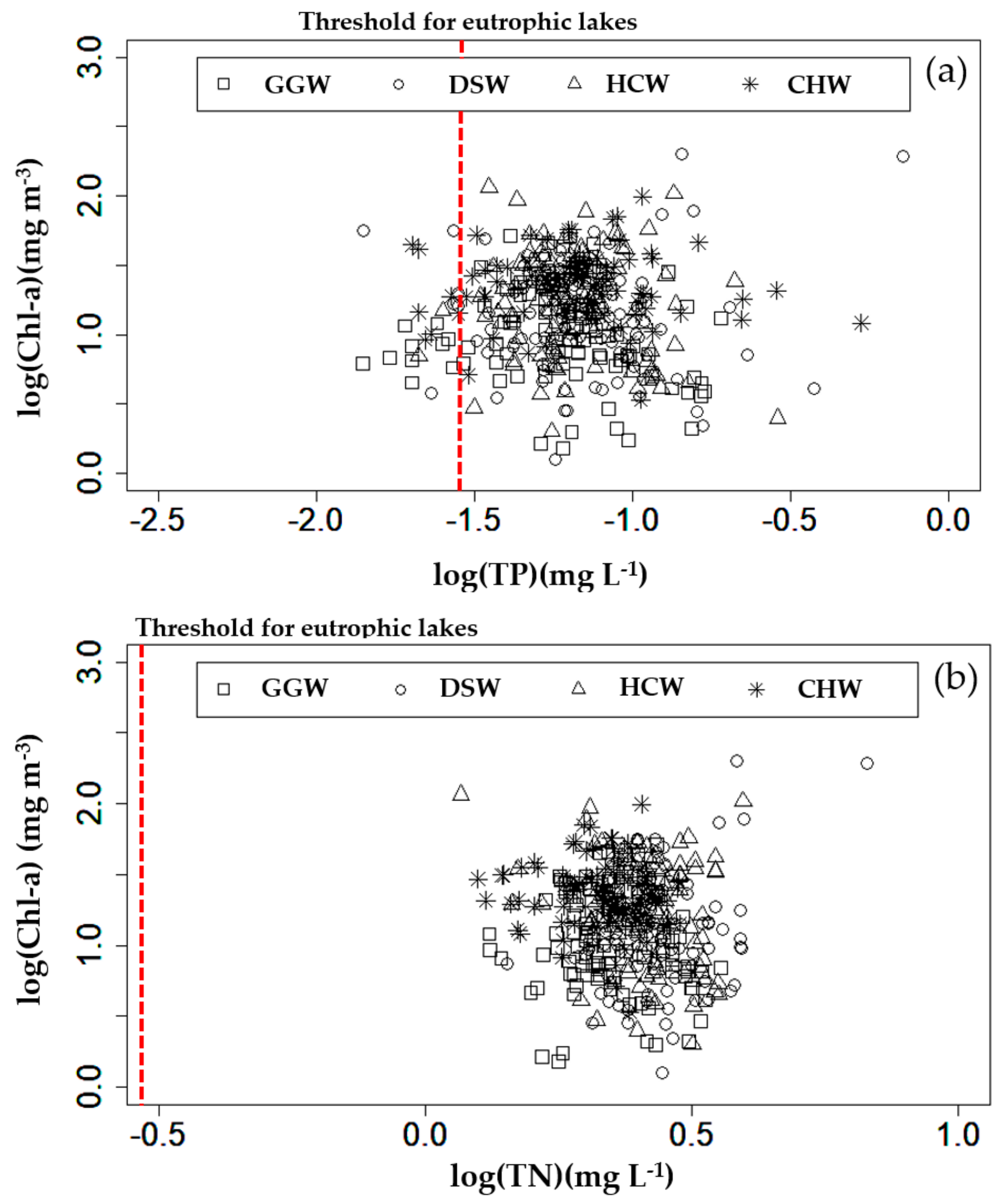
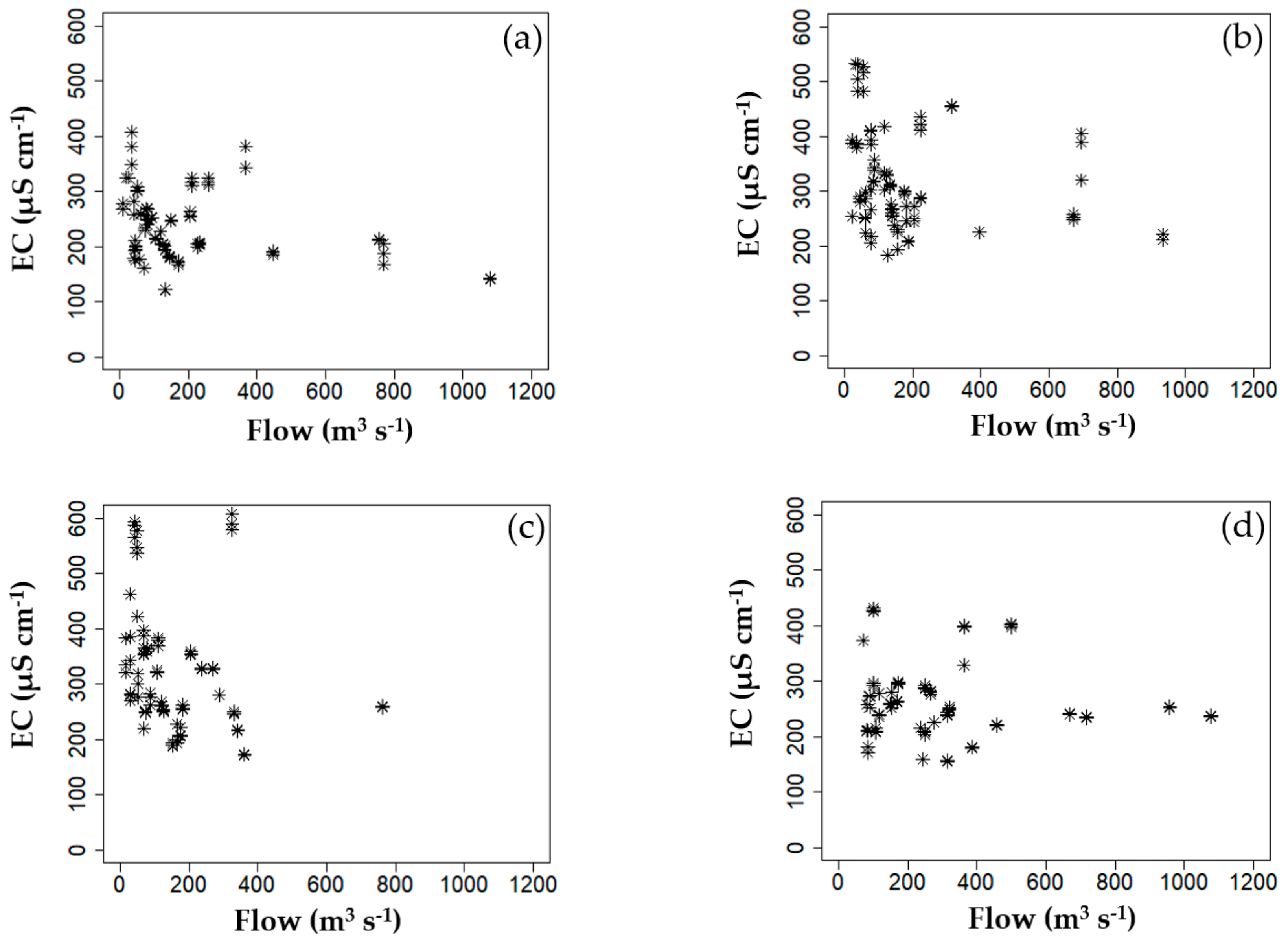
3.2. Correlation Analysis of Environmental Factors
3.3. Selection of Important Environmental Factors Associated with Cyanobacterial Dominance
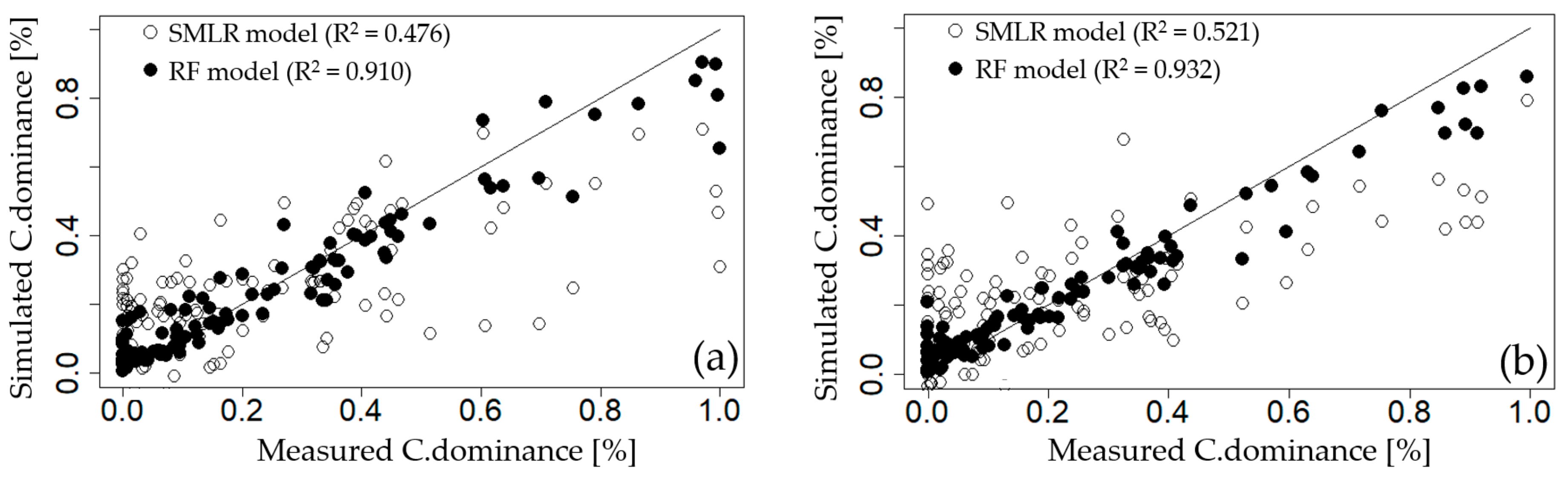
3.3.1. Step-Wise Multiple Linear Regression (SMLR)
3.3.2. Recursive Feature Elimination Based on Random Forest Model (RFE-RF)
3.4. Characterizing Environmental Conditions of High Cyanobacterial Dominance
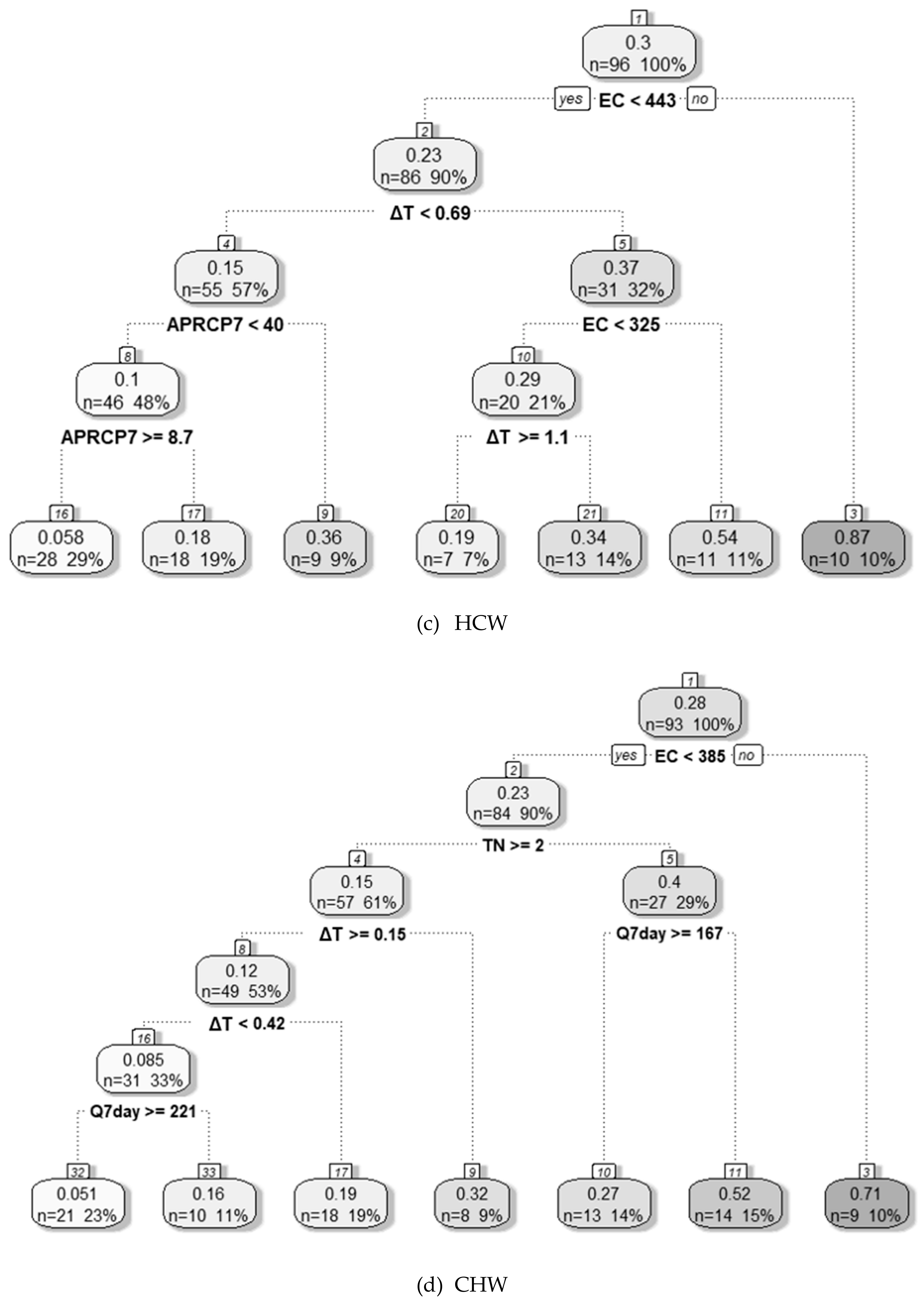
3.4.1. Decision Tree Analysis
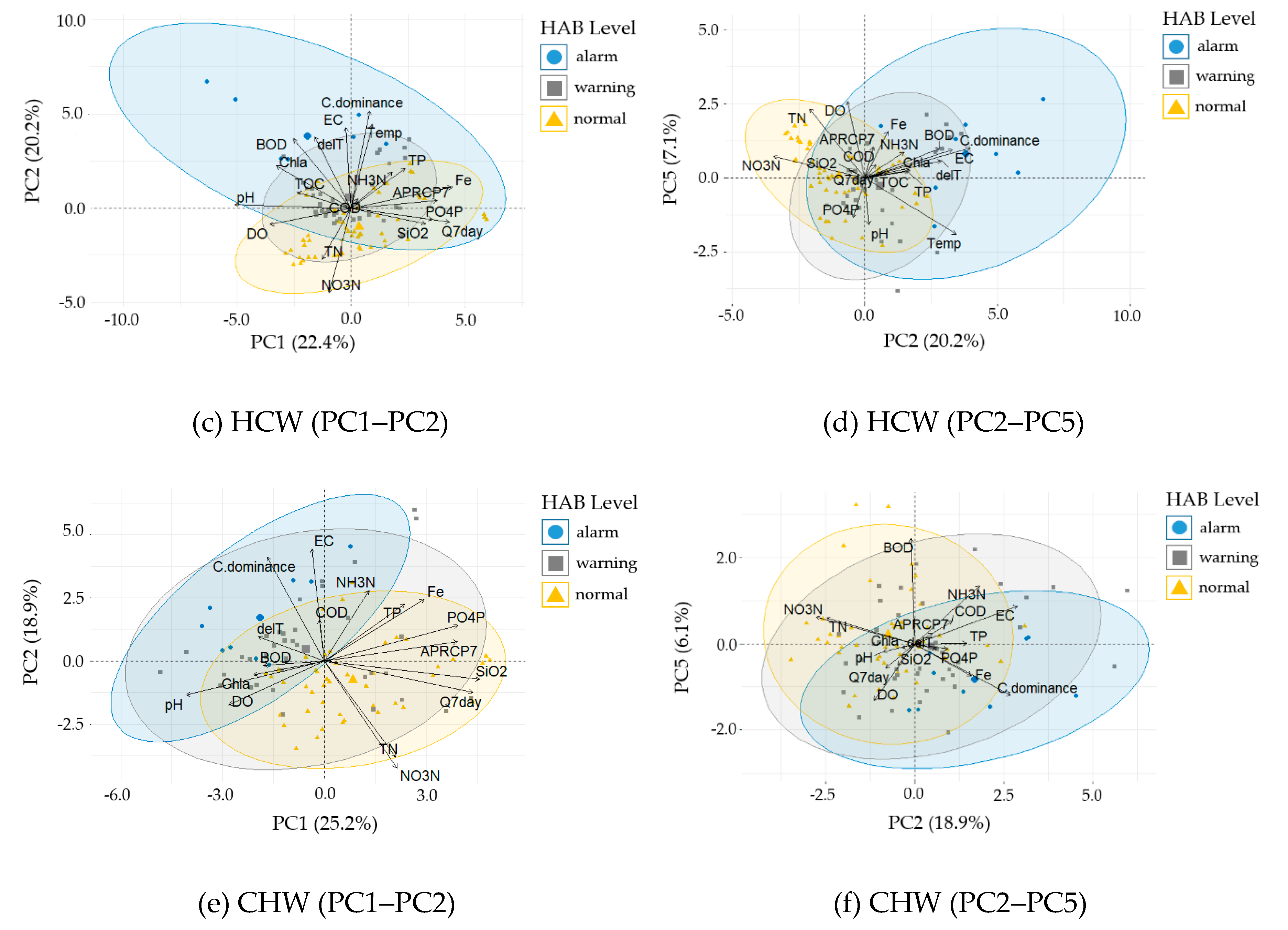
3.4.2. Principal Component Analysis
3.5. Integrated Analysis of Environmental and Control Variables for Cyanobacterial Dominance at Each Weir
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Welch, E.B.; Jacoby, J.M.; Horner, R.R.; Seeley, M.R. Nuisance biomass levels of periphytic algae in streams. Hydrobiologia 1988, 157, 161–168. [Google Scholar] [CrossRef]
- Peter, A.; Köster, O.; Schildknecht, A.; Von, G.U. Occurrence of dissolved and particle-bound taste and order compounds in Swiss lake waters. Water Res. 2009, 43, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Parinet, J.; Rodriguez, M.J.; Serodes, J. Influence of water quality on the presence of off-flavour compounds (geosmin and 2-methylisoborneol). Water Res. 2010, 44, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S. Growth and buoyancy of Microcystis aeruginosa Kutz. Emend. Elenkin in a shallow Eutrophic Lake. Proc. Biol. Sci. 1973, 184, 29–50. [Google Scholar] [CrossRef]
- Srivastava, A.; Ahn, C.Y.; Asthana, R.K.; Lee, H.G.; Oh, H.M. Status, alert system, and prediction of cyanobacterial bloom in South Korea. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G.; Chorus, I.; Falconer, I.; Lin, T.F. Cyanobacteria: Impacts of climate change on occurrence, toxicity and water quality management. Water Res. 2012, 46, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef]
- Webster, I.T.; Jones, G.J.; Oliver, R.L.; Bormans, M.; Sherman, B.S. Control strategies for cyanobacterial blooms in weir pools. Final Gr. Rep. Natl. Res. Manag. Strateg. Tech. Rep. 1995, 119, M3116. [Google Scholar]
- Sherman, B.S.; Webster, I.T. Transitions between Aulacoseira and Anabaena dominance in a turbid river weir pool. Limnol. Oceanogr. 1998, 43, 1902–1915. [Google Scholar] [CrossRef]
- Bormans, M.; Maier, H.; Burch, M. Temperature stratification in the lower River Murray, Australia: Implication for cyanobacterial bloom development. Mar. Freshwater Res. 1997, 48, 647–654. [Google Scholar] [CrossRef]
- Oliver, R.L.; Hart, B.T.; Olley, J.; Grace, M.; Rees, C.; Caitcheon, G. The Darling River: algal growth and the cycling and sources of nutrients. Murray Darling Basin Comm. Proj. M386. Final Rep. 1999, 201. Available online: https://publications.csiro.au/rpr/download?pid=procite:1edb0e8f-ba1b-46d5-b748-aa77850f0f03&dsid=DS1 (accessed on 3 June 2019).
- Mitrovic, S.M.; Oliver, R.L.; Rees, C.; Bowling, R.; Buckney, T. Critical flow velocities for the growth and dominance of Anabaena circinalis in some turbid freshwater rivers. Freshwater Biol. 2003, 48, 164–174. [Google Scholar] [CrossRef]
- Tsujimura, S.; Okubo, T. Development of Anabaena blooms in a small reservoir with dense sediment akinete population, with special reference to temperature and irradiance. J. Plankton Res. 2003, 25, 1059–1067. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Chen, Y. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: An 11-year investigation. Harmful Algae 2010, 10, 337–343. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Walsby, A.E. Water-blooms. Biol. Rev. Camb. Philos. Soc. 1975, 50, 437–481. [Google Scholar] [CrossRef]
- Walsby, A.E. Stratification by cyanobacteria in lakes: A dynamic buoyancy model indicates size limitations met by Planktothrix rubescens filaments. New Phytol. 2005, 168, 365–376. [Google Scholar] [CrossRef]
- Brookes, J.D.; Granf, G.G. Variations in the buoyancy response of Microcystis aeruginosa to nitrogen, phosphorus and light. J. Plankton Res. 2001, 23, 1399–1411. [Google Scholar] [CrossRef]
- Bonnet, M.P.; Poulin, M. Numerical modelling of the planktonic succession in a nutrient-rich reservoir: environmental and physiological factors leading to Microcystis aeruginosa dominance. Ecol. Model. 2002, 156, 93–112. [Google Scholar] [CrossRef]
- Chung, S.W.; Imberger, J.; Hipsey, M.R.; Lee, H.S. The influence of physical and physiological processes on the spatial heterogeneity of a Microcystis bloom in a stratified reservoir. Ecol. Model. 2014, 289, 133–149. [Google Scholar] [CrossRef]
- Moss, B.; Balls, H. Phytoplankton distribution in a floodplain lake and river and river system: Ⅱ, Seasonal changes in the phytoplankton communities and their control by hydrology and nutrient availability. J. Plankton Res. 1989, 11, 839–867. [Google Scholar] [CrossRef]
- Wu, N.; Schmalz, B.; Fohrer, N. Distribution of phytoplankton in a German Lowland river in relation to environmental factors. J. Plankton Res. 2011, 33, 807–820. [Google Scholar] [CrossRef]
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.P.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem measurement. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Ahn, C.Y.; Lee, J.Y.; Oh, H.M. Control of microalgal growth and competition by N:P ratio manipulation. KRIBB 2013, 31, 61–68. [Google Scholar] [CrossRef]
- Bostrom, B.; Jansson, M.; Forsberg, C. Phosphorus release from lake sediments. Arch. Hydrobiol. 1982, 18, 5–59. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, Y.; Zhou, J.; Wu, Y. Phosphorus release from lake sediments: Effects of pH, temperature and dissolved oxygen. KSCE J. Civ. Eng. 2014, 18, 323–329. [Google Scholar] [CrossRef]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Supporting Documentation—Cyanobacterial Toxins-Microcystin-LR. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. 2002. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-cyanobacterial-toxins-document.html/ (accessed on 27 August 2018).
- Brock, T.D. Lower pH limit for the existence of blue-green algae: Evolutionary and ecological implications. Science 1973, 179, 480–483. [Google Scholar] [CrossRef]
- Holm-Hansen, O. Ecology, physiology, and biochemistry of blue-green algae. Annu. Rev. Microbiol. 1968, 22, 47–70. [Google Scholar] [CrossRef]
- Cho, H.J.; Na, J.E.; Jung, M.H.; Lee, H.Y. Relationship between phytoplankton community and water quality in lakes in Jeonnam using SOM. Korean J Limnol. 2017, 50, 148–156. [Google Scholar] [CrossRef]
- Czerny, J.; Barcelos e Ramos, J.; Riebesell, U. Influence of elevated CO2 concentrations on cell division and nitrogen fixation rates in the bloom-forming cyanobacterium Nodularia spumigena. Biogeosciences 2009, 6, 1865–1875. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nakahara, H. The formation and degradation of cyanobacterium Aphanizomenon flos-aquae blooms: the importance of pH, water temperature, and day length. Jpn. J Limnol. 2005, 6, 1–6. [Google Scholar] [CrossRef]
- Walsby, A.E.; Hayes, P.K.; Boje, R. The gas vesicles, buoyancy and vertical distribution of cyanobacteria in the Baltic Sea. Eur. J. Phycol. 1995, 30, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Academic Press: San Diego, CA, USA, 2001; 1006p, ISBN1 0127447601. ISBN2 9780127447605. [Google Scholar]
- Thomas, R.H.; Walsby, A.E. The effects of temperature on recovery of buoyancy by Microcystis. J. Gen. Microbiol. 1986, 132, 1665–1672. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.R. Cascading trophic interactions and lake productivity. Bioscience 1993, 35, 634–639. [Google Scholar] [CrossRef]
- Konopka, A.E.; Klemer, A.R.; Walsby, A.E.; Ibelings, B.W. Effects of macronutrients upon buoyancy regulation by metalimnetic Oscillatoria agardhii in Deming Lake. J. Plankton Res. 1993, 15, 1019–1034. [Google Scholar] [CrossRef]
- Arnold, D.E. Ingestion, assimilation, survival, and reproduction by Daphnia pulex fed seven species of blue-green algae. Limnol. Oceanogr. 1971, 16, 906–920. [Google Scholar] [CrossRef]
- Fulton, R.S.; Paerl, H.W. Toxic and inhibitory effects of the blue-green algae Microcystis aeruginosa on herbivorous zooplankton. J. Plankton Res. 1987, 9, 837–855. [Google Scholar] [CrossRef]
- DeMott, W.R. Optimal foraging theory as a predictor of chemically mediated food selection by suspension-feeding copepods. Limnol. Oceanogr. 1989, 34, 140–154. [Google Scholar] [CrossRef]
- Javier, M.G.; Saul, A. Formation and maintenance of nitrogen fixing cell patterns in filamentous cyanobacteria. Proc. Natl. Acad. Sci. USA 2016, 113, 6218–6223. [Google Scholar] [CrossRef]
- Lee, J.K. Bacterial quorum sensing and quorum quenching for the inhibition of biofilm formation. Korean J. Microbiol. Biotechnol. 2012, 40, 83–91. [Google Scholar] [CrossRef]
- Manefield, M.; Rasmussen, T.B.; Henzter, M.; Andersen, J.B.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 2002, 148, 1119–1146. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Givskov, M. Quorum sensing inhibitors a bargain of effects. Microbiology 2006, 152, 895–904. [Google Scholar] [CrossRef]
- Recknagel, F.; French, M.; Harkonen, P.; Yabunaka, K. Artificial neural network approach for the modelling and prediction of algal blooms. Ecol. Model. 1997, 96, 11–28. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, E. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystem and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Isles, P.D.F.; Rizzo, D.M.; Xu, Y.; Schroth, A.W. Modeling the drivers of interannual variability in cyanobacterial bloom severity using self-organizing maps and high-frequency data. Inland Water 2017, 7, 333–347. [Google Scholar] [CrossRef]
- Tian, D.; Xie, G.; Tian, J.; Tseng, K.H.; Shum, C.K.; Lee, J.Y.; Liang, S. Spatiotemporal variability and environmental factors of harmful algal blooms (HABs) over western Lake Erie. PLoS ONE 2017, 12, 1932–6203. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.J.; Goldman, C.R. Limnology; McGraw-Hill: New York, NY, USA, 1994; p. 465. ISBN 9780070236738. [Google Scholar]
- Sze, P. A Biology of the Algae, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1998; 278p, ISBN1 0697219100. ISBN2 9780697219107. [Google Scholar]
- An, K.G.; Jones, J.R. Factors regulating bluegreen dominance in a reservoir directly influenced by the Asian monsoon. Hydrobiologia 2000, 432, 37–48. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, I.K. Analysis of water quality factor and correlation between water quality and Chl-a in middle and downstream weir section of Nakdong River. J. Korean Soc. Environ. Eng. 2017, 39, 89–96. [Google Scholar] [CrossRef]
- Water Resources Management Information System. Available online: http://www.wamis.go.kr/ (accessed on 27 December 2018).
- Water Environment Information System. Available online: http://water.nier.go.kr/ (accessed on 27 December 2018).
- Ministry of Environment. 2017. Available online: http://www.me.go.kr/ (accessed on 27 December 2018).
- Korea Meteorological Administration (KMA). Available online: http://data.kma.go.kr/ (accessed on 27 December 2018).
- K-Water. Available online: http://English.kwater.or.kr/ (accessed on 27 December 2018).
- Breiman, L.; Cutler, A. Breiman and Cutler’s Random Forests for Classification and Regression. 2015. Available online: http://cran.R-project.org/package=randomForest/ (accessed on 25 May 2018).
- Kuhn, M. Classification and Regression Training. 2011. Available online: https://github.com/topeop/caret/issues (accessed on 3 June 2019).
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Box, G.; Cox, D. An analysis of transformations. J. R. Stat. Soc. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Jung, S.J.; Lee, D.J.; Hwang, K.S.; Lee, K.H.; Choi, K.C.; Im, S.S.; Lee, Y.H.; Lee, J.Y.; Lim, B.J. Evaluation of pollutant characteristics in Yeongsan River using multivariate analysis. Korean J. Limnol. 2012, 45, 368–377. [Google Scholar] [CrossRef]
- Tromas, N.; Fortin, N.; Bedrani, L.; Terrat, Y.; Cardoso, P.; Bird, D.; Greer, C.W.; Shapiro, B.J. Characterising and predicting cyanobacterial blooms in an 8-year amplicon sequencing time course. ISME J. 2017, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Husson, F.; Le, S.; Pages, J. Exploratory Multivariate Analysis by Example Using R; Chapman and Hall: Boca Raton, FL, USA, 2010; ISBN 9780429190032. [Google Scholar]
- Ferreira, F.; Straus, N.A. Iron deprivation in cyanobacteria. J. Appl. Phycol. 1994, 6, 199–210. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by Randomforest. R News 2002, 2, 18–22. [Google Scholar]
- Johnson, T.C.; Odada, E.; Whittaker, K.T. The Limnology, Climatology and Paleoclimatology of the East African Lakes; Gordon and Breach Publishers: Amsterdam, The Netherlands, 1996; ISBN 2884492348. [Google Scholar]
- Moreira, S.; Schultze, M.; Rahn, K.; Boehrer, B. A practical approach to lake water density from electrical conductivity and temperature. Hydrol. Earth Syst. Sci. 2016, 20, 2975–2986. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.R.; Oh, H.M.; Lee, J.A. Ecological study on the toxic Microcystis in downstream of the Nakdong River. Algae 2002, 17, 171–185. [Google Scholar] [CrossRef]
- Paerl, H.W. Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnol. Oceanogr. 1997, 42, 1154–1165. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.R.; Lee, H.W. A study on water quality after construction of the weirs in the middle area in Nakdong River. J. Korean Soc. Environ. Eng. 2014, 36, 258–264. [Google Scholar] [CrossRef]



| Variable | Unit | Weir | |||
|---|---|---|---|---|---|
| GGW | DSW | HCW | CHW | ||
| Sample size | n | 108 | 108 | 96 | 93 |
| Temperature | °C | 23.7 (±4.8) | 23.9 (±4.8) | 23.9 (±5.2) | 25.2 (±4.5) |
| pH | - | 8.2 (±0.6) | 8.2 (±0.8) | 8.1 (±0.8) | 8.2 (±0.8) |
| DO | mg L−1 | 9.01 (±1.79) | 9.20 (±1.90) | 9.07 (±2.15) | 8.63 (±1.74) |
| EC | µS cm−1 | 237.5 (±59.0) | 318.4 (±89.6) | 312.7 (±107.1) | 259.3 (±64.6) |
| SS | mg L−1 | 8.66 (±7.86) | 10.1 (±13.7) | 8.79 (±6.44) | 9.80 (±5.55) |
| BOD | mg L−1 | 1.91 (±0.88) | 2.23 (±2.85) | 1.98 (±1.22) | 2.08 (±1.02) |
| COD | mg L−1 | 4.85 (±0.89) | 6.16 (±5.34) | 5.59 (±0.90) | 5.04 (±0.81) |
| TOC | mg L−1 | 4.30 (±1.14) | 4.45 (±1.16) | 4.36 (±0.96) | 4.01 (±0.78) |
| TN | mg L−1 | 2.29 (±0.48) | 2.83 (±0.85) | 2.61 (±0.50) | 2.18 (±0.41) |
| NH3–N | mg L−1 | 0.121 (±0.094) | 0.144 (±0.102) | 0.102 (±0.076) | 0.107 (±0.085) |
| NO3–N | mg L−1 | 1.65 (±0.43) | 2.03 (±0.50) | 1.88 (±0.50) | 1.48 (±0.46) |
| TP | mg L−1 | 0.068 (±0.036) | 0.087 (±0.100) | 0.072 (±0.039) | 0.078 (±0.063) |
| PO4–P | mg L−1 | 0.037 (±0.069) | 0.035 (±0.033) | 0.027 (±0.021) | 0.028 (±0.022) |
| Chl–a | mg m−3 | 14.7 (±11.3) | 36.5 (±162.7) | 24.0 (±21.0) | 26.2 (±15.6) |
| Cyano | cells mL−1 | 3398 (±9,663) | 9236 (±67,068) | 10,761 (±48,555) | 7,323 (±25,767) |
| Green | cells mL−1 | 3618 (±7,654) | 3239 (±3,037) | 3150 (±4035) | 3,250 (±3472) |
| Diatom | cells mL−1 | 2582 (±2,395) | 3491 (±3,513) | 4139 (±3971) | 5,312 (±3847) |
| Outflow7 | m3 s −1 | 173.91 (±171.43) | 172.83 (±144.54) | 180.19 (±143.73) | 287.08 (±191.96) |
| APRCP7 | mm | 29.8 (±41.7) | 17.6 (±23.4) | 19.5 (±20.7) | 22.7 (±22.4) |
| △T | °C | 2.2 (±1.7) | 1.5 (±1.1) | 1.0 (±1.2) | 0.6 (±0.8) |
| Fe | mg L−1 | 0.10 (±0.06) | 0.01 (±0.01) | 0.08 (±0.05) | 0.07 (±0.04) |
| SiO2 | mg L−1 | 4.93 (±3.43) | 4.17 (±3.29) | 3.59 (±2.71) | 2.81 (±2.80) |
| Weir | Variables | Adj.R2 | RMSE | CP | AIC |
|---|---|---|---|---|---|
| GGW | EC, NO3–N, TN, ΔT | 0.476 | 0.195 | 4.4 | −43.9 |
| DSW | NO3–N, EC, TN, Temp, NH3–N, Q7day | 0.521 | 0.182 | 4.5 | −59.7 |
| HCW | EC, NO3–N, Temp, TN, Q7day, Chl–a, APRCP7, Fe | 0.729 | 0.155 | 7.8 | −86.8 |
| CHW | NO3–N, EC, APRCP7, Q7day, TN | 0.538 | 0.182 | 2.8 | −52.6 |
| Weir | Order of variable importance | RMSE (%) |
|---|---|---|
| GGW | EC > Temp > ΔT > Q7day > TN > PO4–P > APRCP7 | 0.162 |
| DSW | EC > TOC > Temp > TP > TN > Q7day > ΔT | 0.156 |
| HCW | EC > ΔT > Temp > Q7day > APRCP7 > TOC > Fe > PO4-P | 0.145 |
| CHW | EC > TN > APRCP7 > ΔT > Q7day | 0.160 |
| Variable | GGW | DSW | HCW | CHW | ||||
|---|---|---|---|---|---|---|---|---|
| Component | Component | Component | Component | |||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| C.dominance | 6.75 | 6.00 | 3.99 | 5.61 | 0.42 | 19.26 | 2.10 | 16.77 |
| APRCP7 | 3.69 | 16.42 | - | 9.29 | 0.12 | 11.40 | 0.58 | |
| Q7day | 8.97 | 11.53 | - | 12.24 | 0.39 | 14.26 | 1.54 | |
| Temp | 0.39 | 13.41 | - | 0.57 | 14.55 | - | - | |
| ΔT | 6.56 | 1.20 | - | 1.65 | 10.31 | 2.79 | 0.91 | |
| DO | - | - | 8.21 | 0.54 | 5.87 | 2.98 | ||
| pH | 5.40 | 5.07 | 5.15 | 16.98 | 17.04 | 0.03 | 12.23 | 1.81 |
| EC | 14.66 | 2.92 | - | 0.03 | 13.27 | 0.10 | 19.43 | |
| BOD | - | 15.01 | 22.48 | 4.21 | 9.75 | 2.38 | 0.03 | |
| COD | 7.59 | 4.76 | 7.51 | 35.81 | 0.05 | 0.19 | 0.01 | 2.60 |
| TOC | 5.65 | 2.87 | 6.19 | 7.36 | 3.62 | 0.53 | - | - |
| TP | 8.90 | 1.54 | 23.80 | 0.46 | 3.70 | 3.25 | 4.15 | 5.01 |
| PO4–P | 2.76 | 0.02 | - | 8.25 | 0.24 | 11.49 | 1.97 | |
| TN | 8.93 | 13.07 | 20.99 | 0.62 | 1.09 | 5.35 | 3.32 | 14.21 |
| NH3–N | - | - | - | - | 2.15 | 1.28 | 1.28 | 7.73 |
| NO3–N | 8.36 | 12.49 | - | 0.60 | 14.54 | 3.42 | 17.72 | |
| Fe | 1.60 | 1.68 | - | 12.88 | 0.92 | 6.42 | 5.89 | |
| SiO2 | 9.79 | 7.01 | - | 6.98 | 0.47 | 15.55 | 0.52 | |
| Chl–a | - | 17.36 | 10.68 | 7.01 | 3.63 | 3.23 | 0.29 | |
| Weir | Correlation Analysis | Recursive Feature Elimination | Decision Tree | PCA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (r > ∣0.5∣) | (Variable importance rank) | (C.dominance > 50% conditions) | (Clustering) | ||||||||||
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 5th | 1st | 2nd | Positive | Negative | |
| GGW | Chl–a | TOC | ΔT | - | EC | ΔT | TN | Temp | Q7day | EC ≥ 325 μS cm−1 | - | EC, ΔT, Temp | TOC NO3–N TN |
| DSW | Chl–a | SS | TN | TP | EC | TOC | Temp | ΔT | TP | EC ≥ 336 μS cm−1 TP ≥ 0.082 mg L−1 | EC ≥ 336 μS cm−1 TP < 0.082 mg L−1 TN < 2.4 mg L−1 | pH, TOC Chl–a | - |
| HCW | ΔT BOD | - | - | - | EC | ΔT | Temp | Q7day | Fe | EC ≥ 443 μS cm−1 | 325 μS cm−1 ≤ EC < 443 μS cm−1 ΔT ≥ 0.7 °C | EC Temp ΔT BOD NH3–N Chl-a | NO3–N TN |
| CHW | - | - | - | - | EC | ΔT | TN | Q7day | APRCP7 | EC ≥ 385 μS cm−1 | EC < 385 μS cm−1 TN < 2 mg L−1 Q7day < 167 m3 s−1 | EC ΔT Fe | NO3–N TN |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Chung, S.; Park, H.; Cho, Y.; Lee, H. Analysis of Environmental Factors Associated with Cyanobacterial Dominance after River Weir Installation. Water 2019, 11, 1163. https://doi.org/10.3390/w11061163
Kim S, Chung S, Park H, Cho Y, Lee H. Analysis of Environmental Factors Associated with Cyanobacterial Dominance after River Weir Installation. Water. 2019; 11(6):1163. https://doi.org/10.3390/w11061163
Chicago/Turabian StyleKim, Sungjin, Sewoong Chung, Hyungseok Park, Youngcheol Cho, and Heesuk Lee. 2019. "Analysis of Environmental Factors Associated with Cyanobacterial Dominance after River Weir Installation" Water 11, no. 6: 1163. https://doi.org/10.3390/w11061163
APA StyleKim, S., Chung, S., Park, H., Cho, Y., & Lee, H. (2019). Analysis of Environmental Factors Associated with Cyanobacterial Dominance after River Weir Installation. Water, 11(6), 1163. https://doi.org/10.3390/w11061163







